Abstract
Cell suspension cultures of Commiphora wightii, grown in modified MS medium containing 2,4-dichlorophenoxyacetic acid (0.5 mg l−1) and kinetin (0.25 mg l−1), produced ∼5 μg guggulsterone g−1 dry wt. In a 2 l stirred tank bioreactor, the biomass was 5.5 g l−1 and total guggulsterone was 36 μg l−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guggulsterone (as its E- and Z-isomers) is an effective anti-hyperlipidemic agent obtained from the gum resin of guggul tree, Commiphora wightii (Abnott.) Bhandari (syn. C. mukul) (Dev 1999; Wang et al. 2004). Over-exploitation and slow growth of the tree, which is associated with poor seed set, make this plant an endangered species (Kumar and Shankar 1982; Kumar et al. 2003). The biotechnological production of guggulsterone has received attention as a promising alternative production source. Attempts have also been made to develop a micropropogation method through cloning (Barve and Mehta 1993) and somatic embryogenesis (Singh et al. 1997; Kumar et al. 2003) to increase the tree population. Guggulsterone is an effective antagonist of the bile acid receptor (Wu et al. 2002), farnesoid X receptor (Urizar et al. 2002) and a ligand-dependent transcription factor that regulates the expression of CYP 7A1 gene involved in maintaining the cholesterol/bile acid homoeostasis through the bile salt export pump (Owsley and Chiang 2003).
Production of metabolites in cell cultures and development of scale-up technology require standardization of various growth parameters for optimal yield (Ramawat and Mathur 2007; Merillon 2007). Guggulsterone production in callus (0.09–0.22%) and cell cultures (0.18–0.32%) of C. wightii was reported (Phale et al. 1989) but without details of age of cultures, subculture period and procedure for guggulsterone extraction. The callus cultures raised in our laboratory contained 2–8 μg guggulsterone g−1 dry wt, which could be enhanced by morphactin up to 20 μg g−1 (Tanwar et al. 2007). This is the first report describing details of cell cultures grown in shake-flask and stirred tank bioreactor and production of guggulsterones in cell suspension cultures as influenced by growth and inoculum density.
Materials and methods
Cell cultures
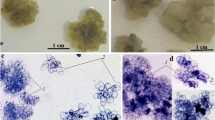
Cell suspension cultures of C. wightii were grown in modified Murashige and Skoog (1962) medium containing 2,4-dichlorophenoxyacetic acid (0.5 mg l−1), kinetin (0.25 mg l−1) and 3% (w/v) sucrose, referred to as CN4 medium with NH4NO3 at 825 mg l−1, KNO3 at 475 mg l−1 and CaCl2·6H2O at 220 mg l−1. This medium was adopted from results obtained with callus cultures (Mathur et al. 2007). A 250 ml Erlenmeyer flask containing 100 ml medium was shaken at 100 rpm at 26°C in the dark. The subculture period and inoculum size were usually 15 days and 125 mg dry wt/ 100 ml medium (10% v/v), respectively. Growth and guggulsterone contents were determined periodically using vessels of different sizes and stirred tank bioreactor as given in the results. Six replicate flasks were used in each treatment and experiments were conducted twice. The details of bioreactor conditions are given in Table 2 (see below).
Guggulsterone extraction and HPLC analysis
The cell cultures were harvested after 15 days, the lyophilized cells (1 g) were finely grounded, extracted overnight with 25 ml methanol as described earlier (Tanwar et al. 2007). In brief, the methanol was evaporated under vacuum; the residue was extracted with ethyl acetate from which a sample was injected in HPLC after filtration through syringe filters (0.45 μm, 4 mm nylon filter). All the results are average of at least two separate analyses. Separation was accomplished on a 250 × 4 mm C18 (5 μm) reverse phase column protected by a guard column of same material using a modified gradient (Tanwar et al. 2007) based on earlier method (Mesrob et al. 1998). The elute was monitored at 245 nm. E- and Z-Guggulsterones were obtained from Chromadex, U.S.A. and Natural Remedies, Bangalore, respectively.
Results and discussion
Cell cultures of C. wightii were established and scaled up to a 2 l bioreactor. Growth of the cell cultures and guggulsterone production was maximal after 15 days (Fig. 1). The ratio of the E- and Z-isomers was approximately constant at 4:1. Best results for cell growth and guggulsterone production was achieved using an inoculum of 10% (v/v) (see Table 1). This was then used in further experiments with cell being continuously harvested at 15 days as the optimum time.
An increase in the size of the flask and corresponding volume of medium decreased the biomass and total guggulsterone yield l−1 (Table 2). No guggulsterone was detected in the spent medium. The total guggulsterone content slightly increased in cells grown in a 2 l stirred tank bioreactor as compared to 2 l flasks. The decrease in biomass production was also associated with a low guggulsterone yield in the medium. The low biomass production might be due to low aerobic conditions in large vessels as vessels of all sizes were agitated at same rpm. About 60% increase in biomass production and about 20% decrease in guggulsterone production were recorded in the cultures during twelve months growth on the medium of same composition. For the initial six months, cultures were almost stable in terms of growth and guggulsterones production, thereafter an increase in growth was recorded.
