Abstract
Chronic obstructive pulmonary disease (COPD) is a complex chronic inflammatory disease of the respiratory system affecting primarily distal respiratory pathways and lung parenchyma. This work was designed as a case–control study aimed at investigating the association of COPD with polymorphisms in inflammatory and immune response genes (JAK1, JAK3, STAT1, STAT3, NFKB1, IL17A, ADIPOQ, ADIPOR1, etc.) in Tatar population from Russia. Ten SNPs (rs310216, rs3212780, rs12693591, rs2293152, rs28362491, rs4711998, rs1974226, rs1501299, rs266729, and rs12733285) were genotyped by the real-time polymerase chain reaction (TaqMan assays) in a case–control study (425 COPD patients and 457 in the control group, from Ufa, Russia). Logistic regression was used to detect the association of SNPs in different models. Linear regression analyses were performed to estimate the relationship between SNPs and lung function parameters and pack-years. In Tatar population, significant associations of JAK1 (rs310216) (P = 0.0002, OR 1.70 in additive model), JAK3 (rs3212780) (P = 0.001, OR 1.61 in dominant model), and IL17A (rs1974226) (P = 0.0037, OR 2.31 in recessive model) with COPD were revealed. The disease risk was higher in carriers of insertion allele of NFKB1 (rs28362491) (P = 0.045, OR 1.22). We found a significant gene-by-environment interaction of smoking status and IL17A (rs1974226) (P interact = 0.016), JAK3 (rs3212780) (P interact = 0.031), ADIPOQ (rs266729) (P interact = 0.013), and ADIPOR1 (rs12733285) (P interact = 0.018). The relationship between the rs4711998, rs1974226, rs310216, rs3212780, rs28362491, and smoking pack-years was found (P = 0.045, P = 0.004, P = 0.0005, P = 0.021, and P = 0.042). A significant genotype-dependent variation of forced vital capacity was observed for NFKB1 (rs28362491) (P = 0.017), ADIPOR1 (rs12733285) (P = 0.043), and STAT1 (rs12693591) (P = 0.048). The genotypes of STAT1 (rs12693591) (P = 0.013) and JAK1 (rs310216) (P = 0.048) were associated with forced expiratory volume in 1 s.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory disease of the respiratory system primarily affecting the distal respiratory pathways and the lung parenchyma (Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, 2011). COPD is one of the most common chronic diseases; in the absence of adequate management, it can significantly impair the patients’ everyday activity and may even lead to a lethal outcome (Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, 2011).
Genome-wide association studies (GWASs) have identified several loci associated with COPD, in particular, in chromosomal regions 15q25.1 near cholinergic receptor, nicotinic, alpha 3/5 (CHRNA3/5), and iron-responsive element binding protein 2 (IREB2); the chromosome 4q22.1 region near family with sequence similarity 13, member A (FAM13A); chromosome 4q31 regions near hedgehog-interacting protein (HHIP); and at 4q28.1, 6p21.31, 6q16.1, 10q22.1, and 10q22.3 regions (Pillai et al. 2009, 2010; Caporaso et al. 2009; Cho et al. 2010; Siedlinski et al. 2011). SNPs of these genes were associated with COPD, lung function parameters, and smoking behavior in different populations (Pillai et al. 2009, 2010; Cho et al. 2010; Siedlinski et al. 2011; Caporaso et al. 2009; Ding et al. 2015a, b).
A key phenomenon in the pathogenesis of COPD is inflammation, which may be induced by tobacco smoke, dust particles in the polluted air, or by viruses and bacteria (Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, 2011; Hackett et al. 2008). Cytokines are a family of polypeptide mediators involved in the formation and regulation of defense reactions. Along with adhesion molecules, acute-phase proteins, and antibacterial peptides, cytokines constitute an important pulmonary signaling system that determines the initiation, the progress, and the outcome of the immune response in the lungs (Hackett et al. 2008). Chronic systemic inflammation in COPD is associated with constant productions of IL-1b, TNFA, IL-8, IL-6, fibrinogen, and C-reactive protein by alveolar macrophages and neutrophils, which correlates with disease progression and frequent exacerbations (Hackett et al. 2008; Bianco et al. 2013). The transformation of local inflammation in the lung tissue to chronic systemic inflammation in COPD can be associated with high permeability of pulmonary blood vessels resulting in a release of proinflammatory factors into the general circulation (Bianco et al. 2013). The key system mediating the manifold effects of cytokines is the JAK/STAT signaling pathway (Janus Kinases–Signal Transducer and Activator of Transcription), which includes cytoplasmic tyrosine kinases of the Janus family (JAK1, JAK2, JAK3, and Tyk2) and cytoplasmic latent transcription factors (in mammals, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6). Cytokines and growth factors act as signaling molecules for the JAK/STAT pathway (Rawlings et al. 2004).
Transcription factor NF-kB controls the expression of numerous genes that encode proteins involved in immune response reactions, apoptosis, cell cycle, differentiation, and stress response. Impaired NF-kB signaling can result in developmental abnormalities, inflammation, and a large number of diseases (Karban et al. 2004; Lawrence 2009). The major form of NF-kB is a heterodimer of the p50 and p65/RelA subunits, which are encoded by the NFKB1 and NFKB2 genes, respectively. The NFKB1 gene located on 4q24 encodes two proteins (p105 and p50) (Karban et al. 2004). The NFKB1 gene exhibits a high level of polymorphism in different human populations of the world (http://www.ncbi.nlm.nih.gov/projects/SNP/). The rs28362491 polymorphism generates a deletion of four nucleotides in the promoter region (−94 ins/del ATTG) causing lowered transcription levels of NFKB1 and consequently partial depletion of p50. The NFKB1 promoter containing the insertion allele (rs28362491) has been shown to have higher promoter activity than a promoter containing the deletion allele (Karban et al. 2004). Lai et al. 2015 indicated that NFKB1 (−94 ins/del ATTG) polymorphism may play a role in coronary artery disease susceptibility in Chinese Uygur population and is functionally associated with IL-6 expression. This functional polymorphism has been associated with malignant tumors (Wang et al. 2014a, b; Wang et al. 2015a, b; Pallavi et al. 2015), myocardial infarction, and congenital heart disease (López-Mejías et al. 2012; Zhang et al. 2013; Yang et al. 2014).
IL-17 is produced by proinflammatory cells, mainly by type 17 T helpers (Th17). The IL-17 family includes six structurally homologous members: A, B, C, D, E, and F. IL-17A stimulates the syntheses of IL-1, TNFA, IL-6, IL-8, granulocyte colony-stimulating factor (G-CSF), and other proinflammatory factors (Korn et al. 2009). In the recent years, Th17 cells have been attracting considerable attention as key mediators involved in the pathogenesis of autoimmune diseases, such as rheumatoid arthritis and psoriasis (Korn et al. 2009; Kurimoto et al. 2013). The roles of Th17 and IL-17 in inflammatory diseases of the respiratory and cardiovascular systems are the subjects of extensive research (Kurimoto et al. 2013; Di Stefano et al. 2009; Eustace et al. 2011). Some proinflammatory functions of IL-17 are mediated by activation of matrix metalloproteases, MMP3 and MMP8, and neutrophil recruitment to the inflammation site. Neutrophils, in turn, activate metalloproteases and serine proteases, which lead to tissue damage (Korn et al. 2009). It is this mechanism that is involved in emphysematous lung tissue damage in COPD. Several studies have established IL-17 association with COPD (Eustace et al. 2011). Kurimoto et al. (2013) demonstrated the role of IL-17A in the development of pulmonary inflammation and lung emphysema in mouse models (Kurimoto et al. 2013).
