Abstract
Diabetic nephropathy (DN) is the main cause of end-stage renal disease. Circular RNA hsa_circ_0004442 (circTLK1) accelerates the progression of renal cell carcinoma. However, the role of circTLK1 in DN pathogenesis is indistinct. The expression of circTLK1, microRNA-126-5p (miR-126-5p), and microRNA-204-5p (miR-204-5p) was tested by quantitative real-time polymerase chain reaction. The levels of interleukin-6 and interleukin-1β were measured by enzyme-linked immunosorbent assay. The levels of reactive oxygen species and malondialdehyde and the activity of superoxide dismutase were determined with corresponding kits. Several protein levels were evaluated with western blotting. The relationship between circTLK1 and miR-126-5p/miR-204-5p was verified by dual-luciferase reporter assay. CircTLK1 was highly expressed in DN patient’s serum and high-glucose (HG)-treated human mesangial cells. Functionally, circTLK1 inhibition reduced HG-induced inflammation, oxidative stress, and ECM accumulation in human mesangial cells. CircTLK1 was verified as a sponge for miR-126-5p and miR-204-5p, which were downregulated in DN patient’s serum and HG-treated human mesangial cells. Both miR-126-5p and miR-204-5p upregulation decreased inflammation, oxidative stress, and ECM accumulation in HG-treated human mesangial cells and circTLK1 silencing-mediated influence on HG-induced human mesangial cell injury was overturned by miR-126-5p or miR-204-5p inhibition. Moreover, circTLK1 knockdown blocked the AKT/NF-κB pathway by sponging miR-126-5p/miR-204-5p. CircTLK1 downregulation alleviated HG-induced inflammation, oxidative stress, and ECM accumulation through blocking the AKT/NF-κB pathway via sponging miR-126-5p/miR-204-5p, providing a new mechanism to comprehend the pathogenesis of DN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
1.
CircTLK1 was upregulated in DN patient’s serum and HG-treated human mesangial cells.
-
2.
Knockdown of circTLK1 relieved HG-induced inflammation, oxidative stress, and ECM accumulation in human mesangial cells.
-
3.
CircTLK1 was verified as a sponge for miR-126-5p and miR-204-5p.
-
4.
CircTLK1 modulated the AKT/NF-κB pathway by sponging miR-126-5p or miR-204-5p.
Introduction
Diabetic nephropathy (DN), the microvascular complications of diabetes, is the main cause of end-stage renal disease (Sun et al. 2019). The excessive production and accumulation of extracellular matrix (ECM) in the glomerulus is characteristic of DN, which may lead to glomerular sclerosis (Bhaskaragoud et al. 2018). DN has a complex pathological progression, which involves different molecules and cells (Bhattacharjee et al. 2016). Mesangial cells are essential to maintain normal glomerular structure and function (Riser et al. 2000). A considerable number of reports revealed that high-glucose (HG) can induce ECM accumulation in mesangial cells (Li et al. 2018; Zhang et al. 2019). Also, the accumulation of ECM in mesangial cells is associated with HG-induced inflammation and oxidative stress (Chen et al. 2018; Wu et al. 2020). Accordingly, exploring the molecular mechanisms related to ECM accumulation, inflammation, and oxidative stress in glomerular mesangial cells is very important to clarify the pathogenesis of DN.
Circular RNAs (circRNAs), a new type of RNA molecules, have closed-loop structures. They are formed by back-splicing from precursor mRNAs (Ebbesen et al. 2017). Accumulated evidence has manifested that circRNAs play a crucial role in some diseases, including metabolic diseases (Wang et al. 2018). Moreover, circRNAs participate in the progression of some diseases by sponging microRNAs (miRNAs), which are implicated in series of cellular biological processes (Achkar et al. 2016; Hsiao et al. 2017). For example, circRNA circ-33186 sponged miR-127-5p to facilitate the pathogenesis of osteoarthritis (Zhou et al. 2019). For another example, circRNA circ-PTN contributed to stemness and proliferation of glioma cells through absorbing miR-145-5p and miR-330-5p (Chen et al. 2019a). CircRNA hsa_circ_0004442 (circTLK1), located at chromosome 2: 171,884,848–171,902,872, is formed by back-splicing of the TLK1 (Tousled Like Kinase 1) mRNA (from exon 9 to exon 10). Li et al. revealed that circTLK1 elevation accelerated cell metastasis and growth by sponging miR-136-5p in renal cell carcinoma (Li et al. 2020). However, the role of circTLK1 in DN pathogenesis is indistinct.
