Abstract
The effect of decreasing the organic (octanol) to aqueous phase volume ratio was evaluated in a two-phase enzymatic process for (R)-phenylacetylcarbinol (PAC) production. Decreasing the ratio from 1:1 to 0.43:1 at 4°C increased PAC in the organic phase from 112 g/l to 183 g/l with a 10% improvement in overall productivity. Interestingly, the rate of enzyme (pyruvate decarboxylase) activity loss was unaffected by the reduced phase ratio over the reaction period (48 h). At 20°C and 0.43:1 phase ratio the organic phase PAC concentration increased to 212 g/l and the overall productivity increased by 30% although the PAC yield (based on pyruvate) declined by about 10% due to greater byproduct acetoin formation at the higher temperature. Product recovery in such a system is facilitated both by the higher PAC concentration and the reduced organic phase volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
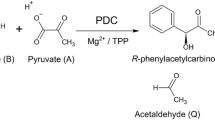
(R)-Phenylacetylcarbinol (PAC) is the chiral precursor for synthesis of the pharmaceuticals (1R, 2S)-ephedrine and (1S, 2S)-pseudoephedrine used to relieve symptoms of congestion associated with asthma, colds and influenza. Traditionally PAC has been produced through the biotransformation of pyruvate and benzaldehyde, catalyzed by pyruvate decarboxylase (PDC) present in baker’s yeast (Fig. 1).
Initial studies by our group demonstrated that the use of partially purified PDC in an aqueous phase system containing pyruvate and benzaldehyde resulted in increased PAC concentrations, yields and productivities when compared to the yeast-based process (Shin and Rogers 1996). PAC production was further improved in an aqueous/benzaldehyde emulsion system (Rosche et al. 2001; 2002a). However the latter process was limited by loss of PDC activity due to soluble benzaldehyde in the aqueous phase (up to 100 mM), and increased benzaldehyde droplet/enzyme interactions at the higher benzaldehyde concentrations.
To address these problems a two-phase organic/aqueous phase system was proposed in which both benzaldehyde and PAC would selectively partition into the organic phase while pyruvate and PDC would be maintained in the aqueous phase, thereby minimizing the loss of PDC activity. Following the screening of a range of organic solvents, octanol was selected in view of its high partition coefficients for benzaldehyde and PAC, and its apparent minimal effects on PDC deactivation. As reported by Rosche et al. (2002b) and Sandford et al. (2005), PAC higher than 100 g/l was achieved in the octanol phase. These experiments were carried out at 4°C as Shin and Rogers (1996) had shown for an aqueous phase system that higher PAC yields were achieved at lower temperatures due to reduced by-product acetoin production, although later studies at 21°C with whole cells of C. utilis (Rosche et al. 2005) demonstrated the greater advantage of higher temperature operation with a 4-fold increase in specific rate of PAC production (based on enzyme activity). The successive progress of PAC production strategies from in vivo to various two-phase systems has been illustrated by Rosche et al. (2005). All of the previously reported two-phase studies were carried out at an octanol to aqueous phase volume ratio of 1:1.
Possibly higher PAC concentrations and productivities may be achieved at lower octanol to aqueous phase volume ratios, and the effects of reducing this ratio are evaluated in the present studies using partially purified PDC at both 4 and 20°C.
Materials and methods
Production of partially purified pyruvate decarboxylase (PDC)
PDC was obtained as a partially purified enzyme from Candida utilis strain UNSW 70940 from the Culture Collection of the School of Biotechnology and Biomolecular Sciences, University of New South Wales (World Directory of Culture Collections No. 248). C. utilis was grown in a fermentation process that was initially fully aerobic to enhance biomass production and subsequently partially anaerobic for induction of PDC activity. PDC was isolated by acetone precipitation as described by Sandford et al. (2005). The PDC activity was expressed as U/ml carboligase activity. One unit (U) of carboligase activity was defined as the amount of enzyme required to form 1 μmol PAC from pyruvate and benzaldehyde in 1 min at 25°C and pH 6.4 in the carboligase assay specified by Rosche et al. (2002a).