Several studies analyzed the relationship between the adipose tissue and the levels of circulating cytokines (TNFA, IL-6), leptin, and adiponectin, which contribute significantly to metabolic disorders developing in COPD and to impairment of the respiratory function (Franssen et al. 2008). Cytokines produced by the adipose tissue attract special attention. Adiponectin (ADIPOQ) is a 30-kDa cytokine secreted by adipocytes; it regulates energy homeostasis and has an anti-inflammatory and anti-atherogenic effect (Bianco et al. 2013; Franssen et al. 2008). Its role in inflammation is controversial: adiponectin plasma concentrations are significantly elevated in metabolic syndrome and in type-II diabetes mellitus, but decreased in some inflammatory diseases, such as rheumatoid arthritis and systemic lupus erythematosus (Korn et al. 2009; Pallavi et al. 2015). Some studies showed that the circulating ADIPOQ levels were increased in COPD and were associated with poor prognosis, in particular, with frequent exacerbations (Yuan et al. 2012). Several genome-wide studies showed that ADIPOQ levels were associated with ADIPOQ polymorphisms (Heid et al. 2010). Yuan et al. (2012) first showed that polymorphic ADIPOQ loci were associated with COPD (Yuan et al. 2012). It was also found that plasma ADIPOQ levels correlated with the respiratory function (Sato et al. 2014). Adiponectin actions are mediated by specific receptors, ADIPOR1 and ADIPOR2 (Bianco et al. 2013).
The frequency distributions of the JAK1, JAK3, STAT1, STAT3, NFKB1, IL17A, ADIPOQ, and ADIPOR1 polymorphisms and their association with COPD was not yet investigated in the populations of Russia. Association studies of polymorphic markers in such candidate genes effecting development and progression of asthma, cancer, cardiovascular, and autoimmune diseases were published (Karban et al. 2004; McGovern et al. 2009; Sperati et al. 2009; Jiang et al. 2011; Wang et al. 2011; Zhong et al. 2012; Yang et al. 2012; Fantuzzi 2013; Yuan et al. 2014; Wang et al. 2014a, b; Rafiei et al. 2013; Carolanet et al. 2014; Kuruma et al. 2014; Lai et al. 2015; Jin et al. 2015). We hypothesized that inflammatory and immune response genes may be associated also with COPD in our Tatar population. To avoid possible problems arising from population and ethical stratification, in our study, we analyzed the association of SNP markers with COPD in ethnically homogenous group—ethnic Tatars, historically dispersed over the territory of the Republic of Bashkortostan (Russia). The Republic of Bashkortostan is located in the southern part of the Ural Mountains and adjacent plains, at the border of Europe and Asia. It is a multinational republic, where representatives of more than hundred ethnic groups live, including Russians (39.3 %), Tatars (28.4 %), and Bashkirs (21.9 %).
The purpose of the present study was to investigate the association of COPD with polymorphisms of JAK1 (rs310216), JAK3 (rs3212780), STAT1 (rs12693591), STAT3 (rs2293152), NFKB1 (rs28362491), IL17A (rs4711998, rs1974226), ADIPOQ (rs1501299, rs266729), and ADIPOR1 (rs12733285) in a population of Tatars from Russia.
Materials and Methods
Prior to implementation, present study was approved by the Local Ethical Committee of Institute of Biochemistry and Genetics of Ufa Scientific Center of Russian Academy of Sciences (IBG USC RAS), Ufa, Russia (Ufa, Protocol No 17, December 7, 2010). Written informed consent was obtained from all individuals. All DNA samples used in the study were anonymous.
Patients and Controls
Study design. This work was designed as a case–control study aimed at investigating the association of COPD with JAK1, JAK3, STAT1, STAT3, NFKB1, IL17A, ADIPOQ, and ADIPOR1 polymorphisms in the ethnically homogenous group—Tatar population from Russia. The case–control groups for our candidate gene approach study were accurately selected and collected from 2010 to 2013 years in the pulmonary departments of Ufa City Hospitals: №13, №18, and №21 (Ufa, Russia). The total number of 882 DNA samples of unrelated individuals, representatives of Tatar population, historically dispersed over the territory of the Republic of Bashkortostan have been analyzed in this study. The Republic of Bashkortostan (the capital city is Ufa) is a sovereign state under the jurisdiction of the Russian Federation. We collected COPD and control groups’ age-, sex- and ethnically matched data. Ethnic origin (up to the third generation) of all the participants was derived from direct interviews with examined persons. The COPD patients were recruited randomly according to the International Classification of Diseases tenth revision (ICD 10) (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision 2015) and following the recommendations of the Global Initiative for Chronic Obstructive Lung Disease (Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, 2011). The study group consisted in total of 425 unrelated COPD patients, recruited during the period from 2010 to 2013 in the pulmonary departments of Ufa City Hospitals, №13, №18, №21 (Ufa, Russia).
The Inclusion and Exclusion Criteria
For all patients with COPD, the diagnose was detected by the hospital specialists on the basis of the medical histories and the results of general, clinical, and special tests (chest X-ray, spirometry measures, and fibrobronchoscopy), physical examination, and laboratory approaches. Patients were excluded from the study if they had diagnosis of asthma and lung cancer. Subjects performed standardized pre-bronchodilator and post-bronchodilator spirometry in accordance with American Thoracic Society/European Respiratory Society (Miller et al. 2005). The spirometry was done in the in pulmonary departments of Ufa City Hospitals №13, №18, №21 (Ufa, Russia) by the hospital specialists, and the predicted values for FVC, FEV1, and FEV1/FVC ratio were generated using previously defined prediction equations as detailed to the European Coal and Steel Community (ECSC) (Quanjer et al. 1993; Roca et al. 1998). All COPD patients had post-bronchodilator FEV1/FVC values of <70 %.
The control group is comprised of 457 unrelated age-, sex- and ethnicity matched to the cases healthy residents of Ufa (Russia) with no history of chronic diseases such as respiratory system pathology and allergic diseases in the anamnesis. All the control subjects were collected among those individuals who attended Ufa City Hospitals №13, 18, 21 (Russia) for regular medical examination. All individuals from control group were unrelated to patients and independent of one another. Control subjects demonstrated normal lung function (FEV1/FVC > 70 %, FEV1 > 80 %). Summary is given in Table 1.
Genotyping
Genomic DNA was isolated from peripheral blood leukocytes using the standard phenol–chloroform extraction procedure (Mathew 1985). We worked with so called candidate genes approach, meaning that we choose for consideration only polymorphisms in genes with known functions and previously shown association with other complex inflammatory diseases. Minor allele frequency (MAF) of ≥5 % in the Caucasian population, parameters set by the SNP database of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/projects/SNP/), were also reviewed. For the current study, ten most widely studied SNPs on JAK1 (rs310216, c.1649-82C>T, intron variant), JAK3 (rs3212780, c.3207+75C>T, intron variant), STAT1 (rs12693591, c.786−557 C>A, intron variant), STAT3 (rs2293152 c.1233+43C>G, intron variant), NFKB1 (rs28362491 c.-798_−795delATTG, 2 KB upstream variant), IL17A (rs471199, c.−877G>A, 2 KB upstream variant 8; rs1974226, c.*1245C>T, 3 prime UTR variant), ADIPOQ (rs1501299, c.214+62G>T, intron variant; rs266729, c.−1124C>G, 2 KB upstream variant), and ADIPOR1 (rs12733285, c.−95+1329G>A, intron variant) were examined by the real-time polymerase chain reaction (PCR), with the use of TaqMan SNP discrimination assays (Applied Biosystems, Foster City, CA). Accumulation of specific PCR-product by hybridization and cleavage of double-labeled fluorogenic probe during amplification was detected with a BioRad CFX96 instrument (Bio-Rad Laboratories Inc., USA). End-point fluorescence and genotype discrimination were determined according to the BioRad CFX96 protocol, using CFX Manager software. For quality control, 5 per cent dummy duplicates and blank controls were also taken up along with the samples in each experiment. The genotyping was blind to case or control status of the samples. Quality control of genotyping data was assessed by subject and by marker. A priori of the association analysis we run strict quality control on our data to exclude genotyping errors, SNPs and individuals with law call rates and other important quality characteristics. Subject data were excluded after examining missingness, reproducibility, and inbreeding. All subjects with a genotype call rate of <95 % were removed. Subsequently SNPs were filtered according to their proportion of missing, minor allele frequency (MAF) or deviation from Hardy–Weinberg–Equilibrium (HWE).