Herein, we discovered that circTLK1 had a higher expression in DN patient’s serum and HG-treated human mesangial cells. Furthermore, circTLK1 silencing mitigated HG-induced human mesangial cell injure through blocking the AKT/NF-κB pathway through absorbing miR-126-5p or miR-204-5p.
Materials and Methods
Case Selection
The study was approved by the Ethics Committee of Weifang People's Hospital. 30 DN patients and 16 healthy volunteers (Control) were recruited from the Weifang People's Hospital and had signed written informed consents. Venous blood samples were obtained from all participants and then centrifuged (3000× g, 4 °C) for 5 min to separate plasma.
Cell Culture and Treatment
Human mesangial cells were purchased from Bena (Suzhou, China) and cultured in Dulbecco’s modified Eagle medium (DMEM) (Thermo Fisher Scientific, Waltham, MA, USA) and supplemented with 10% FBS (fetal bovine serum) (Thermo Fisher Scientific) in a moist atmosphere with 5% CO2 at 37 °C. For HG treatment, human mesangial cells were grown in DMEM containing 10% FBS and d-glucose (30 mmol/L, Sigma, St Louis, MO, USA), and 5.5 mmol/L d-glucose (Sigma) was used as normal glucose (NG). Also, human mesangial cells treated with HG for 24 h were used for functional analysis.
Transient Transfection
Short hairpin (sh) RNA targeting circTLK1 (sh-circTLK1) and matching normal control (NC) (sh-NC), miR-126-5p mimic (miR-126-5p), mimic NC (miR-NC), miR-204-5p mimic (miR-204-5p), miR-126-5p inhibitor (anti-miR-126-5p), inhibitor NC (anti-miR-NC), and miR-204-5p inhibitor (anti-miR-204-5p) were synthesized by RiboBio (Guangzhou, China). Transient transfection was carried out using Lipofectamine 3000 reagent (Thermo Fisher Scientific).
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted using TRIzol™ Reagent (Thermo Fisher Scientific). For purity and integrity assessment, total RNA was analyzed using the Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific) (A260/A280 nm) and agarose gel (Biowest, Kansas, MO, USA) electrophoresis (1%). Reverse transcription was performed using the M-MLV reverse transcriptase (Promega, Madison, WI, USA) or Mir-X miRNA first-strand synthesis kit (Takara, Dalian, China). qRT-PCR was conducted with the SYBR Green mix (Takara) in the Light Cycler 480 II real-time PCR system (Roche, Basel, Switzerland). Relative expression was calculated by the 2−ΔΔCt method and normalized to β-actin or U6 small nuclear RNA (U6). All primer sequences are listed in Table 1.
Actinomycin D and RNase R Treatment
To prevent transcription, actinomycin D (2 mg/mL, Sigma) was added to the growth medium of human mesangial cells, and dimethylsulfoxide (2 mg/mL, Sigma) was utilized as an NC. To verify the stability of circTLK1, total RNA (2 μg) from human mesangial cells was treated with RNase R (3U/ug, Epicentre Technologies, Madison, WI, USA) at 37 °C for 20 min, and DEPC-treated water (Thermo Fisher Scientific) was used as an NC. At the specified time, the expression of circTLK1 and TLK1 mRNA was tested with qRT-PCR.
Subcellular Fractionation Assay
Subcellular fractionation assay was performed used a PARIS™ Kit (Ambion, Austin, TX, USA). In short, human mesangial cells (1 × 104 cells) were re-suspended in the cell fractionation buffer and incubated for 10 min on ice. Then, centrifugation was performed to separate the supernatant and the nuclear pellet for RNA extraction. The level of circTLK1 in the cytoplasmic RNA and nuclear RNA was analyzed with qRT-PCR, and U6 and 18S rRNA were used as controls for nuclear RNA or cytoplasmic RNA.
Enzyme-Linked Immunosorbent Assay (ELISA)
The levels of IL-6 (interleukin-6) and IL-1β (interleukin-1β) were detected using human IL-6 and IL-1β ELISA kits (Solarbio, Beijing, China). After transfection for 48 h, centrifugation (1000× g, 10 min) was performed to obtain the supernatant of human mesangial cells. The standard and test samples were added into the reaction wells and incubated for 90 min at 37 °C. Thereafter, the detection reagent and substrate solution were added in sequence in the light of the manufacturer’s instructions. After adding the stop solution, the measurement was immediately performed with a Microplate Reader (Bio-Rad, Hercules, CA, USA) at 450 nm.
Measurement of Reactive Oxygen Species (ROS), Malondialdehyde (MDA), and Superoxide Dismutase (SOD)
The level of ROS was analyzed using the 2′,7′-dichlorofluorescin diacetate (DCFH-DA) probe (Beyotime, Shanghai, China) in accordance with the manufacturer’s procedures. The level of MDA and the activity of SOD in the supernatant of cell medium were detected with the MDA detection kit (Beyotime) and SOD activity detection kit (Beyotime).
