Abstract
The white grub species Phyllophaga polyphylla and Anomala cincta (Coleoptera: Melolonthidae) are economically important species that affect many crops in Mexico. A series of experiments to study the pathogenic interaction between isolates of Beauveria bassiana and Metarhizium anisopliae and these two insect species were undertaken. First, the susceptibility of third instar P. polyphylla larvae to each of seven isolates representing both species of fungus was evaluated by dipping the insects in 1 × 108 conidia ml−1 suspensions. A second study examined the differences in the susceptibility of P. polyphylla and A. cincta larvae to two selected isolates for each of the fungal species. Finally, the susceptibility of A. cincta larvae to one M. anisopliae isolate when incubated in soil collected from four different sites was assessed. No significant differences in proportion of infection of P. polyphylla larvae were observed amongst the fungal isolates tested and mortality due to fungal infection was never greater than 20% after 36 days incubation. Anomala cincta larvae were more susceptible than P. polyphylla larvae, with greater than 90% infection when inoculated with isolates of M. anisopliae whereas mortalities of only 20% where achieved against P. polyphylla larvae. The soil type in which A. cincta were incubated following inoculation with M. anisopliae affected their susceptibility to infection. The results demonstrated that there is a complex interaction amongst entomopathogenic fungi, white grub larvae and soil properties, and points to the need of further investigation of this system in order to optimize the efficacy of entomopathogenic fungi against these insect species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The species complex of white grubs is a serious problem in many parts of México (Morón 2010). White grub larvae feed on the roots of crops such as maize (Zea mays L.), sorghum (Sorghum vulgare Pers.), sugar cane (Saccharum officinarum L.), beans (Phaseolus vulgaris L.), onion (Allium cepa L.), tomato (Physalis ixocarpa B.), potato (Solanum tuberosum L.) (Morón 1986) and agave (Agave tequilana Weber) (García et al. 2009). Efforts have been made to control this soil-dwelling pest through the use of chemical insecticides, but results are becoming increasingly variable due to increased levels of pesticide resistance (Loera-Gallardo et al. 2010). Of the reported 285 species of white grub in the genus Phyllophaga found in México, only 10% occur on agricultural crops (Morón 2010). In Guanajuato state, México, 60% of the land is in agricultural use, and of that, 80% is used to grow maize, sorghum, wheat and brassicas. White grubs are one of the main pests attacking these crops (Marin-Jarillo and Bujanos-Muñiz 2003). Phyllophaga polyphylla (Bates) is one of the most economically important white grub species in Guanajuato (Marin-Jarillo and Bujanos-Muñiz 2003). A second white grub, A. cincta (Say), has also been reported in Guanajuato, although at present this species is not considered as economically important as Phyllophaga species (Marin-Jarillo and Bujanos-Muñiz 2003). Anomala cincta is found in the same geographical region and subject to the same entomopathogenic fungi in the soil.
Local growers in Guanajuato have reported white grub larvae on maize apparently infected by entomopathogenic fungi. Therefore, these microorganisms may represent a potential control strategy against this pest. In addition, extensive research on other soil-dwelling pests such as Melolontha melolontha (L.) and the entomopathogenic fungus Beauveria brongniartii (Sacc.) Petch in Europe (e.g. Dolci et al. 2006; Enkerli et al. 2004; Keller et al. 1999; Kessler et al. 2003, 2004; Sevim et al. 2010) confirms the enormous potential that entomopathogenic fungi have to control soil-dwelling pests.
Based on this information, the susceptibility of P. polyphylla and A. cincta larvae to infection with native Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metsch.) isolates was evaluated. Understanding the interaction between entomopathogenic fungi and these two pest species will contribute to increase our knowledge of the ecology of native entomopathogenic fungi, and their role as alternative control methods against white grubs. First, the susceptibility of third instar larvae of P. polyphylla to isolates of B. bassiana and M. anisopliae was determined. In addition, the virulence of a reduced number of isolates against A. cincta larvae was determined. Finally, the effect of soil on the interaction between an isolate of M. anisopliae and A. cincta larvae was assessed.
Materials and methods
Phyllophaga polyphylla and A. cincta larvae
A large number of insects was required to perform all experiments. Although laboratory-reared insects are usually recommended, the long life cycles of insects, such as these two insect species (annual life cycles), can make it laborious and impractical to maintain in laboratory culture (Jackson and Saville 2000; Klein et al. 2000). Therefore, only field collected larvae were used for the experiments. Third instar larvae of both species were collected from maize and sorghum crops near San Lorenzo in Guanajuato, Mexico from August to November 2009. This site was selected due to the large larval population. Larvae were collected manually and deposited in a 70 × 40 × 20 cm plastic container filled with damp peat moss (Growing Mix®, Canada). Collected larvae were transported to the laboratory and separated by genus based on the presence of palidia in the last abdominal segment (raster) and anal aperture morphology (Morón 1986; Ritcher 1966). Subsequently each larva was confined individually in transparent 100 ml plastic containers filled with damp peat moss and a piece of carrot (as a food source). To determine the species of collected larvae, ~50 larvae from each collecting date (three collecting dates) were allowed to reach adult stage and identified to the level of species using taxonomic keys developed by Morón (1988, 1994) and Morón et al. (2000). As fewer Anomala larva were present during experimental period, ten larvae from each collecting date were allowed to reach adult stage and species determination was carried out as described above. Amongst all the Phyllophaga larvae that reached adult stage, three individuals were of the species Phyllophaga dentex (Bates), eight of P. rubella (Bates) and five of P. vetula (Horn). For the Anomala larvae, all individuals were of the species A. cincta.