Statistical Analysis
The sample size was calculated by Quanto software (http://biostats.usc.edu/software). We examined eight candidate genes and used the most significant reported SNPs with a high minor allele frequency for each gene. On the basis of our calculations using the Power and Sample Size software program, our sample (N = 882) was considered adequate to study the selected SNPs. The sample size (N = 425 for case group and N = 457 for control group) was sufficient to detect the association of examined SNPs and COPD with more than 80 % power (Power: 95.53 %, Disease prevalence, 7 %, error: 5 %).
For the quantitative traits, the mean values and standard deviations (M ± SD) were calculated; the group comparison was performed with a nonparametric Mann–Whitney U-test. The frequencies of qualitative traits were compared using the Pearson’s X 2. Statistical analysis was carried out with the Statistica v. 6.0 program (StatSoft Inc., Tulsa, OK, USA). A minor allele frequencies (MAF) and the agreement of the genotype distribution to the Hardy–Weinberg equilibrium (X 2), the association analysis using the basic allele test and the calculation of the odds ratio (OR) for the rare allele of each locus and the significance of intergroup differences in allele and genotype frequencies (X 2 test for sample heterogeneity and the P value), and Cochran–Armitage trend test were performed with PLINK v. 1.07 (Purcell et al. 2007). Differences were considered significant if their corresponding P values were less than 0.05. To control Type-I error rate, Bonferroni correction for multiple comparison was performed meaning that P value was multiplied by the number of SNP loci studied (n = 10) to obtained the new P cor value, false discovery rate (FDR) (Benjamini Hochberg) was calculated using the online software program http://www.sdmproject.com/utilities/?show=FDR. Logistic regression was used to detect the association of SNPs loci in different models, accounting for quantitative and binary traits (gender, age, pack-years, smoking status, body mass index). The significance of the obtained model accounting for all variables was verified by the significance of the likelihood ratio test (P adj). The best model was chosen using the Akaike’s information criterion (AIC). For each significant locus (P < 0.05), the model with the lowest AIC was chosen. Linear regression analyses were performed to estimate the relationship between SNPs and quantitative phenotypes, such as lung function parameters and pack-years. The regression analysis was performed with PLINK v. 1.07 (Purcell et al. 2007) and SNPStats packages (Solé et al. 2006).
Results
Before candidate gene polymorphisms were analyzed for associations with COPD, we checked whether their genotype frequency distributions agreed with the Hardy–Weinberg equilibrium and evaluated minor allele frequencies (MAF) both in the combined group of patients and healthy subjects and in either group individually. For the control group, the following results were obtained: JAK1 (rs310216) (P = 0.064, MAF = 0.128), JAK3 (rs3212780) (P = 0.053, MAF = 0.291), STAT1 (rs12693591) (P = 0.38, MAF = 0.140), STAT3 (rs2293152) (P = 0.11, MAF = 0.306), NFKB1 (rs28362491) (P = 0.27, MAF = 0.378), IL17A (rs4711998) (P = 0.98, MAF = 0.326) and (rs1974226) (P = 0.94, MAF = 0.223), ADIPOQ (rs1501299) (P = 0.17, MAF = 0.217) and (rs266729) (P = 0.27, MAF = 0.298), ADIPOR1 (rs12733285) (P = 0.44, MAF = 0.271).
Association of Candidate Polymorphic Loci with COPD
Data on the allele and genotype frequency distributions for the loci in question, the significance of their differences between the groups, and odd ratio vales calculated for the minor allele, and Cochran–Armitage trend test of each locus are shown in Tables 2 and 3. Significant differences between the groups studied were identified for the following polymorphic loci: JAK1 (rs310216), JAK3 (rs3212780), NFKB1 (rs28362491), IL17A (rs1974226). Table 4 presents the characteristics of the detected significant associations with COPD: the regression coefficient (beta), its exponent interpreted as odds ratio (OR) in the logistic model, the corresponding 95 % confidence intervals, and the level of significance, calculated while taking into account the patients’ sex, age, smoking status, BMI, and smoking index in different models.
The frequency of the minor T allele of JAK1 (rs310216) was significantly higher in COPD patients than in controls (19.88 vs 12.8 %; P = 0.0001, OR 1.69 (95 % CI 1.31–2.18)). The portion of TT homozygotes in the group of COPD patients was as high as 5.41 %, in contrast to 0.66 % in healthy subjects (P = 0.0001, OR 10.71 (95 % CI 2.48–46.33)) in the recessive model). Significant association with COPD was established in the additive (P = 0.0002, OR 1.70 (95 % CI 1.28–2.26)) and in the dominant model (P = 0.005, OR 1.58 (95 % CI 1.15–2.19)).
The minor T allele of JAK3 (rs3212780) was shown to be associated with COPD (P = 0.0001, OR 1.43 (95 % CI 1.17–1.75)). In the dominant model, JAK3 (rs3212780) association with COPD was more informative (P = 0.001, OR 1.61 (95 % CI 1.21–2.14)), since the portion of homozygous and heterozygous carriers of the T allele was 59.5 % in COPD patients in comparison to 47.5 % in healthy controls.
The frequency of the Del allele of the NFKB1 insertion/deletion polymorphism rs28362491 was lower in COPD patients than in controls (33.18 vs 37.86 %; P = 0.045, OR 0.81 (95 % CI 0.67–0.99)). The more frequent Ins allele was the marker associated with the disease (OR 1.22 (95 % CI 1.05–1.49)). However, regression analysis failed to detect a significant association between NFKB1 (rs28362491) and COPD.
The minor allele of IL17A (rs1974226) was also shown to be associated with COPD (P = 0.048, OR 1.25 (95 % CI 1.01–1.56)); its frequency was 26.47 % in the group of patients, in contrast to 22.32 % in healthy controls. A regression analysis established IL17A’s (rs1974226) association with COPD in the recessive model (P = 0.0037, OR 2.31 (95 % CI 1.28–4.16)). The observed association was because the portion of homozygous and heterozygous carriers of the minor T allele was higher in COPD patients.
Analysis of Genotype-by-Environment Interactions in COPD
Significant interactions with the smoking status were observed for the loci IL17A (rs1974226) (P interact = 0.016), JAK3 (rs3212780) (P interact = 0.031), ADIPOQ (rs266729) (P interact = 0.013), and ADIPOR1 (rs12733285) (P interact = 0.018). We analyzed the contribution of candidate gene genotypes to the variation of the quantitative trait characterizing smoking intensity (smoking index, packs/year) and found that the smoking index was affected by the genotypes by IL17A (rs4711998, rs1974226), JAK1 (rs310216), JAK3 (rs3212780), and NFKB1 (rs28362491) (Table 5).
In particular, the smoking index was significantly higher in carriers of IL17 genotypes AA (rs4711998) and TT (rs1974226) (P = 0.045 and P = 0.004, respectively). In TT homozygotes by the minor allele of JAK1 (rs310216), the smoking index was as high as 43.48 packs/years, in contrast to 23.31 packs/year in heterozygotes and CC homozygotes (P = 0.0005). The CC genotype by JAK3 (rs3212780) was associated with lower smoking index values (P = 0.021), whereas the homozygous insertion variant (Ins/Ins) of NFKB1 (rs28362491) was associated with an elevated smoking index (P = 0.042).