Western Blotting
Total protein was extracted from human mesangial cells using the radio-immunoprecipitation assay (RIPA) buffer (Sigma) and was quantified with the BCA assay kit (Pierce, Rockford, IL, USA). Total protein was isolated by 12% sodium dodecyl sulfate–polyacrylamide gels. Subsequently, the separated proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Sigma) and sealed with 5% non-fat milk powder. Thereafter, the membranes were incubated with primary proteins, including anti-FN (fibronectin) (#PA5-29578, 1:1000, Thermo Fisher Scientific), Col I (collagen I) (#PA1-26204, 1:1000, Thermo Fisher Scientific), Col IV (collagen IV) (#PA5-86939, 1:500, Thermo Fisher Scientific), p (phosphorylated)-AKT (protein kinase B) (#66444–1-IG, 1:2000, Thermo Fisher Scientific), AKT (10176–2-AP, 1:1000, Thermo Fisher Scientific), p-p65 (#44-711G, 1:1000, Thermo Fisher Scientific), p65 (#33-9900, 1 µg/mL, Thermo Fisher Scientific), and anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (#MA5-15738-D800, 1:1000, Thermo Fisher Scientific) antibodies. Then, the membranes were incubated with a goat anti-mouse (#31430, 1:10000, Thermo Fisher Scientific) or anti-rabbit (#A32731, 1:10000, Thermo Fisher Scientific) IgG secondary antibodies. The blots were detected by enhanced chemiluminescence (ECL) substrates (Thermo Fisher Scientific). GAPDH was deemed as a loading control.
Dual-Luciferase Reporter Assay
MiRNAs possessed complementary sequences to circTLK1 were predicted with the starBase and circBank databases. The sequence of wild-type (wt) circTLK1 (wt-circTLK1) and its mutant (mut) sequences (mut-circTLK1 site1 and mut-circTLK1 site2) were inserted into the pMIR-REPORT vectors (Applied Biosystems, Foster City, CA, USA), respectively. Human mesangial cells were co-transfected with a luciferase reporter vector carrying wt-circTLK1, mut-circTLK1 site1, or mut-circTLK1 site2 and miR-NC, miR-126-5p, or miR-204-5p using Lipofectamine 3000 reagent (Thermo Fisher Scientific). The luciferase activities were tested with a luciferase reporter assay kit (Promega) in a TD20/20 Luminometer (Turner Biosystems, Sunnyvale, CA, USA).
Statistical Analysis
Statistical analysis was carried out using GraphPad Prism 7.0 (Graph-Pad Software, La Jolla, CA, USA). All data were presented as the mean ± standard deviation, which were from 3 replicate experiments. The correlation between circTLK1 and miR-126-5p or miR-204-5p in the serum of DN patients was determined by Pearson’s correlation analysis. The differences among 3 or more groups were tested with a one-way variance analysis (ANOVA) followed by Turkey’s post hoc test. The difference between 2 groups was analyzed with an unpaired Student’s t-test. Statistical significance was accepted when P < 0.05.
Results
CircTLK1 was Highly Expressed in the Serum of Patients with DN and HG-Stimulated Human Mesangial Cells
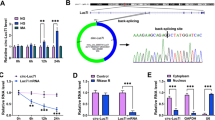
It has reported that circTLK1 plays a promoting impact on renal cell carcinoma growth (Li et al. 2020). To survey the function of circTLK1 in DN, we detected circTLK1 expression in the serum of patients with DN (n = 30) and healthy volunteers (n = 16). qRT-PCR presented that circTLK1 had a higher expression in the serum of patients with DN compared with healthy volunteers (Fig. 1A). Then, we evaluated circTLK1 expression in human mesangial cells with HG (30 mmol/L) or NG (5.5 mmol/L) treatment. As presented in Fig. 1B, circTLK1 was upregulated in HG-stimulated human mesangial cells in a time-dependent manner, and human mesangial cells treated with HG (30 mmol/L) for 24 h were used for function analysis. To validate the stability of circTLK1 in human mesangial cells, we utilized actinomycin D to repress RNA transcription. The results exhibited that the half-life of TLK1 mRNA was less than 12 h, while the half-life of circTLK1 exceeded 24 h, suggesting that circTLK1 was more stable (Fig. 1C). Also, TLK1 mRNA was degraded after RNase R treatment, but circTLK1 did not change significantly (Fig. 1D). We also investigated the distribution of circTLK1 in human mesangial cells. The results showed that circTLK1 was preferentially located in the cytoplasm of human mesangial cells, manifesting that circTLK1 might play a regulatory role at the post-transcriptional level (Fig. 1E). These data indicated that circTLK1 expression was elevated in DN.