Biotransformations
The biotransformations were conducted at a 10 ml scale in 20 ml glass vials at 4(±1)°C and 20(±1)°C with magnetic stirring to maintain an aqueous/octanol emulsion, and the effects of organic:aqueous phase volume ratios of 1:1, 0.67:1, 0.43:1 and 0.25:1 were evaluated. The experiments were designed in such a manner that the initial substrate amounts per total reaction volume (TRV) were approximately the same for each phase ratio. The concentrations and reaction conditions are specified in the figure legends. Samples of 0.75 ml were centrifuged for phase separation (20,800g, 4°C, 5 min). The remaining PDC carboligase activity in the aqueous phase was determined as described earlier (Rosche et al. 2002a) after filtration through Bio-gel P6 columns (BioRad Laboratories, Hercules, USA) to remove substrates and products from the enzyme solution. Addition of 10% (w/v) trichloroacetic acid to another aliquot of the unfiltred aqueous phase stopped the reaction. Precipitated material was removed by centrifugation (20,800g, 4°C, 5 min) prior to HPLC analysis. For analyses of the organic phase, 10 μl of the phase was extracted with 990 μl water. The given data points are mean values obtained from one biotransformation and error bars show the highest and lowest values of 2–3 measurements.
Analytical methods
PAC and benzaldehyde concentrations were determined by HPLC with an Alltima C8 column and UV detection at 283 nm (Rosche et al. 2001). Pyruvate and acetaldehyde concentrations were quantified enzymatically using lactate dehydrogenase and alcohol dehydrogenase respectively (Rosche et al. 2002a). Acetoin concentrations were determined using a gas chromatographic method (Rosche et al. 2002a).
Results
Effect of decreasing organic to aqueous phase volume ratio
Experiments were carried out at 4°C in which the organic to aqueous phase volume ratios were decreased from 1:1 to 0.43:1. A phase volume ratio of 0.25:1 was evaluated also; however this system proved impractical due to problems of organic phase separation.
As shown in Fig. 2A, after the reaction had proceeded for 48 h, the organic phase PAC concentration increased with decreased phase volume ratios. This was accompanied by a smaller increase of the aqueous phase PAC concentration. Productivity also increased with the reduced phase volume ratios (Fig. 2B), with the value for 0.43:1 being approximately 10% higher compared to that at 1:1.
Effect of decreasing the organic to aqueous phase volume ratio in aqueous/octanol-benzaldehyde emulsion system at 4°C, initial pH 6.5: (A) PAC concentrations in the organic and aqueous phase at 48 h and (B) Overall PAC volumetric productivity at 48 h. Agitation rates of 220–250 rpm were selected to maintain an emulsion, initial concentrations in the total reaction volume of both phases: 680 mM benzaldehyde, 630–650 mM pyruvate, 2.8 U/ml PDC carboligase activity, 2.5 M MOPS buffer, 1 mM Mg2+, 1 mM thiamine pyrophosphate (TPP)
The PDC activity decreased with time in each case (Fig. 3), although the rates of decrease were almost identical for the three different phase volume ratios.
For a phase volume ratio of 0.43:1 at 4°C, detailed kinetics are shown in Fig. 4 for substrate and product concentrations in both the organic and aqueous phases. From the results it can be seen that PAC concentrations continued to increase in both phases over 48 h, although with a declining overall rate. A final organic phase concentration of approximately 1,200 mM (180 g/l) PAC was achieved. A higher PAC concentration may have been possible if the reaction time had been extended as residual pyruvate, benzaldehyde and PDC activity remained. Acetoin was the major by-product with similar concentrations of 20–30 mM evident in both phases. Benzaldehyde concentrations in the aqueous phase remained relatively low throughout the biotransformation (less than 30 mM) and this would have been important in reducing the extent of PDC deactivation.