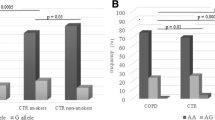
Genotype-by-environment interactions were also analyzed by comparing odds ratio values calculated for the candidate genes in subgroups formed according to the presence or absence of the environmental factor ((Table 6). We found that the polymorphisms IL17A (rs1974226), JAK1 (rs310216), and NFKB1 (rs28362491) were significantly associated with COPD only in the group of smokers. IL17A (rs1974226) was associated with COPD in smokers (P = 0.017, OR 1.39 (95 % CI 1.06–1.83) in the additive model). In the subgroup of smokers, the risk of COPD was associated with JAK1 (rs310216) (P = 0.0001, OR 1.94 (95 % CI 1.37–2.74)) in the additive model, while NFKB1 (rs28362491) was associated with COPD in smokers in the dominant model (P = 0.032, OR 0.66 (95 % CI 0.45–0.97)).
In nonsmokers, significant associations were established for JAK3 (rs3212780) (P = 0.0016, OR 2.63 (95 % CI 1.43–4.84) in the dominant model), STAT3 (rs2293152) (P = 0.0046, OR 1.93 (95 % CI 1.22–3.06) in the additive model), ADIPOQ (rs266729) (P = 0.019, OR 1.64 (95 % CI 1.08–2.48) in the additive model), and ADIPOR1 (rs12733285) (P = 0.017, OR 0.46 (95 % CI 0.24–0.89) for the GA genotype) (Table 6).
Contribution of Candidate Gene Polymorphisms to the Variation of Quantitative Characteristics of the Respiratory Function
We also analyzed whether quantitative characteristics of the respiratory function: vital capacity (VC), forced vital capacity (FVC), and forced expiration volume in 1 s (FEV1), depended on the genotypes at the loci studied in COPD patients. Polymorphisms of JAK3 (rs3212780), STAT3 (rs2293152), IL17A (rs4711998, rs1974226), and ADIPOQ (rs1501299, rs266729) were not significantly associated with quantitative characteristics of the respiratory function.
Carriers of the NFKB1 (rs28362491) genotype Del/Del exhibited higher FVC values (P = 0.017) (Table 5), which further confirmed that this locus was associated with COPD. Patients heterozygous at the ADIPOR1 (rs12733285) also had higher FVC (P = 0.043). FEV1 was found to depend on JAK1 (rs310216) and STAT1 (rs12693591) genotypes. Patients homozygous for the T allele of JAK1 (rs310216) had lower FEV1 than those with CC and CT genotypes (P = 0.013). In AA homozygotes by STAT1 (rs12693591), FVC values were significantly decreased (P = 0.048).
Discussion
The objective of this study was to evaluate the contribution of JAK1 (rs310216), JAK3 (rs3212780), STAT1 (rs12693591), STAT3 (rs2293152), NFKB1 (rs28362491), IL17A (rs4711998, rs1974226), ADIPOQ (rs1501299, rs266729), and ADIPOR1 (rs12733285) polymorphisms to susceptibility to COPD in a Tatar population from Russia. We also investigated the associations of these candidate gene loci with quantitative parameters characterizing COPD progression and analyzed interactions between genetic and environmental factors.
Several STAT1, STAT3, JAK1, and JAK3 SNPs were reported to be significantly associated with cervical cancer (Wang et al. 2011), nonsmall cell lung cancer (Jiang et al. 2011), leukemia (Zhong et al. 2012), gastric cancer (Yuan et al. 2014), Crohn’s disease (Wang et al. 2014a, b), and cardiovascular diseases (Sperati et al. 2009). Based on previous studies, our case–control study detected the effect of JAK1 (rs310216, c.1649-82C>T), JAK3 (rs3212780, c.3207+75C>T), STAT1 (rs12693591, c.786-557G>T), STAT3 (rs2293152, c.1233+43C>G) gene polymorphisms on COPD in Tatar population from Russia. The most significant associations have been established for the polymorphisms of JAK1 and JAK3. Minor alleles of JAK1 (rs310216) and JAK3 (rs3212780) were associated with COPD in general. JAK1 (rs310216) was also associated with variation in respiratory function characteristics reflecting the severity of airway obstruction. In particular, homozygous carrier of the minor T allele exhibited lower FEV1 values. JAK3 (rs3212780) showed a significant interaction with the smoking status. JAK3 (rs3212780) association with COPD was confirmed only in nonsmokers, whereas JAK1 (rs310216) was associated with COPD in smokers. It should be noted that TT homozygotes by the minor allele of JAK1 (rs310216) had a significantly higher smoking index. At the same time, homozygous carriers of the frequent C allele of JAK3 (rs3212780) exhibited lower smoking index values. JAK1 and JAK3 encode cytoplasmic tyrosine kinases of the Janus family whose function is to mediate interferon- and cytokine-induced signaling. The distinctive feature of Janus kinases differing them from other mammalian tyrosine kinases is the presence of a tandem of a kinase (JH1) and pseudokinase (JH2) domains (Rawlings et al. 2004). Mutations of the JH2 domain can inhibit or stimulate the catalytic activity of Janus kinases depending on their particular location (Rawlings et al. 2004). Another function of the pseudokinase domain is to provide binding sites for STAT proteins. JAK1 is expressed in different tissues and can be activated by many cytokines, while JAK3 expression is specific to certain tissues. It is constitutively expressed in natural killer cells and thymocytes, and can be induced in T and B cells, as well as in myeloid cells (Rawlings et al. 2004). JAK3 (rs3212780) was associated with cardiovascular complications in patients on dialysis (Sperati et al. 2009). JAK1 polymorphisms were significantly associated with Vogt–Koyanagi–Harada disease and with Behcet’s disease in a Chinese Han population (Hu et al. 2013; Hou et al. 2013). Previously, JAK1 polymorphisms were associated with bronchial asthma (Hsieh et al. 2011), and hepatocellular carcinoma (Xie et al. 2009).
For signal transduction and activation of transcription, protein 1 (STAT1) is a transcription factor mediating constitutive transcription of numerous genes (Ramana et al. 2000). It is also involved in signal transduction from a variety of different ligands, including interferons (type 1, IFNA and IFNB; and type 2, IFNG), cytokines (IL-6, IL-22, TNF, and IL-10), MAP kinases, peptide hormones, and lipopolysaccharides. The STAT1-encoding gene is located on 2q32.2 and comprises 25 exons. The promoter region of STAT1 contains binding sites for transcription factors STAT5B, Max1, AP-1, IRF-1, S8, COMP1, FOXO1a, RSRFC4, and c-Myc. STAT1 polymorphisms have been associated with an increased risk of hepatocellular carcinoma (Zhu et al. 2010) and with atopic sensibilization (Pinto et al. 2007). STAT3 is the main signal transducer for gp130-like receptors. It is activated by phosphorylation induced by cytokines of the IL-6 and IL-10 families, and leptin. This transcription factor is involved in the signaling pathways induced by cytokines and chemokines, nerve growth factor, and leptin, as well as in Notch signaling, and the Th17 differentiation pathway (Geraghty et al. 2013). Through mediating the effects of different cell stimuli on the expressions of numerous genes, STAT3 plays an important role in a variety of cell processes. In particular, STAT3 regulates all key processes involved in airway damage and lung tissue destruction in COPD: inflammation, apoptosis, and protease expression (Geraghty et al. 2013). It was shown in a mouse model that exposure to tobacco smoke caused STAT3 activation (Geraghty et al. 2013). Expression of the STAT3 gene located on chromosome 17q21.2 gives rise to two STAT3 isoforms by alternative splicing. STAT3 polymorphisms have been associated with Crohn’s disease (Wang et al. 2014a, b), gastric cancer (Yuan et al. 2014), autoimmune thyroiditis (Xiao et al. 2013), and multiple sclerosis (Lill et al. 2012). In the present work, STAT3 (rs2293152) exhibited an association with COPD in nonsmokers. The genotype by STAT1 (rs12693591) affected the FVC parameter corresponding to COPD severity: it was significantly decreased in homozygous carriers of the minor allele.