Expression pattern of circTLK1 in the serum of DN patients and HG-stimulated human mesangial cells. A Assessment of the level of circTLK1 in the serum of DN patients (n = 30) and healthy volunteers (n = 16) (Control) by qRT-PCR. B Analysis of the level of circTLK1 in human mesangial cells treated with HG (30 mmol/L) or NG (5.5 mmol/L) by qRT-PCR. C and D After actinomycin D (C) and RNase R (D) treatment, the levels of circTLK1 and TLK1 mRNA were detected using qRT-PCR. E The level of circTLK1 in the nuclear and cytoplasmic fractions of human mesangial cells was determined by qRT-PCR, and 18S rRNA and U6 were used as controls for cytoplasm or nucleus RNA. *P < 0.05
Inhibition of circTLK1 Decreased HG-Induced Inflammation, Oxidative Stress, and ECM Accumulation in Human Mesangial Cells
Subsequently, we performed loss-of-function experiments to explore the role of circTLK1 in HG-stimulated human mesangial cells. After sh-circTLK1 transfection, circTLK1 expression was apparently decreased in human mesangial cells (Fig. 2A). The influence of circTLK1 downregulation on inflammation, oxidative stress, and ECM accumulation in HG-stimulated human mesangial cells was explored. We observed that the elevation of the levels of IL-6 and IL-1β in HG-stimulated human mesangial cells was restored after sh-circTLK1 transfection, indicating that circTLK1 inhibition reduced HG-induced inflammation in human mesangial cells (Fig. 2B, C). Moreover, the levels of ROS and MDA were elevated in HG-stimulated human mesangial cells, but the activity of SOD had an opposing tendency. However, the introduction of sh-circTLK1 reversed these trends in HG-stimulated human mesangial cells (Fig. 2D–F). Also, the knockdown of circTLK1 overturned the upregulation of FN, Col I, and Col IV in HG-stimulated human mesangial cells (Fig. 2G). Collectively, these results manifested that circTLK1 repression reduced HG-induced inflammation, oxidative stress, and ECM accumulation in human mesangial cells.
Impacts of circTLK1 silencing on inflammation, oxidative stress, and ECM accumulation of HG-stimulated human mesangial cells. A Analysis of the knockdown efficiency of circTLK1 by qRT-PCR. B–G Human mesangial cells were transfected with sh-circTLK1 and sh-NC. B and C Analysis of the levels of IL-6 and IL-1β in human mesangial cells under HG treatment by ELISA. D–F The levels of ROS and MDA and the activity of SOD in human mesangial cells under HG treatment were determined with corresponding kits. G Assessment of the protein levels of FN, Col I, and Col IV in human mesangial cells under HG treatment by western blotting. *P < 0.05
CircTLK1 was Validated as an Efficient miRNA Sponge for miR-126-5p and miR-204-5p in Human Mesangial Cells
Considering that circTLK1 was preferentially located in the cytoplasm of human mesangial cells, we predicted miRNAs that might bind to circTLK1. Bioinformatics prediction (starBase and circBank databases) showed that 4 miRNAs (miR-126-5p, miR-204-5p, miR-136-5p, and miR-211-5p) possessed complementary sequence to circTLK1 (Fig. 3A). We observed that miR-126-5p and miR-204-5p were downregulated in HG-stimulated human mesangial cells, but miR-136-5p and miR-211-5p did not change (Fig. 3B). Moreover, miR-126-5p and miR-204-5p expression were lowly expressed in the serum of DN patients (Fig. 3C, D). Also, there was a negative correlation between miR-126-5p or miR-204-5p and circTLK1 in the serum of DN patients (Fig. 3E, F). The complementary sequences between miR-126-5p or miR-204-5p and circTLK1 are displayed in Fig. 3G. The overexpression efficiencies of miR-126-5p and miR-204-5p are presented in Fig. 3H and I. Furthermore, miR-126-5p and miR-204-5p overexpression decreased the luciferase activity of the wt-circTLK1 reporter. However, the luciferase activities of the mut-circTLK1 site1 (within the assumed binding site for miR-126-5p) and mut-circTLK1 site2 (within the assumed binding site for miR-204-5p) reporters were reduced by miR-204-5p mimic and miR-126-5p mimic, respectively (Fig. 3J). In addition, circTLK1 inhibition elevated the levels of miR-126-5p and miR-204-5p in human mesangial cells (Fig. 3K). Together, these results manifested that circTLK1 served as a sponge for miR-126-5p and miR-204-5p in human mesangial cells.
