Abstract
Several populations of the stem-mining weevil Mecinus janthinus Germar species complex (Mecinini, Curculionidae), identified based on morphological characteristics, have been introduced in North America for the biological control of invasive toadflaxes of European origin: Linaria vulgaris Miller and L. dalmatica (L.) Miller (Plantaginaceae). According to the mitochondrial cytochrome oxidase subunit II (COII) gene haplotype divergence of Mecinus janthinus species complex, a total of 20 M. janthinus s.s., 3 M. janthinus s.l. of the ‘speciosa’ genotype and 29 M. janthiniformis haplotypes have been recorded across their native range in central and southeastern Europe. A polymerase chain reaction followed by restriction fragment length polymorphism (PCR-RFLP) diagnostic assay of COII gene using Hpy188III and MnlI enzyme-mix, was developed for fast and cost-effective discrimination of these morphologically very similar cryptic weevil species. It is shown that digestion generates unique 4-fragment restriction profile in M. janthinus s.s., 2-fragment profile in M. janthiniformis and 3-fragment profile in M. janthinus s.l. ‘speciosa’ group of haplotypes, allowing precise identification of each species or genotype. The proposed method represents a practical tool for fast and accurate identification of the target biocontrol agents and should prevent using inappropriate weevil species in redistribution programs for biological control of invasive toadflax species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Broad-leaved Dalmatian toadflax [Linaria dalmatica (L.) Miller], narrow-leaved Dalmatian toadflax [L. genistifolia (L.) Miller] and yellow toadflax (L. vulgaris Miller) (Plantaginaceae) are perennial weeds of European origin that have become naturalized in North America (Sutton 1988). Introduced as an ornamental in the middle of the 17th century, yellow toadflax is now found in every state in the USA and across southern Canada (Mack 2003). Dalmatian toadflax was introduced at the end of the 19th century and has spread across every Canadian province and the northern and western USA (Vujnović and Wein 1997) where it has become a major problem on dry, rocky or gravelly soils. There is still much uncertainty regarding toadflax taxonomy, particularly the L. genistifolia/dalmatica complex of species and their hybrids. Chater et al. (1972) treated Linaria dalmatica as a subspecies of L. genistifolia. Hartl (1974) and Davis (1978) treated L. dalmatica as a separate species closely related to L. genistifolia, while Sutton (1988) resumed Davis’s statement and comments that morphological types called L. dalmatica may belong to various hybrid forms between L. genistifolia and L. grandiflora Desf. More recently, Niketić and Tomović (2008) identified 36 names used in the literature for L. genistifolia taxa and Ward et al. (2009) demonstrated that hybridization is occurring between yellow toadflax and Dalmatian toadflax in North America, and that the hybrid progeny is viable and fertile. The uncertainty about the taxonomy of invasive toadflaxes in North America and their ability to hybridize makes the selection of effective biocontrol agents a challenging task, in particular when they prove to be highly host specific.
A biological control program for alien invasive toadflax species in North America was initiated in 1987 and the first introduction of the stem-mining weevil Mecinus janthinus Germar was made in 1991 (De Clerck-Floate and Harris 2002; McClay and De Clerck-Floate 2002). Population development and the impact of M. janthinus in North America have varied widely between different release areas and host plants. Although the great majority of all weevils released in North America originated from L. vulgaris, collected in South Germany and North Switzerland (Toševski and Gassmann, unpublished data), the general consensus is that releases on L. dalmatica in North America led to a rapid buildup of outbreak-level populations of M. janthinus, with substantial impact on this weed (Peterson et al. 2005; Wilson et al. 2005; van Hezewijk et al. 2010; Schat et al. 2011). In contrast, only scarce and low-density populations of this weevil were recently reported on yellow toadflax in Canada (McClay and Hughes 2007; De Clerck-Floate, personal communication) and USA (Sing, personal communication).