In our study, the insertion allele of the NFKB1 (rs28362491, c.-798_-795delATTG) was associated both with COPD in general and with decreased lung function parameters characterizing the severity of the disease. The rs28362491 genotype was found to affect the smoking index: it was significantly higher in homozygous carriers of the Ins allele. The rs28362491 (−94 insertion/deletion ATTG) functional NFKB1 polymorphism is located between two putative key promoter regulatory elements. The study of in vitro promoter expression indicated that the ATTG insertion allele may increase the mRNA expression of the NFKB1, resulting in the production of p50/p105 NF-kB protein (Karban et al. 2004). The NFKB1 (rs28362491, −94 insertion/deletion ATTG) polymorphism may influence the susceptibility to inflammatory diseases by inducing imbalance in the pro-inflammatory and anti-inflammatory responses (Karban et al. 2004; Lai et al. 2015). The deletion allele of NFKB1 (rs28362491) is a marker of an increased risk of malignant tumors and dilated cardiomyopathy, but a decreased risk of myocardial infarction, pathogenesis of which also involves an inflammatory component (Yang et al. 2014; Zhou et al. 2009). The role of inflammation in the pathogenesis of COPD is well known (Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease 2011; Hackett et al. 2008), and NFKB1 is therefore a reliable candidate gene for COPD. Moreover, NFKB1 (rs28362491) is located in 4q24, and it is possible that this polymorphism is in linkage with other polymorphisms that are associated with COPD. Resent GWASs and replication studies have identified several SNPs in chromosomal region 4q22.1 (FAM13A), 4q31 region (HHIP), and at 4q28.1 region, which were associated with COPD, lung function parameters, and smoking behaviors in different populations (Pillai et al. 2009, 2010; Cho et al. 2010; Siedlinski et al. 2011; Caporaso et al. 2009; Ding et al. 2015a,b). Caporaso et al. (2009) observed association between rs10489113 located on 4q28.1 near NFKB1 and smoking behavior. Ding et al. 2015a studied rs950063 on 4q28.1 in case–control study of COPD in Hainan region, and did not find any significant association between the rs950063 and risk of COPD, but detected that SNPs from FAM13A (rs7671167) on 4q22.1 are associated with COPD in Chinese Li minority population (Ding et al. 2015b).
IL-17 is a proinflammatory cytokine encoded by IL17A, which is located on chromosome 6p12. The human IL17A gene is composed of three exons and two introns covering 4, 252 bases of genomic DNA (Nakada et al. 2011). IL-17 stimulates the expression of IL-6 and cyclooxygenase 2 (COX2) (Dariusz et al. 2014). It was recently demonstrated that, apart from Th17 lymphocytes, IL-17 is produced by other types of cells, including macrophages, neutrophils, and mast cells (Korn et al. 2009). By stimulating macrophages, IL-17 promotes the production of proinflammatory cytokines and matrix metalloproteases and thus can be involved in the pathogenesis of not only autoimmune, but also other inflammatory diseases, including COPD. Our case–control study detected the effect of the IL17A (rs4711998, c.-877A>G) the 2 KB upstream variant, and the IL17A (rs1974226, c.*1245C>T) the 3 prime UTR variant on COPD in Tatar population from Russia. The variant IL17A (rs4711998, c.−877A>G) located within 5′UTR region possibly was associated with a change in transcriptional activity of IL17A (Nakada et al. 2011). A study by McGovern et al. (2009) confirmed the contribution of IL17 and IL23 polymorphisms to Crohn’s disease, a classical autoimmune condition (McGovern et al. 2009). At the same time, a polymorphism in the IL17A promoter was shown to be significantly associated with gastric cancer in an Iranian population (Rafiei et al. 2013) and with postmenopausal osteoporosis in a Polish population (Dariusz et al. 2014). A number of IL17A polymorphisms were analyzed for association with brucellosis (Rasouli et al. 2013). Jin et al. (2015) in systematic review showed that IL17A (rs4711998) may be potential risk factors for asthma susceptibility (Jin et al. 2015). The transcript of IL17A (1859 bp) has a relatively long 3′UTR region (1345 bp), where the IL17A (rs1974226, c.*1245C>T) polymorphism is located (Nakada et al. 2011). The potential mechanism of the rs1974226 effect is alteration of gene regulation (Nakada et al. 2011). Chen et al. (2006) in their review have provided evidence for the role of the 3′ UTR region in regulation of gene expression, such as mRNA stability and/or degradation as well as translation efficiency (Chen et al. 2006). In our work, rs1974226, (c.*1245C>T) polymorphism of IL17A has for the first time been shown to be associated with COPD; this association was further confirmed in the subgroup of smokers. Characteristically, rs1974226 exhibited a significant gene-by-environment interaction with the smoking status (P interact = 0.016), and the smoking index (describing the number of cigarettes smoked) depended on the IL17A polymorphisms, rs1974226 and rs4711998. In study by Chang et al. (2014) was demonstrated that IL-17A plays important roles in the initial inflammatory response to cigarette smoke exposure and on alveolar epithelial cell damage. These findings support an important role for IL-17 in the pathogenesis of COPD (Chang et al. 2014). The data by Roos et al. suggest IL-17A involvement in COPD progression and in the formation of secondary lymph nodes in the lung tissue of patients with very severe COPD (Roos et al. 2015). The role of cytokines IL-9, IL-17, IL-22, IL-25, and IL-33 in airway inflammation in bronchial asthma is discussed in (Farahani et al. 2014). The fact that the associations of IL17A loci, rs1974226 and rs4711998, with COPD were confirmed only in smokers, along with the detected relationship between IL17A polymorphisms and smoking index and smoking status, seems to support the notion that IL-17 acts by activating matrix metalloproteases and serine proteases and destroys the lung tissue similarly to cartilage degradation in rheumatoid arthritis. It is known that smoking is the principal factor that triggers the chronic inflammation cascade in COPD and leads to emphysematous degradation of lung tissue. It is not unlikely that autoimmune mechanisms are also involved in this processes (Eustace et al. 2011).
Adiponectin (ADIPOQ) is a cytokine that is secreted by adipocytes and regulates energy homeostasis (Bianco et al. 2013; Franssen et al. 2008). Circulating ADIPOQ levels are significantly decreased in obesity (Franssen et al. 2008). Increased systemic ADIPOQ levels were observed in patients with inflammatory and autoimmune diseases (Fantuzzi 2013). Peripheral blood biomarkers can serve as accurate indicators predicting COPD phenotypes and other systemic manifestations of the disease. One of such biomarkers is ADIPOQ, whose level clearly correlates with COPD severity and emphysematous changes in the lungs, it may play an important role in the pathogenesis of smoking-induced pulmonary diseases (Carolan et al. 2014). It was also found that significant amounts of ADIPOQ are expressed in bronchoalveolar lavage and in epithelial cells of the respiratory pathways of patients with COPD and lung emphysema, but not in controls (Miller et al. 2009). Some polymorphisms have been found at the ADIPOQ gene (http://www.ncbi.nlm.nih.gov/projects/SNP/). We chose genotypes, rs266729 and rs1501299, because these SNP are the most common SNPs and have been studied extensively by others as to their functionality and in relation to other complex inflammatory diseases (Yuan et al. 2012; Yang et al. 2012; Sato et al. 2014; Leu et al. 2011; Laumen et al. 2009; de Faria et al. 2015). Among them, intron variant rs1501299 (c.214+62G>T) is the one most extensively studied. ADIPOQ (rs1501299, c.214+62G>T), situated in the intron 2 region, has opposite effects on up-regulating adiponectin levels (Zhang et al. 2009; de Faria et al. 2015). The T allele of the rs1501299 is associated with high adiponectin levels in plasma (Woo et al. 2006; Vasseur et al. 2002). It is possible that this intron variant (rs1501299) is in linkage with other functional polymorphisms. The rs1501299 locus was associated with pancreatic cancer in a Japanese population (Kuruma et al. 2014), and with reduced prevalence of depression (Wang et al. 2015).