CircTLK1 was verified as a sponge for miR-126-5p and miR-204-5p in human mesangial cells. A A schematic illustration presented the putative sites of the miRNAs associated with circTLK1. B Analysis of the expression of the candidate miRNAs in human mesangial cells under HG treatment by qRT-PCR. C and D Expression levels of miR-126-5p and miR-204-5p in the serum of DN patients and healthy volunteers were determined with qRT-PCR. E and F Pearson’s correlation analysis exhibited the correlation between miR-126-5p or miR-204-5p and circTLK1 in the serum of DN patients. G The complementary sequences between wt-circTLK1, mut-circTLK1 site1, or mut-circTLK1 site2 and miR-126-5p or miR-204-5p. H and I qRT-PCR was carried out to validate the overexpression efficiencies of miR-126-5p and miR-204-5p in human mesangial cells. J Dual-luciferase reporter assay was performed in human mesangial cells co-transfected with luciferase reporter vector carrying wt-circTLK1, mut-circTLK1 site1, or mut-circTLK1 site2 and miR-126-5p mimic, miR-204-5p mimic, or miR-NC. K Analysis of the expression of miR-126-5p and miR-204-5p in human mesangial cells after sh-NC or sh-circTLK1 transfection by qRT-PCR. *P < 0.05
Both miR-126-5p and miR-204-5p Overexpression Reduced Inflammation, Oxidative Stress, and ECM Accumulation of HG-Stimulated Human Mesangial Cells
Given that miR-126-5p and miR-204-5p were downregulated in the serum of DN patients and HG-stimulated human mesangial cells, we conducted gain-of-function experiments to verify the roles of miR-126-5p and miR-204-5p in DN. The results exhibited that both miR-126-5p and miR-204-5p overexpression restored the upregulation of IL-6 and IL-1β in HG-stimulated human mesangial cells (Fig. 4A, B). Furthermore, either miR-126-5p elevation or miR-204-5p overexpression antagonized the elevation of ROS and MDA levels and the decrease of SOD activity in HG-treated human mesangial cells (Fig. 4C–E). Additionally, the promoting influence of HG on the protein levels of FN, Col I, and Col IV in human mesangial cells was reversed after miR-126-5p or miR-204-5p elevation (Fig. 4F). Thus, these findings indicated that both miR-126-5p and miR-204-5p elevation could weaken inflammation, oxidative stress, and ECM accumulation of human mesangial cells under HG stimulation.
Effects of miR-126-5p or miR-204-5p elevation on inflammation, oxidative stress, and ECM accumulation of human mesangial cells under HG stimulation. A–F Human mesangial cells were transfected with miR-126-5p, miR-204-5p, or miR-NC. A and B ELISA was performed to detect the levels of IL-6 and IL-1β in human mesangial cells with or without HG treatment. C–E The levels of ROS and MDA and the activity of SOD in human mesangial cells with or without HG treatment were assessed with corresponding kits. F Western blotting was carried out to measure the levels of FN, Col I, and Col IV in human mesangial cells with or without HG treatment. *P < 0.05
CircTLK1 Regulated Inflammation, Oxidative Stress, and ECM Accumulation of HG-Stimulated Human Mesangial Cells by Sponging miR-126-5p or miR-204-5p
After verifying the role of miR-126-5p and miR-204-5p in DN, we further verified whether circTLK1 regulated inflammation, oxidative stress, and ECM accumulation of human mesangial cells under HG stimulation by sponging miR-126-5p or miR-204-5p. The expression of miR-126-5p and miR-204-5p was reduced in human mesangial cells after transfection with anti-miR-126-5p or anti-miR-204-5p (Fig. 5A, B). Moreover, the decrease of miR-126-5p or miR-204-5p could overturn circTLK1 inhibition-mediated influence on inflammation of HG-stimulated human mesangial cells (Fig. 5C, D). Also, the inhibitory effect of circTLK1 silencing on oxidative stress and ECM accumulation of HG-treated human mesangial cells was abolished after miR-126-5p or miR-204-5p downregulation (Fig. 5E–H). Together, these results indicated that circTLK1 silencing alleviated inflammation, oxidative stress, and ECM accumulation of HG-stimulated human mesangial cells by sponging miR-126-5p or miR-204-5p.