The need to revise the taxonomical status of M. janthinus associated with Linaria spp. in Europe and to determine the exact European origin of the successful weevil populations used as biological control agents against invasive toadflaxes in North America increased after reports on high levels of genetic diversity within and among invasive yellow and Dalmatian toadflax populations in North America (Ward et al. 2008, 2009). A genetic study of European M. janthinus (s.l.) populations using mithochondrial DNA sequences, revealed extensive genetic divergence between populations associated with different Linaria spp. and conspicuous clustering by their host-plant affiliations (Toševski et al. 2011). In both maximum parsimony and statistical parsimony analyses, weevil COII haplotypes retrieved from a particular host-plant taxon cluster together. Two haplotype groups were assigned full species status: M. janthinus is associated with yellow toadflax and M. janthiniformis Toševski and Caldara with broad-leaved and narrow-leaved Dalmatian toadflaxes. In addition, three haplotypes (referred to as ‘speciosa’ group sequences) were identified occurring sympatrically with populations of M. janthinus on L. vulgaris populations from very distant sites in central and southeast Europe.
The possible existence of two cryptic weevil species within the populations of M. janthinus introduced in North America raised the question of how to distinguish those species in redistribution programs in order to improve their selection and effectiveness as biological control agents. Restriction fragment length polymorphism (RFLP) is a powerful tool which provides relatively simple and precise method when applied for species identification, especially if it is applied on DNA products obtained by polymerase chain reaction (PCR) (Gaskin et al. 2011). In general, molecular methods are still not routine in biological control programs and for this reason there are only few studies in the literature that have characterized genetic variation within or among populations of weed biocontrol agents (Rauth and Hufbauer 2009). In contrast, PCR-RFLP-based methods are in wide use as a diagnostic tool for economically important or quarantine pests (Scheffer et al. 2001; Brunner et al. 2002; Salazar et al. 2002; Barr et al. 2006; McKern and Szalanski 2007). In the case of cryptic species, PCR-RFLP should be the method of choice since it is inexpensive, simple, reliable, and repeatable and can be used on the insect during any developmental stage (Gaskin et al. 2011). Here we report the development of a PCR-RFLP-based diagnostic tool to differentiate between Mecinus weevils which are being used for the biological control of toadflaxes in North America.
Materials and methods
Material for DNA study
For this study we used previously reported DNA material of M. janthinus s.l. (Toševski et al. 2011) as well as newly collected specimens (Table 1). From the previously reported material we used the DNA of selected specimens representing each of the formerly identified haplotypes upon the mtDNA cytochrome oxidase subunit II (COII) gene sequences of M. janthinus s.l. collected in central and southeastern Europe (Table 2). Template DNA of these specimens was used for de novo PCR amplification of the COII gene and submitted to RFLP analyses, along with newly collected specimens.
Between 2011 and 2012, we conducted additional sampling of M. janthinus and M. janthiniformis in order to get a better overview of the haplotype diversity and frequency in the native range of both weevil species (Table 1). With this sampling we attempted to increase the number of haplotypes for further analyses and cover majority of the possible haplotypes that could be expected in the introduced environment of North America. A total of 137 specimens were collected in the following European countries: Switzerland, northern Italy, eastern Serbia, Montenegro, northeastern Bulgaria, southern Macedonia and northern Greece (Table 1). Newly collected weevil specimens were kept in 96 % ethanol and stored at −20 °C until DNA extraction. Individual weevils were punctured on the ventro-lateral side of the second thoracic segment and total DNA was extracted using DNeasy® Blood & Tissue Kit (QIAGEN) according to the manufacturer’s instructions.
PCR amplification and sequencing
The mitochondrial COII gene was chosen as a marker for the identification of host-plant associated species or genotype within the M. janthinus species complex. This gene has been previously proven to be an appropriate marker to determine host plant affiliation within related species from the tribe Mecinini (Caldara et al. 2008; Hernández-Vera et al. 2010) and was used for genetic differentiation of cryptic species within the M. janthinus species complex (Toševski et al. 2011).
MtDNA fragments including the complete COII gene were amplified using TL2-J-3038 (5′-TAATATGGCAGATTAGTGCATTGGA-3′) (Emerson et al. 2000) and TK-N-3782 (5′-GAGACCATTACTTGCTTTCAGTCATCT-3′) (Harrison Laboratory, Cornell University, Ithaca, NY, USA) primers located in the adjacent tRNA genes. Amplification reactions were performed in a 20-μl final reaction volume containing Kapabiosystems high yield reaction buffer A with Mg (1×), 3.5 mM MgCl2, 0.8 mM of each dNTP, 0.75 μM of each primer, 0.75 U of KAPATaq DNA polymerase (Kapa Biosystems, Inc., Woburn, MA, USA) and 1 μl of DNA extract. PCR cycles were carried out in a Mastercycler ep gradient S (Eppendorf) applying the following thermal steps: initial denaturation for 5 min at 95 °C followed by 40 cycles of denaturation step at 95 °C for 1 min, annealing at 45 °C for 1 min and elongation step at 72 °C for 2 min. Final elongation was performed at 72 °C for 10 min.