The ADIPOQ (rs266729, c.-1124C>G), located in the promoter region and has been associated with down-regulation of ADIPOQ (Zhang et al. 2009). The presence of the G allele of the rs266729 polymorphism results in loss of transcriptional stimulatory protein (SP1) binding site and consequently affects ADIPOQ regulation and expression (Zhang et al. 2009 Laumen et al. 2009). In a study by Yuan et al. (2012), ADIPOQ polymorphisms were for the first time shown to be associated with COPD (Yuan et al. 2012). In the present work, the rs266729 (c.-1124C>G) polymorphism of ADIPOQ was found to be associated with COPD in nonsmoking subjects; at the same time, no association with COPD was observed for rs1501299 in the population studied. Carriers of the heterozygous ADIPOR1 (rs12733285, c.-95+1329G>A) genotype had higher FVC values, i.e., this genotype acts as protective against COPD in nonsmokers. The loci ADIPOQ (rs266729) and ADIPOR1 (rs12733285) exhibited significant gene-by-environment interactions with smoking status in COPD.
The present study also had some potential limitations. Our study was only restricted to a population of Tatars from Russia. Further large sample size studies with more diverse ethnic populations are required to replicate our results. Second, although our study suggested that JAK1 (rs310216), JAK3 (rs3212780), NFKB1 (rs28362491), and IL17A (rs1974226) were associated with the risk of COPD, more biological background data and functional studies are needed to explain the results.
In summary, our analysis of COPD association with polymorphisms of candidate genes involved in systemic inflammatory response in a population of Tatars from Russia has for the first time shown that polymorphisms of JAK1 (rs310216), JAK3 (rs3212780), NFKB1 (rs28362491), and IL17A (rs1974226) are implicated in this disease in our population. Association of ADIPOQ (rs1501299 and rs266729) polymorphisms with COPD has also been confirmed in a Tatar population. Further research aimed at elucidating the mechanisms underlying the development of systemic inflammatory reaction in COPD will contribute to the understanding of the hereditary factors predisposing to the disease and will help specify new targets for its therapy.
References
Bianco A, Mazzarella G, Turchiarelli V, Nigro E, Corbi G, Scudiero O, Sofia M, Daniele A (2013) Adiponectin: an attractive marker for metabolic disorders in chronic obstructive pulmonary disease (COPD). Nutrients 5(10):4115–4125. doi:10.3390/nu5104115
Caporaso N, Gu F, Chatterjee N, Sheng-Chih J, Yu K, Yeager M, Chen C, Jacobs K, Wheeler W, Landi MT, Ziegler RG, Hunter DJ, Chanock S, Hankinson S, Kraft P, Bergen AW (2009) Genome-wide and candidate gene association study of cigarette smoking behaviors. PLoS One 4(2):e4653. doi:10.1371/journal.pone.0004653
Carolan BJ, Hughes G, Morrow J, Hersh CP, O’Neal WK, Rennard S, Pillai SG, Belloni P, Cockayne DA, Comellas AP, Han M, Zemans RL, Kechris K, Bowler RP (2014) The association of plasma biomarkers with computed tomography-assessed emphysema phenotypes. Respir Res 15:127. doi:10.1186/s12931-014-0127-9
Chang Y, Al-Alwan L, Audusseau S, Chouiali F, Carlevaro-Fita J, Iwakura Y, Baglole CJ, Eidelman DH, Hamid Q (2014) Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. Am J Physiol Lung Cell Mol Physiol 306(2):L132–L143. doi:10.1152/ajplung.00111.2013
Chen JM, Ferec C, Cooper DN (2006) A systematic analysis of disease-associated variants in the 3′ regulatory regions of human protein-coding genes I: general principles and overview. Hum Genet 120:1–21. doi:10.1007/s00439-006-0180-7
Cho MH, Boutaoui N, Klanderman BJ, Sylvia JS, Ziniti JP, Hersh CP et al (2010) Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet 42:200–202. doi:10.1038/ng.535
Dariusz B, Agnieszka SM, Daniel K, Anna B, Adam KS (2014) Polymorphism of interleukin-17 and its relation to mineral density of bones in perimenopausal women. Eur J Med Res 19(1):69. doi:10.1186/s40001-014-0069-1
de Faria AP, Modolo R, Sabbatini AR, Barbaro NR, Corrêa NB, Brunelli V, Tanus-Santos JE, Fontana V, Moreno H (2015) Adiponectin −11377C/G and +276G/T polymorphisms affect adiponectin levels but do not modify responsiveness to therapy in resistant hypertension. Basic Clin Pharmacol Toxicol 117(1):65–72. doi:10.1111/bcpt.12368
Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D’Anna SE, Zanini A, Brun P, Casolari P, Chung KF, Barnes PJ, Papi A, Adcock I, Balbi B (2009) T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 157(2):316–324. doi:10.1111/j.1365-2249.2009.03965.x
Ding Y, Niu H, Zhou L, Zhou W, Chen J, Xie S, Geng T, Ouyang Y, He P, Sun P, Feng T, Jin T (2015a) Association of multiple genetic variants with chronic obstructive pulmonary disease (COPD) susceptibility in Hainan region. Clin Respir J. doi:10.1111/crj.12407
Ding Y, Yang D, Zhou L, Xu J, Chen Y, He P, Yao J, Chen J, Niu H, Sun P, Jin T (2015b) Variants in multiple genes polymorphism association analysis of COPD in the Chinese Li population. Int J Chron Obstr Pulmon Dis 10:1455–1463. doi:10.2147/COPD.S86721
Eustace A, Smyth LJ, Mitchell L, Williamson K, Plumb J, Singh D (2011) Identification of cells expressing IL-17A and IL-17 F in the lungs of patients with COPD. Chest 139:1089–1100
Fantuzzi G (2013) Adiponectin in inflammatory and immune-mediated diseases. Cytokine 64(1):1–10
Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R (2014) Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res 3:127. doi:10.4103/2277-9175.133249
Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM (2008) Obesity and the lung: 5. obesity and COPD. Thorax 63(12):1110–1117
Geraghty P, Wyman AE, Garcia-Arcos I, Dabo AJ, Gadhvi S, Foronjy R (2013) STAT3 modulates cigarette smoke-induced inflammation and protease expression. Front Physiol 4:267. doi:10.3389/fphys.2013.00267
Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease, Global Initiative for Chronic Obstructive Lung Disease (GOLD) (Updated 2011). http://www.goldcopd.org/
Hackett TL, Holloway R, Holgate ST, Warner JA (2008) Dynamics of pro-inflammatory and anti-inflammatory cytokine release during acute inflammation in chronic obstructive pulmonary disease: an ex vivo study. Respir Res 29(9):47. doi:10.1186/1465-9921-9-47
Heid IM, Henneman P, Hicks A, Coassin S, Winkler T, Aulchenko YS et al (2010) Clear detection of ADIPOQ locus as the major gene for plasma adiponectin: results of genome-wide association analyses including 4659 European individuals. Atherosclerosis 208(2):412–420
Hou S, Qi J, Zhang Q, Liao D, Li Q, Hu K, Zhou Y, Kijlstra A, Yang P (2013) Genetic variants in the JAK1 gene confer higher risk of Behcet’s disease with ocular involvement in Han Chinese. Hum Genet 132(9):1049–1058