CircTLK1 sponged miR-126-5p or miR-204-5p to regulate inflammation, oxidative stress, and ECM accumulation in HG-stimulated human mesangial cells. A and B The silencing efficiencies of miR-126-5p and miR-204-5p in human mesangial cells were validated by qRT-PCR. C–H Human mesangial cells were transfected with sh-NC, sh-circTLK1, sh-circTLK1+anti-miR-NC, sh-circTLK1+anti-miR-126-5p, or sh-circTLK1+anti-miR-204-5p. C and D ELISA revealed the levels of IL-6 and IL-1β in human mesangial cells under HG treatment. E–G The levels of ROS and MDA and the activity of SOD in human mesangial cells under HG stimulation were measured using corresponding kits. H Western blotting presented the levels of FN, Col I, and Col IV in human mesangial cells under HG treatment. *P < 0.05
CircTLK1 Knockdown Blocked the AKT/NF-κB Pathway by Sponging miR-126-5p or miR-204-5p in HG-Stimulated Human Mesangial Cells
Previous studies have reported that the AKT/NF-κB pathway is associated with the progression of DN (Ji et al. 2016; Jiang et al. 2018). Thus, we further surveyed whether circTLK1 modulated the AKT/NF-κB pathway through sponging miR-126-5p or miR-204-5p in HG-stimulated human mesangial cells. We observed that HG treatment elevated the ratio of p-AKT/AKT and p-p65/p65 in human mesangial cells, while this impact was reversed after circTLK1 inhibition. Moreover, the influence circTLK1 silencing on the ratio of p-AKT/AKT and p-p65/p65 in HG-stimulated human mesangial cells was overturned by repressing the expression of miR-126-5p or miR-204-5p (Fig. 6A). These results indicated that circTLK1 sponged miR-126-5p and miR-204-5p to regulate the AKT/NF-κB pathway in HG-stimulated human mesangial cells.
CircTLK1 modulated the AKT/NF-κB pathway via absorbing miR-126-5p or miR-204-5p in HG-stimulated human mesangial cells. A The levels of p-AKT, AKT, p-p65, and p65 in human mesangial cells transfected with sh-NC, sh-circTLK1, sh-circTLK1+anti-miR-NC, sh-circTLK1+anti-miR-126-5p, or sh-circTLK1+anti-miR-204-5p with or without HG treatment were analyzed with western blotting. B Schematic diagram of the mechanism of circTLK1 regulated the pathogenesis of DN. HG-induced circTLK1 activated the AKT/NF-κB pathway by absorbing miR-126-5p or miR-204-5p, thereby elevating inflammation, oxidative stress, and ECM accumulation of human mesangial cells. *P < 0.05
Discussion
It was reported that DN accounted for more than 30% of complications in diabetes patients worldwide (Lim 2014). With the increasing incidence of diabetes, DN has attracted people’s attention (Guariguata et al. 2014). Increasing evidence has suggested that circRNAs are related to the pathogenesis of DN. Previous report uncovered that circRNA circ-156598 aggravated the accumulation of ECM through regulation of the miR-185/TGF-β1 pathway in mesangial cells (Hu et al. 2019). Report of Chen et al. suggested that the inhibition of circLRP6 decreased mesangial cell injure through inactivating the TLR4/NF-κB pathway (Chen et al. 2019b). Herein, circTLK1 expression was elevated in DN patient’s serum and HG-treated human mesangial cells. Moreover, circTLK1 downregulation relieved inflammation, oxidative stress, and ECM accumulation in human mesangial cells treated with HG. Report of Li et al. indicated that circTLK1 facilitated renal cell carcinoma advancement via absorbing miR-136-5p (Li et al. 2020). Moreover, circTLK1 elevation exacerbated neuronal injury and neurological deficits through regulation of the miR-335-3p/TIPARP axis after ischemic stroke (Wu et al. 2019). Also, circTLK1 overexpression aggravated myocardial ischemia/reperfusion injury via the TNF pathway through modulating the miR-214/RIPK1 axis (Song et al. 2020). Thus, we concluded that circTLK1 played a critical role in the pathogenesis of DN.
Given that circTLK1 was mainly distributed in the cytoplasm of human mesangial cells, we discovered that circTLK1 was a sponge of miR-126-5p and miR-204-5p. Previous study manifested that the decrease of circulating miR-126 was related to the development of DN in T2D patients and might be a promising biomarker for DN risk assessment (Al-Kafaji et al. 2016). Moreover, miR-126 overexpression delayed the senescence of human mesangial cells through blocking the JAK/STAT and telomere-p53-p21-Rb pathways (Cao et al. 2018). Chen et al. revealed that miR-204-5p played a protective effect on the kidney against chronic damage caused by diabetes and hypertension (Cheng et al. 2020). Also, miR-204-5p targeted IL6R to repress IL6-mediated chemokine generation and inflammatory response in renal tubular epithelial cells (Li et al. 2019). Herein, miR-126-5p and miR-204-5p upregulation reduced HG-induced inflammation, oxidative stress, and ECM accumulation in human mesangial cells. Furthermore, circTLK1 silencing-mediated impact on HG-induced inflammation, oxidative stress, and ECM accumulation in human mesangial cells was antagonized by repressing the expression of miR-126-5p or miR-204-5p. Therefore, we inferred that circTLK1 regulated HG-induced human mesangial cell injure through absorbing miR-126-5p or miR-204-5p.