The PCR amplicons were purified using the QIAquick PCR purification Kit (QIAGEN, Hilden, Germany) and sequenced on automated equipment by BMR Service (Padova, Italy) or Macrogen Inc. (Seoul, South Korea) using one, or both primers used for amplification. For most of the analyzed specimens sequences of full length COII gene (678-bp) were obtained with the forward primer only, while for a number of specimens, reading was done with both primers in order to obtain sequences of full length PCR products (784-bp). The obtained sequences were edited using FinchTV v.1.4.0 (http://www.geospiza.com) and aligned using ClustalW program integrated into MEGA5 software (Tamura et al. 2011).
Sequences of M. janthinus s.l. COII haplotypes detected in this study for the first time were deposited in the GenBank database under the accession numbers JX631141-55. Accession numbers of the complete COII gene sequences of all recorded haplotypes are presented in Table 2.
Virtual restriction analysis and gel plotting
In silico restriction analysis was performed for all registered COII gene haplotypes to identify suitable restriction enzymes that could be used for species and ‘speciosa’ genotype differentiation within the M. janthinus species complex. Sequences of all haplotypes were aligned and presence of species-specific or ‘speciosa’ genotype-specific SNPs (single nucleotide polymorphisms) in the recognition sites for restriction endonucleases were determined using the pDRAW32 software (AcaClone Software, http://www.acaclone.com). Full-length sequences, corresponding to the exact size of TL2-J-3038/TK-N3782 PCR fragment, were exported to the pDRAW32 program and virtually digested with the selected restrictive enzymes using the option ‘explicitly select’ for single-enzyme digestion or ‘enzyme-mix’ for double-enzyme digestion and separated on a 1.5 % agarose gel. Virtual restriction patterns were compared with actual enzymatic RFLP patterns of amplicons obtained from specimens representing each COII haplotype.
RFLP analysis
Based on the putative restriction map of the M. janthinus species complex COII gene haplotypes, Hpy188III and MnlI endonucleases were used for the in vitro digestion. TL2-J-3038/TK-N3782 PCR amplified DNA fragments of all 52 specimens representing unique haplotypes (Table 2) were digested with both enzymes in reactions with a single enzyme and in a reaction with mix of both enzymes. All digestion reactions were performed at 37 °C for 16 h using 1× NEBuffer 4, according to manufacturer’s instructions (New England BioLabs, Inc., USA). Restriction products were separated by electrophoresis on 13 % polyacrylamide gels in TBE buffer (Tris–Borate 90 mM, EDTA 1 mM), stained with ethidium bromide and visualised with a UV transilluminator.
Results
Haplotype diversity
To determine haplotype diversity on mtCOII gene, altogether 137 newly collected specimens from central and southeastern Europe belonging to the M. janthinus species complex associated with L. vulgaris, L. genistifolia/dalmatica and specimens associated with L. vulgaris that belong to ‘speciosa’ genotype were analyzed and compared with formerly identified haplotypes (Table 2). In the previous study (Toševski et al. 2011), a total of 15 M. janthinus haplotypes associated with L. vulgaris (v1–v15), 19 M. janthiniformis haplotypes associated with L. genistifolia/dalmatica (g1–g10, d1–d5, gd1–gd4), and three haplotypes designated as ‘speciosa’ group genotype associated with L. vulgaris (s1–s3) were recorded. The analysis of additional material revealed the existence of another five haplotypes within M. janthinus, ten within M. janthiniformis (seven associated with L. dalmatica and three with L. genistifolia), but no new haplotypes within the ‘speciosa’ group genotype (Table 1). In total, 20 M. janthinus s.s haplotypes have been recorded out of 106 specimens from L. vulgaris sampled from a wide range of its central and southeastern European distribution, 29 M. janthiniformis haplotypes out of 198 specimens from its southeastern European distribution associated with L. genistifolia and L. dalmatica and three haplotypes belonging to the ‘speciosa’ genotype in ten sequenced specimens that originated from northern Switzerland, central Hungary and eastern Serbia (Table 2).