Hsieh YY, Chang CC, Hsu CM, Wan L, Chen SY, Lin WH, Tsai FJ (2011) JAK-1 rs2780895 C-related genotype and allele but not JAK-1 rs10789166, rs4916008, rs2780885, rs17127114, and rs3806277 are associated with higher susceptibility to asthma. Genet Test Mol Biomark 15:841–847
Hu K, Hou S, Li F, Xiang Q, Kijlstra A, Yang P (2013) JAK1, but not JAK2 and STAT3, confers susceptibility to Vogt-Koyanagi-Harada (VKH) syndrome in Han Chinese population. Invest Ophthalmol Vis Sci 54(5):3360–3365
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (2015) (ICD-10 http://www.who.int/classifications/icd/en/)
Jiang B, Zhu ZZ, Liu F, Yang LJ, Zhang WY, Yuan HH, Wang JG, Hu XH, Huang G (2011) STAT3 gene polymorphisms and susceptibility to non-small cell lung cancer. Genet Mol Res 10(3):1856–1865. doi:10.4238/vol10-3gmr1071
Jin Y, Deng Z, Cao C, Li L (2015) IL-17 polymorphisms and asthma risk: a meta-analysis of 11 single nucleotide polymorphisms. J Asthma 52(10):981–988. doi:10.3109/02770903.2015
Karban AS, Okazaki T, Panhuysen CI, Gallegos T, Potter JJ, Bailey-Wilson JE et al (2004) Functional annotation of a novel NFKB1promoter polymorphism that increases risk for ulcerative colitis. Hum Mol Genet 13:35–45
Korn T, Bettelli E, Oukka M, Kuchroo VK (2009) IL-17 and Th17 Cells. Ann Rev Immunol 27:485–517
Kurimoto E, Miyahara N, Kanehiro A, Waseda K, Taniguchi A, Ikeda G, Koga H, Nishimori H, Tanimoto Y, Kataoka M, Iwakura Y, Gelfand EW, Tanimoto M (2013) IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir Res 14:5. doi:10.1186/1465-9921-14-5
Kuruma S, Egawa N, Kurata M, Honda G, Kamisawa T, Ueda J, Ishii H, Ueno M, Nakao H et al (2014) Case-control study of diabetes-related genetic variants and pancreatic cancer risk in Japan. World J Gastroenterol 20(46):17456–17462
Lai H-M, Li X-M, Yang Y-N, Ma Y-T, Xu R, Pan S et al (2015) Genetic variation in NFKB1 and NFKBIA and susceptibility to coronary artery disease in a chinese uygur population. PLoS One 10(6):e0129144. doi:10.1371/journal.pone.0129144
Laumen H, Saningong AD, Heid IM, Hess J, Herder C, Claussnitzer M et al (2009) Functional characterization of promoter variants of the adiponectin gene complemented by epidemiological data. Diabetes 58:9849–9891. doi:10.2337/db07-1646
Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1(6):a001651. doi:10.1101/cshperspect.a001651
Leu HB, Chung CM, Lin SJ, Jong YS, Pan WH, Chen JW (2011) Adiponectin gene polymorphism is selectively associated with the concomitant presence of metabolic syndrome and essential hypertension. PLoS One 6:e19999. doi:10.1371/journal.pone.0019999
Lill CM, Schjeide BM, Akkad DA, Blaschke P, Winkelmann A, Gerdes LA, Hoffjan S, Luessi F, Dörner T, Li SC et al (2012) Independent replication of STAT3 association with multiple sclerosis risk in a large German case-control sample. Neurogenetics 13(1):83–86
López-Mejías R, García-Bermúdez M, González-Juanatey C, Castañeda S, Miranda-Filloy JA, Gómez-Vaquero C, Fernández-Gutiérrez B, Balsa A, Pascual-Salcedo D, Blanco R, González-Álvaro I, Llorca J, Martín J, González-Gay MA (2012) NFKB1-94ATTG ins/del polymorphism (rs28362491) is associated with cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 224(2):426–429
Mathew CG (1985) The isolation of high molecular weight eukaryotic DNA. Methods Mol Biol 2:31–34
McGovern DP, Rotter JI, Mei L, Haritunians T, Landers C, Derkowski C, Dutridge D, Dubinsky M, Ippoliti A, Vasiliauskas E, Mengesha E, King L, Pressman S, Targan SR, Taylor KD (2009) Genetic epistasis of IL23/IL17 pathway genes in Crohn’s disease. Inflamm Bowel Dis 15(6):883–889
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al (2005) Standardisation of spirometry. Eur Respir J 26:319–338
Miller M, Cho JY, Pham A, Ramsdell J, Broide DH (2009) Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol 182:684–691
Nakada TA, Russell JA, Boyd JH, Walley KR (2011) IL17A genetic variation is associated with altered susceptibility to Gram-positive infection and mortality of severe sepsis. Crit Care 15(5):R254. doi:10.1186/cc10515
Open database of single nucleotide polymorphisms (SNPs) and multiple small-scale variations that include insertions/deletions, microsatellites, and non-polymorphic variants. Bethesda (MD): The National Center for Biotechnology Information advances science and health by providing access to biomedical and genomic information (US). http://www.ncbi.nlm.nih.gov/projects/SNP/
Pallavi S, Anoop K, Showket H, Alo N, Mausumi B (2015) NFKB1/NFKBIa polymorphisms are associated with the progression of cervical carcinoma in HPV-infected postmenopausal women from rural area. Tumour Biol 36(8):6265–6276
Pillai SG, Ge D, Zhu G, Kong X, Shianna KV, Need AC et al (2009) A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 5:e1000421. doi:10.1371/journal.pgen.1000421
Pillai SG, Kong X, Edwards LD, Cho MH, Anderson WH, Coxson HO et al (2010) Loci identified by genome-wide association studies influence different disease-related phenotypes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182:1498–1505. doi:10.1164/rccm.201002-0151OC
Pinto LA, Steudemann L, Depner M, Klopp N, Illig T, Weiland SK, von Mutius E, Kabesch M (2007) STAT1 gene variations, IgE regulation and atopy. Allergy 62(12):1456–1461
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC (2007) PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet 81:559–575
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC (1993) Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European community for steel and coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 16:5–40
Rafiei A, Hosseini V, Janbabai G, Ghorbani A, Ajami A, Farzmandfar T, Azizi MD, Gilbreath JJ, Merrell DS (2013) Polymorphism in the interleukin-17A promoter contributes to gastric cancer. World J Gastroenterol 19(34):5693–5699
Ramana CV, Chatterjee-Kishore M, Nguyen H, Stark GR (2000) Complex roles of Stat1 in regulating gene expression. Oncogene 19(21):2619–2627
Rasouli M, Asaei S, Kalani M, Kiany S, Moravej A (2013) Interleukin-17A genetic variants can confer resistance to brucellosis in Iranian population. Cytokine 61(1):297–303
Rawlings JS, Rosler KM, Harrison DA (2004) The JAK/STAT signaling pathway. J Cell Sci 117(Pt 8):1281–1283
Roca J, Burgos F, Barberà JA, Sunyer J, Rodriguez-Roisin R, Castellsagué J et al (1998) Prediction equations for plethysmographic lung volumes. Respir Med 92:454–460
Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS (2015) IL-17A is elevated in end-stage COPD and contributes to cigarette smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med 191(11):1232–1241