AKT can accelerate the transcription of NF-κB (Gilmore 2006). Mounting studies have demonstrated that the AKT/NF-κB pathway is involved in the inflammation, oxidative stress, and ECM accumulation in DN (Ji et al. 2016; Xu et al. 2019, 2020). Xu et al. revealed that daphnetin reduced ECM accumulation, oxidative stress, and inflammation in HG-stimulated human mesangial cells by regulating the AKT/NF-κB and Nrf2/keap1 pathways (Xu et al. 2019). Also, the inhibition of CTRP6 inactivated the AKT/NF-κB pathway in HG-treated human mesangial cells, leading to reduced oxidative stress, inflammation, and ECM accumulation (Xu et al. 2020). Herein, circTLK1 inhibition also inactivated the AKT/NF-κB pathway via absorbing miR-126-5p or miR-204-5p. Accordingly, we concluded that HG-induced circTLK1 sponged miR-126-5p or miR-204-5p to activate the AKT/NF-κB pathway, which aggravated inflammation, oxidative stress, and ECM accumulation in human mesangial cells (Fig. 6B).
Together, HG-induced circTLK1 activated the AKT/NF-κB pathway via sponging miR-126-5p or miR-204-5p in human mesangial cells, thereby facilitating inflammation, oxidative stress, and ECM accumulation, which caused cell damage. The study offered a novel mechanism to understand the pathogenesis of DN.
References
Achkar NP, Cambiagno DA, Manavella PA (2016) miRNA biogenesis: a dynamic pathway. Trends Plant Sci 21(12):1034–1044
Al-Kafaji G, Al-Mahroos G, Al-Muhtaresh HA, Skrypnyk C, Sabry MA, Ramadan AR (2016) Decreased expression of circulating microRNA-126 in patients with type 2 diabetic nephropathy: a potential blood-based biomarker. Exp Ther Med 12(2):815–822
Bhaskaragoud G, Geetha V, Sharanappa T, Mohan Kumar AS, Hema Kumar C, Suresh Kumar G (2018) Hypolipidemic and antioxidant properties of oryzanol concentrate in reducing diabetic nephropathy via SREBP1 downregulation rather than β-oxidation. Mol Nutr Food Res 62(8):e1700511
Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P (2016) Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol 791:8
Cao D-W, Jiang C-M, Wan C, Zhang M, Zhang Q-Y, Zhao M, Yang B, Zhu D-L, Han X (2018) Upregulation of MiR-126 delays the senescence of human glomerular mesangial cells induced by high glucose via telomere-p53-p21-Rb signaling pathway. Curr Med Sci 38(5):758–764
Chen F, Zhu X, Sun Z, Ma Y (2018) Astilbin inhibits high glucose-induced inflammation and extracellular matrix accumulation by suppressing the TLR4/MyD88/NF-κB pathway in rat glomerular mesangial cells. Front Pharmacol 9:1187
Chen J, Chen T, Zhu Y, Li Y, Zhang Y, Wang Y, Li X, Xie X, Wang J, Huang M, Sun X, Ke Y (2019a) circPTN sponges miR-145-5p/miR-330-5p to promote proliferation and stemness in glioma. J Exp Clin Cancer Res 38(1):398
Chen B, Li Y, Liu Y, Xu Z (2019b) circLRP6 regulates high glucose-induced proliferation, oxidative stress, ECM accumulation, and inflammation in mesangial cells. J Cell Physiol 234(11):21249–21259
Cheng Y, Wang D, Wang F, Liu J, Huang B, Baker MA, Yin J, Wu R, Liu X, Regner KR, Usa K, Liu Y, Zhang C, Dong L, Geurts AM, Wang N, Miller SS, He Y, Liang M (2020) Endogenous miR-204 protects the kidney against chronic injury in hypertension and diabetes. J Am Soc Nephrol 31(7):1539–1554
Ebbesen KK, Hansen TB, Kjems J (2017) Insights into circular RNA biology. RNA Biol 14(8):1035–1045
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25(51):6680–6684
Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE (2014) Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 103(2):137–149
Hsiao K-Y, Sun HS, Tsai S-J (2017) Circular RNA—new member of noncoding RNA with novel functions. Exp Biol Med 242(11):1136–1141