Sequence comparison and putative restriction maps
Sequence comparison of full length COII gene (678-bp) of the 52 haplotypes identified within the M. janthinus species complex revealed genetic variability represented with 59 variable, 27 singleton and 32 parsimony-informative sites. Out of these, only 11 sites could be used as informative for the discrimination of M. janthinus from M. janthiniformis and/or ‘speciosa’ genotypes or for the discrimination of M. janthiniformis from the ‘speciosa’ genotype (Table 3). All new haplotypes of M. janthinus and M. janthiniformis had a specific set of eight nucleotides defined as being species-specific in the original description (Toševski et al. 2011). These positions were used for the identification of restriction enzymes that could be used in the diagnostic assay.
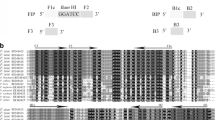
In silico restriction analyses allowed us to identify Hpy188III and MnlI as suitable enzymes for the practical and reliable procedure of molecular identification and differentiation of M. janthinus s.s. from M. janthiniformis. In addition, this procedure enables the discrimination of individuals belonging to the ‘speciosa’ genotype. Based on the putative restriction map of the COII gene sequences (Fig. 1), all M. janthinus s.s. and ‘speciosa’ specimens have two recognition sites for Hpy188III endonuclease (TC′nn_GA), at the 433- and 548-bp positions of the TL2-J-3038/TK-N3782 amplicons, while sequences of M. janthiniformis have only one recognition sequence for this enzyme at the 433-bp position. They lack the 548-bp restriction site due to a nucleotide substitution from A to G at the 489-bp of the COII gene (Fig. 1; Table 3). This nucleotide position is interspecific informative and its substitution is species-specific for M. janthiniformis (Table 3). Consequently, this enzyme enables the discrimination of M. janthinus from M. janthiniformis, but not M. janthinus from ‘speciosa’ group genotypes. For the MnlI restriction enzyme (CCTCnnnnnn_n′) all three groups of sequences have one recognition site at 745-bp position generating fragments of 745- and 39-bp in length. However, only M. janthinus s.s. has an additional MnlI restriction site at the 225-bp position, which makes its restriction pattern unique. This additional MnlI restriction site on the COII gene is a consequence of a nucleotide substitution from A to G at position 171, which distinguishes M. janthinus from M. janthiniformis (Toševski et al. 2011; Table 3).
Putative restriction map for MnlI (CCTCNNNNNN_N′) and Hpy188III (TC′NN_GA) endonucleases based on TL2-J-3038/TK-N3782 delineated sequences of mtDNA fragments encompassing complete COII gene (indicated with vertical arrows) of Mecinus janthinus s.s., M. janthiniformis and M. janthinus s.l. ‘speciosa’ genotype. Asterisks are indicating nucleotide positions of informative sites (accounting nucleotides from the beginning of the COII gene) enabling species-specific identification of M. janthinus and M. janthiniformis and differentiation from ‘speciosa’ genotype
RFLP diagnostic procedure
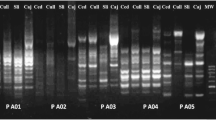
Based on in silico and in vitro restriction analysis of TL2-J-3038/TK-N3782 COII amplicons with Hpy188III and MnlI endonucleases, we propose a diagnostic method using PCR-RFLP for the identification and separation of the M. janthinus, M. janthiniformis and ‘speciosa’ genotypes. Comparison of the sequence virtual analysis with actual digestion patterns of all 52 haplotypes confirmed the accuracy and reproducibility of the proposed methodology (Fig. 2a, b). All haplotypes had the expected restriction profiles which enabled their assignment to a species or genotype, solely based on the RFLP pattern. Single digestion with Hpy188III or MnlI endonuclease was insufficient for the precise recognition of all three entities (Fig. 2). Restriction digestion with Hpy188III could separate only M. janthinus from M. janthiniformis, but not from the ‘speciosa’ genotype (Fig. 2). On the contrary, digestion with MnlI enzyme enabled differentiation of M. janthinus from M. janthiniformis, but not M. janthiniformis from the ‘speciosa’ genotype. Therefore, only double digestion facilitates precise identification of each species or genotype.