Substrate, PAC and byproduct concentration profiles in the (A) organic phase and (B) aqueous phase for an organic:aqueous phase volume ratio of 0.43:1 at 4°C, initial pH 6.5, agitation rate 220 rpm, initial concentrations: 2.26 M organic phase benzaldehyde, the aqueous phase contained 0.93 M pyruvate, 4 U/ml PDC carboligase activity, 2.5 M MOPS buffer, 1 mM Mg2+, 1 mM TPP. (□) Benzaldehyde, (◇) pyruvate, (▲) PAC, (○) acetaldehyde, (×) acetoin
To investigate the effect of increasing temperature, an experiment was carried out at 20°C with conditions very similar to those reported for the previous study at 4°C. The results are presented in Fig. 5 and show a comparatively faster rate of PAC production with full utilization of pyruvate, and essentially completion of the reaction by 24 h. Under these conditions, a maximum organic phase PAC concentration of approximately 1,400 mM (210 g/l) was achieved. The concentration of acetoin in both phases at 48 h was approximately 2-fold higher at the higher temperature with a value close to 70 mM in the organic phase and 40 mM in the aqueous phase. Similar acetaldehyde concentrations at 48 h of 12–15 mM (organic phase) and 3–4 mM (aqueous phase) were evident at both temperatures.
Substrate, PAC and by-product concentration profiles in the (A) organic phase and (B) aqueous phase at organic:aqueous phase volume ratio of 0.43:1 at 20°C, initial pH 6.5, agitation rate 220 rpm, initial concentrations: 2.47 M organic phase benzaldehyde, the aqueous phase contained 0.92 M pyruvate, 4 U/ml PDC carboligase activity, 2.5 M MOPS buffer, 1 mM Mg2+, 1 mM TPP. Refer to Fig. 4 for symbols
The results for the different sets of experiments at 4 and 20°C are summarized in Table 1 which also includes calculated values for PAC yields (based on benzaldehyde and pyruvate consumed) as well as mass balances for these two substrates. From the data it is clear that both phase volume ratio and temperature are critical process variables. In the present study for example, the highest organic phase PAC concentration (212 g/l), the highest PAC productivity (4 g/l/h) and the highest specific PAC production (29 mg/U initial carboligase activity) were all achieved at the lowest phase ratio (0.43:1) and the higher temperature (20°C). The only problem is that acetoin production was also maximal under these conditions (5.5 g/l), an effect mainly due to the increase in operating temperature from 4 to 20°C.
Calculation of PAC yields on benzaldehyde consumed gave values close to theoretical indicating negligible conversion of benzaldehyde to by-products such as benzyl alcohol, which is produced in significant amounts in commercial yeast-based processes. PAC yields on pyruvate consumed were less than theoretical due to production of acetoin and acetaldehyde and were more affected by the increase in temperature than by any change in phase volume ratios. At 20°C and a phase ratio of 0.43:1, a total yield loss of 16% was determined. The mass balance calculations were well within acceptable limits (given their dependence on a number of analytical procedures) and indicated that all of the major byproducts had been identified.
Discussion and conclusions
The present study extends our earlier research on PAC production in a two phase reactor by evaluating the effect of changing organic to aqueous phase volume ratios on process characteristics. Its effect on the equilibrium conversion of a biphasic bi-molecular process, with the catalytic reaction taking place only in one phase, has been modeled recently by Eckstein et al. (2006). From this study the authors concluded that the effect can be complex and will be dependent on the partition coefficients of the substrates and products for the selected solvent.
For model validation, an experimental study was carried out by the above authors using an (R)-selective alcohol dehydrogenase from Lactobacillus brevis with reactants acetophenone and 2-propanol, and products 1-phenylethanol and acetone. Various non-reactive solvents were selected for the organic phase with the reaction itself occurring only in the aqueous phase. The solvents, MTBE and hexane, were evaluated for studies on the effect of changing phase volume ratios on the equilibrium conversions. From the results it was concluded for the phase volume ratio range 0.1–10, that a reasonably good prediction of the experimental conversions could be achieved by the model. The experimental data confirmed that lower phase volume ratios resulted in higher equilibrium conversions for both solvents.