Hu W, Han Q, Zhao L, Wang L (2019) Circular RNA circRNA_15698 aggravates the extracellular matrix of diabetic nephropathy mesangial cells via miR-185/TGF-β1. J Cell Physiol 234(2):1469–1476
Ji X, Li C, Ou Y, Li N, Yuan K, Yang G, Chen X, Yang Z, Liu B, Cheung WW, Wang L, Huang R, Lan T (2016) Andrographolide ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated renal oxidative stress and inflammation via Akt/NF-κB pathway. Mol Cell Endocrinol 437:268–279
Jiang Y, Liu J, Zhou Z, Liu K, Liu C (2018) Diosmetin attenuates Akt signaling pathway by modulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/inducible nitric oxide synthase (iNOS) in streptozotocin (STZ)-induced diabetic nephropathy mice. Med Sci Monit 24:7007–7014
Li H, Wang Y, Chen B, Shi J (2018) Silencing of PAQR3 suppresses extracellular matrix accumulation in high glucose-stimulated human glomerular mesangial cells via PI3K/AKT signaling pathway. Eur J Pharmacol 832:50–55
Li H, Wang J, Liu X, Cheng Q (2019) MicroRNA-204-5p suppresses IL6-mediated inflammatory response and chemokine generation in HK-2 renal tubular epithelial cells by targeting IL6R. Biochem Cell Biol 97(2):109–117
Li J, Huang C, Zou Y, Ye J, Yu J, Gui Y (2020) CircTLK1 promotes the proliferation and metastasis of renal cell carcinoma by sponging miR-136-5p. Mol Cancer 19(1):103
Lim AK (2014) Diabetic nephropathy—complications and treatment. Int J Nephrol Renovasc Dis 7:361–381
Riser BL, Cortes P, Yee J (2000) Modelling the effects of vascular stress in mesangial cells. Curr Opin Nephrol Hypertens 9(1):43–47
Song Y-F, Zhao L, Wang B-C, Sun J-J, Hu J-L, Zhu X-L, Zhao J, Zheng D-K, Ge Z-W (2020) The circular RNA TLK1 exacerbates myocardial ischemia/reperfusion injury via targeting miR-214/RIPK1 through TNF signaling pathway. Free Radic Biol Med 155:69–80
Sun H-J, Wu Z-Y, Cao L, Zhu M-Y, Liu T-T, Guo L, Lin Y, Nie X-W, Bian J-S (2019) Hydrogen sulfide: recent progression and perspectives for the treatment of diabetic nephropathy. Molecules 24(15):2857
Wang T, Pan W, Hu J, Zhang Z, Li G, Liang Y (2018) Circular RNAs in metabolic diseases. Adv Exp Med Biol 1087:275–285
Wu F, Han B, Wu S, Yang L, Leng S, Li M, Liao J, Wang G, Ye Q, Zhang Y, Chen H, Chen X, Zhong M, Xu Y, Liu Q, Zhang JH, Yao H (2019) Circular RNA aggravates neuronal injury and neurological deficits after ischemic stroke via miR-335-3p/TIPARP. J Neurosci 39(37):7369–7393
Wu X, Pan C, Chen R, Zhang S, Zhai Y, Guo H (2020) BML-111 attenuates high glucose-induced inflammation, oxidative stress and reduces extracellular matrix accumulation via targeting Nrf2 in rat glomerular mesangial cells. Int Immunopharmacol 79:106108
Xu K, Guo L, Bu H, Wang H (2019) Daphnetin inhibits high glucose-induced extracellular matrix accumulation, oxidative stress and inflammation in human glomerular mesangial cells. J Pharmacol Sci 139(2):91–97
Xu E, Yin C, Yi X, Liu Y (2020) Knockdown of CTRP6 inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells through regulating the Akt/NF-κB pathway. Clin Exp Pharmacol Physiol 47(7):1203–1211
Zhang H-X, Yuan J, Li Y-F, Li R-S (2019) Thalidomide decreases high glucose-induced extracellular matrix protein synthesis in mesangial cells via the AMPK pathway. Exp Ther Med 17(1):927–934
Zhou Z-B, Huang G-X, Fu Q, Han B, Lu J-J, Chen A-M, Zhu L (2019) circRNA.33186 contributes to the pathogenesis of osteoarthritis by sponging miR-127–5p. Mol Ther 27(3):531–541
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qiu, B., Qi, X. & Wang, J. CircTLK1 Downregulation Attenuates High Glucose-Induced Human Mesangial Cell Injury by Blocking the AKT/NF-κB Pathway Through Sponging miR-126-5p/miR-204-5p. Biochem Genet 60, 1471–1487 (2022). https://doi.org/10.1007/s10528-021-10146-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-021-10146-8