Actual (a) and virtual (b) restriction profiles of the 784-bp COII gene amplified or in silico delineated by TL2-J-3038 and TK-N-3782 primer pair, single and double digested with MnlI and Hpy188III restriction endonucleases. Abbreviations: Mecinus janthinus s.s. (M.j.), Mecinus janthiniformis (M.jf.), Mecinus janthinus ‘speciosa’ genotype (M.j.s.). Molecular weight markers: phiX174/HaeIII digested (Fermentas) and 50 bp DNA Ladder (Invitrogen). Fragment sizes of the RFLP profiles and phiX174/HaeIII marker are indicated on virtual gel image. Dotted lines in an in silico profile represent bands not visible or barely visible in actual experimental profile
In summary, double digestion of TL2-J-3038/TK-N3782 COII amplicons with Hpy188III and MnlI enzymes generates a 4-fragment restriction profile in M. janthinus s.s., a 2-fragment profile in M. janthiniformis and a 3-fragment profile in the ‘speciosa’ group of haplotypes (Fig. 2a). The fragment of 39-bp length is not included in the diagnostic RLFP patterns, since all restriction profiles possess this fragment and due to its length it is expected that it will be diffuse and hardly visible on actual RFLP gel (Fig. 2a, b). Thus, the RFLP pattern in specimens belonging to M. janthinus s.s. consists of four clearly separated and visible fragments of 225, 208, 197 and 115 bp in length, M. janthiniformis two fragments of 433 and 312 bp and M. janthinus ‘speciosa’ genotype three fragments of 433, 197 and 115 bp (Fig. 2b).
Discussion
Methods developed for molecular identification of organisms have become important tools that can help to improve security and efficacy in using natural enemies in classical biological control of weeds (Gaskin et al. 2011). The presence of cryptic or sibling species within introduced and established populations of selected biological control agents are probably rare events, but it is an increasing point of concern. Since little effort has been dedicated to performing population genetic studies prior to the introduction of beneficial insect as biocontrol agents, the discovery of cryptic species within introduced arthropod agents has to be expected. Rauth et al. (2011) quoted only six studies that have characterized genetic variation within or among populations of weed biological control agents prior to their release. Moreover, little is done for the re-evaluation of the population genetics of introduced and already established agents. One of the rare examples of such a re-evaluation is a recently published study by Roehrdanz et al. (2011) on the evaluation of the genetic population status of several Aphthona species (Halticinae, Chrysomelidae) introduced in North America for the biological control of leafy spurge. The authors concluded that some populations of Aphthona lacertosa, one of the introduced species, are divergent enough to merit consideration of cryptic species and proposed further studies in order to determine physical traits, ecology and geographic origin of these specimens.
Historically, in nearly all the cases, the determination and taxonomic position of a potential biological control agent was based on traditional taxonomy and the typological species concept, whereby the identification was primarily based on morphological characters and on the authority of taxonomists specializing in that particular taxa group. The same approach was applied in the selection of M. janthinus as a biological control agent against invasive toadflaxes, where identification of voucher specimens was confirmed by the taxonomist Dr L. Dieckmann, Eberswalde, Germany (Jeanneret and Schroeder 1991). The typological species concept and a general knowledge of the biology of M. janthinus that was available in literature during the time of pre-release studies has led to the introduction in North America of two different, host-associated weevil species from central and southeastern Europe (Toševski et al. 2011). M. janthinus and M. janthiniformis are morphologically distinguishable from each other by only a few very subtle characters, in contrast to strong genetic differentiation which was consistent also in specimens collected from sympatric populations of L. vulgaris and L. genistifolia. In addition, host plant associated genetic differentiations are supported by substantially higher offspring survival of the two Mecinus species on their respective host-plant species (Toševski et al. 2011). This example emphasizes the need for pre-release population genetic studies when selecting potential biological control agents.
The existence of cryptic species associated with L. vulgaris and the L. genistifolia/dalmatica species complex in Europe with distinct biological characteristics would appear to at least partly explain both the successes and failures of biological control of toadflaxes in North America and a study is ongoing to determine the taxonomic status and European origin of invasive toadflaxes in North America. The redistribution and improvement of the biological control capacity of Mecinus weevils will be required to go through a proper selection of these cryptic species. Furthermore, genetic properties of the weevils should be complemented by the recognition of the genetic variability of the targeted toadflax populations. The proposed PCR-RFLP tool can avoid the use of inadequate weevil populations and minimize failure in redistribution and establishment of these beneficial weevils.