Sato K, Shibata Y, Abe S, Inoue S, Igarashi A, Yamauchi K, Aida Y, Nunomiya K et al (2014) Association between plasma adiponectin levels and decline in forced expiratory volume in 1 s in a general Japanese population: the Takahata study. Int J Med Sci 11(8):758–764
Siedlinski M, Cho MH, Bakke P, Gulsvik A, Lomas DA, Anderson W et al (2011) Genome-wide association study of smoking behaviours in patients with COPD. Thorax 66:894–902. doi:10.1136/thoraxjnl-2011-200154
Solé X, Guinó E, Valls J, Iniesta R, Moreno V (2006) SNPStats: a web tool for the analysis of association studies. Bioinformatics 22:1928–1929
Sperati CJ, Parekh RS, Berthier-Schaad Y, Jaar BG, Plantinga L, Fink N, Powe NR, Smith MW, Coresh J, Kao WH (2009) Association of single-nucleotide polymorphisms in JAK3, STAT4, and STAT6 with new cardiovascular events in incident dialysis patients. Am J Kidney Dis 53(5):845–855
Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S et al (2002) Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet 11:2607–2614
Wang K, Zhou B, Zhang J, Xin Y, Lai T, Wang Y, Hou Q, Song Y, Chen Y, Quan Y, Xi M, Zhang L (2011) Association of signal transducer and activator of transcription 3 gene polymorphisms with cervical cancer in Chinese women. DNA Cell Biol 30(11):931–936. doi:10.1089/dna.2010.1179
Wang Z, Liu QL, Sun W, Yang CJ, Tang L, Zhang X, Zhong XM (2014a) Genetic polymorphisms in inflammatory response genes and their associations with breast cancer risk. Croat Med J 55(6):638–646
Wang Z, Xu B, Zhang H, Fan R, Zhou J, Zhong J (2014b) Association between STAT3 gene polymorphisms and Crohn’s disease susceptibility: a case-control study in a Chinese Han population. Diagn Pathol 9:104. doi:10.1186/1746-1596-9-104
Wang Q, Zhu XC, Liu H, Ran MS, Fang DZ (2015a) A longitudinal study of the association of adiponectin gene rs1501299 with depression in Chinese Han adolescents after Wenchuan earthquake. J Affect Disord 175:86–91. doi:10.1016/j.jad.2014.12.056
Wang X, Peng H, Liang Y, Sun R, Wei T, Li Z, Gong Y, Gong R, Liu F, Zhang L, Zhu JA (2015b) functional insertion/deletion polymorphism in the promoter region of the NFKB1 gene increases the risk of papillary thyroid carcinoma. Genet Test Mol Biomark 19(3):167–171
Woo JG, Dolan LM, Deka R, Kaushal RD, Shen Y, Pal P, Daniels SR, Martin LJ (2006) Interactions between noncontiguous haplotypes in the adiponectin gene ACDC are associated with plasma adiponectin. Diabetes 55:523–529
Xiao L, Muhali FS, Cai TT, Song RH, Hu R, Shi XH, Jiang WJ, Li DF, He ST, Xu J, Zhang JA (2013) Association of single-nucleotide polymorphisms in the STAT3 gene with autoimmune thyroid disease in Chinese individuals. Funct Integr Genomics 13(4):455–461
Xie HJ, Bae HJ, Noh JH, Eun JW, Kim JK, Jung KH, Ryu JC et al (2009) Mutational analysis of JAK1 gene in human hepatocellular carcinoma. Neoplasma 56:136–140
Yang Y, Zhang F, Ding R, Wang Y, Lei H, Hu D (2012) Association of ADIPOQ gene polymorphisms and coronary artery disease risk: a meta-analysis based on 12 465 subjects. Thromb Res 130:58–64. doi:10.1016/j.thromres.2012.01.018
Yang YN, Zhang JY, Ma YT, Xie X, Li XM, Liu F, Chen BD, Dong XH, Zheng YY, Pan S, Zhai H, Li DZ, Chen QJ (2014) 94 ATTG insertion/deletion polymorphism of the NFKB1 gene is associated with coronary artery disease in Han and Uygur women in China. Genet Test Mol Biomark 18(6):430–438
Yuan Y, Jiang H, Kuang J, Hou X, Feng Y, Su Z (2012) Genetic variations in ADIPOQ gene are associated with chronic obstructive pulmonary disease. PLoS One 7(11):e50848. doi:10.1371/journal.pone.0050848
Yuan K, Liu H, Huang L, Ren X, Liu J, Dong X, Tian W, Jia Y (2014) rs744166 polymorphism of the STAT3 gene is associated with risk of gastric cancer in a Chinese population. Biomed Res Int. doi:10.1155/2014/527918
Zhang D, Ma J, Brismar K, Efendic S, Gu HF (2009) A single nucleotide polymorphism alters the sequence of SP1 binding site in the adiponectin promoter region and is associated with diabetic nephropathy among type 1 diabetic patients in the genetics of kidneys in diabetes study. J Diabetes Complicat 23:265–272. doi:10.1016/j.jdiacomp.2008.05.004
Zhang D, Li L, Zhu Y, Zhao L, Wan L, Lv J, Li X, Huang P, Wei L, Ma M (2013) The NFKB1-94 ATTG insertion/deletion polymorphism (rs28362491) contributes to the susceptibility of congenital heart disease in a Chinese population. Gene 516(2):307–310
Zhong Y, Wu J, Chen B, Ma R, Cao H, Wang Z, Cheng L, Ding J, Feng J (2012) Investigation and analysis of single nucleotide polymorphisms in Janus kinase/signal transducer and activator of transcription genes with leukemia. Leuk Lymph 53(6):1216–1221. doi:10.3109/10428194.2011.645212
Zhou B, Rao L, Peng Y, Wang Y, Li Y, Gao L, Chen Y, Xue H, Song Y, Liao M, Zhang L (2009) Functional polymorphism of the NFKB1 gene promoter is related to the risk of dilated cardiomyopathy. BMC Med Genet 10:47. doi:10.1186/1471-2350-10-47
Zhu ZZ, Di JZ, Gu WY, Cong WM, Gawron A, Wang Y, Zheng Q, Wang AZ, Zhu G, Zhang P, Hou L (2010) Association of genetic polymorphisms in STAT1 gene with increased risk of hepatocellular carcinoma. Oncology 78(5–6):382–388
Acknowledgments
This work was supported by the Russian Foundation for Basic Research Grants (Nos. 13-04-00287, 14-04-97006, and 14-06-97003), and the Russian Foundation for Humanities Grant No. 16-06-00162).
Author Contributions
The conception and study design of the work: G. F. Korytina, T.V. Viktorova, Sh.Z. Zagidullin. The patients and control—medical examination and selection: Y.G. Aznabaeva, Sh.Z. Zagidullin. Performing the experiment (case and control—DNA-collecting; real-time PCR): G. F. Korytina, L. Z. Akhmadishina, O.V. Kochetova. Genetic association analysis: G. F. Korytina, L. Z. Akhmadishina, O.V. Kochetova. Statistical analysis: G. F. Korytina. Interpretation of data for the work: G. F. Korytina, T.V. Viktorova, Z. Akhmadishina, O.V. Kochetova, Y.G. Aznabaeva, Sh.Z. Zagidullin. Manuscript writing: G. F. Korytina, T.V. Viktorova, L. Z. Akhmadishina, Y.G. Aznabaeva, Sh.Z. Zagidullin, O.V. Kochetova. Review of the manuscript: G. F. Korytina, T.V. Viktorova, L. Z. Akhmadishina, Y.G. Aznabaeva, Sh.Z. Zagidullin., O.V. Kochetova.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has conflicts of interest to report with regard to this manuscript.
Rights and permissions
About this article
Cite this article
Korytina, G.F., Akhmadishina, L.Z., Kochetova, O.V. et al. Inflammatory and Immune Response Genes Polymorphisms are Associated with Susceptibility to Chronic Obstructive Pulmonary Disease in Tatars Population from Russia. Biochem Genet 54, 388–412 (2016). https://doi.org/10.1007/s10528-016-9726-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10528-016-9726-0