Other researchers have found also that changing the organic to aqueous phase volume ratio in such systems can affect the equilibrium conversions and rates of biocatalytic reactions. For example, Panintrarux et al. (1995) reported for β-glucosidase catalyzed biphasic production of n-alkyl-β-d glucosides, that the equilibrium conversions from glucose and n-alcohols were decreased at lower phase volume ratios (range 10–50), while Yi et al. (1998) observed increasing reaction rates of hexyl β-d glucoside production with decreasing hexanol to water volume ratios (range 5–15) although they did not report their effect on final equilibrium conversions. The different conclusions of the above studies (in very different systems) support the statements of Eckstein et al. (2006) that the effect of changing the phase volume ratio is a complex one with dependence on reactants, products and solvents and their potential interaction with the enzymes selected. Recent investigations have also shown that two-phase systems can be advantageous for decreasing by-product formation, for example by Zymomonas mobilis in the production of (S)-citronellal (Müller et al. 2006).
In the present study, a lower phase ratio has been shown to be beneficial for PAC production. This has been confirmed at both 4 and 20°C although increased by-product (acetoin) formation occurred at 20°C. The lowest phase ratio evaluated of 0.43:1, resulted in the highest PAC concentrations and productivities, and may have the additional advantages of facilitating PAC recovery from smaller organic phase volumes as well as enhancing solvent recovery and recycling.
References
Eckstein MF, Peters M, Lembrecht J, Speiss AC, Greiner L (2006) Maximize equilibrium conversion in biphasic catalysed reactions: mathematical description and practical guidelines. Adv Synth Catal 348:1591–1596
Müller A, Hauer B, Rosche B (2006) Enzymatic reduction of the alpha, beta-unsaturated carbon bond in citral. J Mol Catal B: Enzym 38:126–130
Panintrarux C, Adachi S, Araki Y, Kimura Y, Matsuno R (1995) Equilibrium yield of n-alkyl-β-D-glucoside through condensation of glucose and n-alcohol by β-glucosidase in a biphasic system. Enzym Microb Technol 17:32–40
Rosche B, Breuer M, Hauer B, Rogers P (2005) Cells of Candida utilis for in vitro (R)-phenylacetylcarbinol production in an aqueous/octanol two-phase reactor. Biotechnol Lett 27:575–581
Rosche B, Leksawasdi N, Sandford V, Breuer M, Hauer B, Rogers PL (2002a) Enzymatic (R)-phenylacetylcarbinol production in benzaldehyde emulsions. Appl Microbiol Biotechnol 60:94–100
Rosche B, Sandford V, Breuer M, Hauer B, Rogers PL (2002b) Enhanced production of R-phenylacetylcarbinol (R-PAC) through enzymatic biotransformation. J Mol Catal B: Enzym 19:109–115
Rosche B, Sandford V, Breuer M, Hauer B, Rogers PL (2001) Biotransformation of benzaldehyde into (R)-phenylacetylcarbinol by filamentous fungi or their extracts. Appl Microbiol Biotechnol 57:309–315
Sandford V, Breuer M, Hauer B, Rogers P, Rosche B (2005) (R)-Phenylacetylcarbinol production in aqueous/organic two-phase systems using partially purified pyruvate decarboxylase from Candida utilis. Biotechnol Bioeng 91:190–198
Shin HS, Rogers PL (1996) Production of l-phenylacetylcarbinol (l-PAC) from benzaldehyde using partially purified pyruvate decarboxylase (PDC). Biotechnol Bioeng 49:52–62
Yi Q, Sarney DB, Khan JA, Vulfson EN (1998) A novel approach to biotransformations in aqueous-organic two-phase systems: enzymatic synthesis of alkyl β-[D]-glucosides using microencapsulated β-glucosidase. Biotechnol Bioeng 60:385–390
Acknowledgements
The authors like to dedicate this publication to Josephine. The work was supported by an Endeavour International Postgraduate Research Scholarship at the UNSW (CG) and additional funding from BASF-AG, Germany is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gunawan, C., Breuer, M., Hauer, B. et al. Improved (R)-phenylacetylcarbinol production with Candida utilis pyruvate decarboxylase at decreased organic to aqueous phase volume ratios. Biotechnol Lett 30, 281–286 (2008). https://doi.org/10.1007/s10529-007-9525-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9525-0