References
Barr NB, Copeland RS, De Meyer M, Masiga D, Kibogo HG, Billah MK, Osir E, Wharton RA, McPheron BA (2006) Molecular diagnostics of economically important Ceratitis fruit fly species (Diptera: Tephritidae) in Africa using PCR and RFLP analyses. Bull Entomol Res 96:505–521
Brunner PC, Fleming C, Frey E (2002) A molecular identification for economically important thrips species (Thysanoptera: Thripidae) using direct sequencing and a PCR-RFLP-based approach. Agric For Entomol 4:127–136
Caldara R, Desančić M, Gassmann A, Legarreta L, Emerson B, Toševski I (2008) On the identity of Rhinusa hispida (Brullé) and its current synonyms (Coleoptera: Curculionidae). Zootaxa 1805:61–68
Chater AO, Valdes B, Webb DA (1972) 14. Linaria Miller. In: Tutin TG, Heywood VH, Burges NA, Moon DM, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea, Vol. 3. Cambridge University Press, Cambridge, UK
Davis PH (1978) Flora of Turkey, vol 6. Edinburgh University Press, Edinburgh, UK
De Clerck-Floate RA, Harris P (2002) Linaria dalmatica (L.) Miller, Dalmatian toadflax (Scrophulariaceae). In: Mason PG, Huber JT (eds) Biological Control Programmes in Canada, 1981–2000, CABI Publishing, Wallingford, Oxon, UK, pp 368–374
Emerson BC, Oromi P, Hewitt GM (2000) Interpreting colonization of the Calathus (Coleoptera: Carabidae) on the Canary Islands and Madeira through the application of the parametric bootstrap. Evolution 54:2081–2090
Gaskin JF, Bon MC, Cock MJW, Cristofaro M, De Biase A, De Clerck-Floate R, Ellison CA, Hinz H, Hufbauer R, Julien M, Sforza R (2011) Applying molecular-based approaches to classical biological control of weeds. Biol Control 58:1–21
Hartl D (1974) Familie Scrophulariaceae. In: Hegi G, Hartl D, Wagenitz G (eds) Illustrierte Flora von Mittleleuropa, Bd. VI, Part 1, 2nd edn. Carl Hanser, Munich, Germany
Hernández-Vera G, Mitrović M, Jović J, Toševski I, Caldara R, Gassmann A, Emerson BC (2010) Host associated genetic differentiation in a seed parasitic weevil Rhinusa antirrhini (Coleptera: Curculionidae) revealed by mitochondrial and nuclear sequence data. Mol Ecol 19:2286–2300
Jeanneret P, Schroeder D (1991) Mecinus janthinus Germar (Coleoptera: Curculionidae): a candidate for the biological control of Dalmatian and yellow toadflax in North America. Final report. International Institute of Biological Control, CAB International, European Station, Delemont, Switzerland
Mack RN (2003) Plant naturalizations and invasions in the Eastern United States: 1634–1860. Ann Mo Bot Gard 90:77–90
McClay AS, De Clerck-Floate RA (2002) Linaria vulgaris Miller, yellow toadflax (Scrophulariaceae). In: Mason PG, Huber JT (eds) Biological Control Programmes in Canada, 1981–2000, CABI Publishing, Wallingford, Oxon, UK, pp 375–382
McClay AS, Hughes RB (2007) Temperature and host-plant effects on development and population growth of Mecinus janthinus (Coleoptera: Curculionidae), a biological control agent for invasive Linaria spp. Biol Control 40:405–410
McKern J, Szalanski A (2007) Molecular diagnostics of economically important clearwing moths (Lepidoptera: Sesiidae). Fla Entomol 90:475–479
Niketić M, Tomović G (2008) Taxonomy and nomenclature of the Linaria genistifolia complex (Plantaginaceae–Antirrhineae) in S.E Europe and Anatolia. Taxon 57:619–629
Peterson R, Sing S, Weaver D (2005) Differential physiological responses of Dalmatian toadflax, Linaria dalmatica L. Miller, to injury from two insect biological control agents: implications for decision-making in biological control. Environ Entomol 34:899–905
Rauth JS, Hufbauer AR (2009) PCR-RFLP assays for discerning three weevil stem feeders (Ceutorhynchus spp.) (Col.: Curculionidae) on garlic mustard (Alliaria petiolata). Biocontrol Sci Technol 19:999–1005