The cultures of C. wightii are maintained on MS medium with varying salt (Mathur et al. 2007) and plant growth regulator combinations (Tanwar et al. 2007) to find out the best combination of growth medium. The apparent high guggulsterone content recorded by Phale et al. (1989) might be due to density of other metabolites present in the extract used in spectrophotometric method. The HPLC method based on Mesorb et al. (1998) clearly separated the resin components. In several plant cell cultures, improvement in accumulation of metabolites with high cell densities has been recorded (Zhang and Zhong 1997, Wang et al. 1997), however the mechanism underlying this effect is far from clear. In the present work with cell cultures of C. wightii, increased vessel/medium volume and inoculum size grown in growth medium resulted in low net biomass and guggulsterone accumulation. The present results will be helpful in further deciding the medium and time period for cultivation of the cells in the growth and production media.
References
Barve DM, Mehta AR (1993) Clonal propagation of mature elite trees of Commiphora wightii. Plant Cell Tiss Org Cult 35:237–244
Dev S (1999) Ancient-modern concordance in Ayurvedic plants: some examples. Environ Health Perspect 107:783–789
Kumar S, Shankar V (1982) Medicinal plants of the Indian Desert: Commiphora wightii (Arnott) Bhand. J Arid Environ 5:1–11
Kumar S, Suri SS, Sonie KC et al (2003) Establishment of embryonic cultures and somatic embryogenesis in callus culture of guggul-Commiphora wightii (Arnott.) Bhandari. Indian J Exp Biol 41:69–77
Mathur M, Jain AK, Dass S, Ramawat KG (2007) Optimization of guggulsterone production in callus cultures of Commiphora wightii (Arnott.) Bhandari. Indian J Biotechnol Bt 835 (in press)
Merillon JM (2007) Large-scale production in bioreactors. In: Ramawat KG, Merillon JM (eds) Biotechnology secondary metabolites. Sci Pub, Enfield, USA pp 331–357
Mesrob B, Nesbitt C, Misra R et al (1998) High-performance liquid chromatographic method of fingerprinting and quantitative determination of E- and Z-guggulsterones in Commiphora mukul resin and its products. J Chromatogr B 720:189–196
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Owsley E, Chiang JYL (2003) Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7α-hydroxylase gene. Biochem Biophys Res Commun 304:191–195
Phale P, Subramani J, Bhatt PN et al (1989) Viability and guggulsterone production in immobilized tissue cultured cells of Commiphora wightii. Indian J Exp Biol 27:338–340
Ramawat KG, Mathur M (2007) Factors affecting production of secondary metabolites. In: Ramawat KG, Merillon JM (eds) Biotechnology secondary metabolites. Sci Pub, Enfield, USA pp 59–101
Singh AK, Suri SS, Ramawat KG (1997) Somatic embryogenesis from immature zygotic embryos of Commiphora wightii, a woody medicinal plant. Gartenbau 62:44–48
Tanwar YS, Mathur M, Ramawat KG (2007) Morphactin influences guggulsterone production in callus cultures of Commiphora wightii. Plant Growth Regul 51:93–98
Urizar NL, Liverman AB, Dodds DT et al (2002) A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 269:1703–1706
Wang X, Greilberger J, Ledinski G et al (2004) The hypolipidemic natural product Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis 172:239–246
Wang HQ, Zhong JJ, Yu JT (1997) Enhance production of taxol in suspension cultures of Taxus chinensis by controlling inoculum size. Biotechnol Lett 19:353–355
Wu J, Xia C, Meier J et al (2002) The hypolipidemic natural product guggulsterone acts as an antagonist of bile acid receptor. Molec Endocrin 16:1590–1597
Zhang YH, Zhong JJ (1997) Hyper production of ginseng saponin and polysaccharide by high-density cultivation of Panax notoginseng. Enzyme Microb Technol 21:59–63
Acknowledgement
This work was supported by financial assistance (grant No. BT/PR3214/PBD/17/210/2002) from the DBT, Government of India, New Delhi, and partially by DST-FIST programme for infrastructure development and UGC-DRS under special assistance programme for medicinal plant research to KGR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mathur, M., Ramawat, K.G. Guggulsterone production in cell suspension cultures of the guggul tree, Commiphora wightii, grown in shake-flasks and bioreactors. Biotechnol Lett 29, 979–982 (2007). https://doi.org/10.1007/s10529-007-9342-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9342-5





 Dry weight,
Dry weight, 
 guggulsterone-Z,
guggulsterone-Z,  total guggulsterone
total guggulsterone