Rauth S, Hinz H, Gerber E, Hufbauer R (2011) The benefits of pre-release population genetics: a case study using Ceutorhynchus scrobicollis, a candidate agent of garlic mustard, Alliaria petiolata. Biol Control 56:67–75
Roehrdanz R, Bourchier R, Cortilet A, Olson D, Sears S (2011) Phylogeny and genetic diversity of flea beetles (Aphthona sp.) introduced to North America as biological control agents for leafy spurge. Ann Entomol Soc Am 104:966–975
Salazar M, Theoduloz C, Vega A, Poblete F, González E, Badilla R, Meza-Basso L (2002) PCR-RFLP identification of endemic Chilean species of Rhagoletis (Diptera: Tephritidae) attacking Solanaceae. Bull Entomol Res 92:337–341
Schat M, Sing S, Peterson R, Menalled F, Weaver D (2011) Growth inhibition of Dalmatian toadflax, Linaria dalmatica (L.) Miller, in response to herbivory by the biological control agent Mecinus janthinus Germar. J Entomol Sci 46:232–246
Scheffer SJ, Wijeskara A, Visser D, Hallett RH (2001) PCR-RFLP method to distinguish Liriomyza huidobrensis from L. langei applied to three recent leafminer invasions. J Econ Entomol 94:1177–1182
Sutton DA (1988) A revision of the tribe Antirrhineae. Oxford University Press, London & Oxford, UK
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Toševski I, Caldara R, Jović J, Hernández-Vera G, Naviera C, Gassmann A, Emerson BC (2011) Morphological, molecular and biological evidence reveal two cryptic species in Mecinus janthinus Germar (Coleoptera, Curculionidae), a successful biological control agent of Dalmatian toadflax, Linaria dalmatica (Lamiales, Plantaginaceae). Syst Entomol 36:741–753
van Hezewijk BH, Bourchier RS, De Clerck-Floate RA (2010) Regional-scale impact of the weed biocontrol agent Mecinus janthinus on Dalmatian toadflax (Linaria dalmatica). Biol Control 55:197–202
Vujnović K, Wein RW (1997) The biology of Canadian weeds. 106. Linaria dalmatica (L.) Mill. Can J Plant Sci 77:483–491
Ward SM, Scott DR, Harrington J, Sutton J, Beck GK (2008) Genetic variation in invasive populations of yellow toadflax (Linaria vulgaris) in the western United States. Weed Sci 56:394–399
Ward SM, Fleischmann CE, Turner MF, Sing SE (2009) Hybridization between invasive populations of Dalmatian toadflax (Linaria dalmatica) and yellow toadflax (Linaria vulgaris). Invasive Plant Sci Manag 2:369–378
Wilson LM, Sing SE, Piper GL, Hansen RW, De Clerck-Floate R, MacKinnon DK, Randall C (2005) Biology and biological control of Dalmatian and yellow toadflax. USDA Forest Service, FHTET-05-13, Morgantown, USA
Acknowledgments
We thank the Ministry of Forests and Range, British Columbia Provincial Government, the Wyoming Biological Control Steering Committee, USDA-APHIS-CPHST, the USDA Forest Service and the Montana Noxious Weed Trust Fund through the Montana State University, and the California Department of Food and Agriculture who supported this programme. We gratefully acknowledge the support of Dr De Clerck-Floate (AAFC, Lethbridge, Canada) and Dr Andrew Norton (Colorado State University, USA), the coordinators of the toadflax consortium in North America. This research was partly funded by Grant III43001 (The Ministry of Education, Science and Technological Development of the Republic of Serbia).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: John Scott
Rights and permissions
About this article
Cite this article
Toševski, I., Jović, J., Krstić, O. et al. PCR-RFLP-based method for reliable discrimination of cryptic species within Mecinus janthinus species complex (Mecinini, Curculionidae) introduced in North America for biological control of invasive toadflaxes. BioControl 58, 563–573 (2013). https://doi.org/10.1007/s10526-013-9506-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-013-9506-2