For experimentation, all larvae were maintained at room temperature for 30 days. Only larvae that survived the 30 days observation period were used for experiments.
Fungal isolates
Isolates used here were previously obtained in a survey of Guanajuato State to collect native isolates of entomopathogenic fungi from infected white grubs. The selection of isolates used was based on preliminary infection assays carried out on P. polyphylla and A. cincta larvae (data not shown).
Experiments were carried out using seven monosporic isolates, four B. bassiana and three M. anisopliae (Table 1). All isolates were identified based on conidia size and partial sequence of the Elongation Factor 1-α gene. The full description of the material, methods and results of conidia size and phylogenetic analysis will be described in a separate paper. Briefly, conidia size was obtained by measuring the radial distances (length and width) of an average of 300 conidia per isolate from digital photographs using the software Image Tool for Windows 3.0 (Wilcox et al. 2002). Partial sequences of the Elongation Factor 1-α gene were obtained using the primers 983F and 2218R (Rehner and Buckley 2005) for the Metarhizium isolates, and the primers 983F (Rehner and Buckley 2005) and 1567R (Rehner, S.A. Personal communication) for the Beauveria isolates. The conidia sizes for the seven isolates used in the first experiment are given in Table 1. Partial sequences of the Elongation Factor 1-α for all isolates were deposited in GenBank, here we present the accession numbers only of the four isolates used in all experiments, JN617962 and JN617970 for the Metarhizium isolates GC01 and GC02, respectively, and JN617941 and JN617953 for the Beauveria isolates GC03 and GC15, respectively. The phylogenetic analysis performed suggests that the morphologically identified M. anisopliae isolates could be classified as M. pingshaense Q.T. Chen & H.L. Guo, and the B. bassiana isolates could be classified as B. bassiana Clade C (Rehner and Buckley 2005). However, until these results are formally published, we will refer to the isolates as B. bassiana and M. anisopliae as taxonomical entities (morphospecies).
For experimentation, conidia suspensions were produced using the same method. Each isolate was grown in sterile Petri dishes containing 20 ml of sabouraud dextrose agar (SDA) and incubated at 25°C in complete darkness. Conidia were harvested with a sterile scalpel after 15 days, deposited into a sterile 50 ml volume centrifuge tube (NEPTUNE™, Continental Lab Products Inc. San Diego Cal., USA) containing 20 ml of 0.03% Tween 80 and were vortexed for 5 min. The suspension was filtered through a sterile cloth into a new sterile centrifuge tube. The conidia concentration was estimated using a haemocytometer, adjusted to the concentration required for experimentation and maintained at 4°C until required, but never for more than 24 h.
Susceptibility of P. polyphylla larvae against B. bassiana and M. anisopliae isolates
Larval inoculation procedures were the same for isolates of both species (seven isolates) (Table 1). Groups of 24 larvae were placed in a 15 cm diameter Büchner funnel containing two 15 cm diameter filter papers, and inoculated with 100 ml of conidial suspension (1 × 108 conidia ml−1). Larvae were immersed in the suspension for 20 s after which the suspension was removed by suction filtration using a vacuum pump. Inoculated larvae were transferred individually to each of 12 wells of a cell culture plate (COSTAR®, Corning Inc. NY, USA) which contained a 2 cm diameter filter paper which had been moistened with 80 μl of sterile distilled water. Two culture plates were used for each isolate bioassay. A small piece of carrot was added to each well for food. A control treatment had larvae immersed in 100 ml of 0.03% Tween 80 solution. The culture plates were incubated at 25°C in complete darkness and mortality assessed every three days for 36 days. Dead insects were left in their cell culture well, all remaining carrot removed, and a new filter paper circle was deposited and moistened with 80 μl of sterile distilled water to promote sporulation and allowing infection to be confirmed. The eight treatments were carried out the same day, and the complete experiment was repeated on three different occasions.
A completely randomized experimental design was used. Logistic regression, with blocking structure for occasion and culture plate (and allowing for over-dispersion where necessary), was used to assess whether proportional mortality differed between the control and the combined isolates treatments, and then proportion of infection between the isolates. All the statistical analyses were carried out using the statistical package GenStat v. 8.0 (Payne et al. 2005).
Susceptibility of P. polyphylla and A. cincta larvae to selected B. bassiana and M. anisopliae isolates
This experiment compared the susceptibility of P. polyphylla and A. cincta to two B. bassiana (GC03 and GC015) and two M. anisopliae (GC01 and GC02) isolates (Table 1). Twenty-four third instar larvae from both species were inoculated in a cylindrical cage (10 cm diameter × 7 cm tall). Cages were made of copper wire and covered with insect-proof mesh, except in the upper part. Cages with P. polyphylla larvae were immersed into a 1 l glass beaker containing 100 ml of conidial suspension (1 × 108 conidia ml−1) for 20 s. The same suspension was then used to inoculate A. cincta larvae contained in a second cage. The control treatment had larvae from both species immersed in 100 ml of 0.03% Tween 80 solution. Larvae were transferred to culture plates and incubated as described above. Mortality was recorded every three days for 36 days. The five treatments (including control treatment) for each white grub species were carried out the same day and the complete experiment was repeated on three different occasions. On each occasion, the order of white grub species first inoculated was alternated to avoid any effect of reduction in conidia number of the suspension.
A completely randomized experimental design was used. Logistic regression, with blocking structure for occasion and culture plate was used. First, the control treatment was compared to the combined isolates treatments. Secondly the effect of isolate, white grub species and the possible interactions between these factors was assessed.
Effect of soil type on the susceptibility of A. cincta larvae against a M. anisopliae isolate
A series of experiments were conducted to assess whether the soil type in which larvae were incubated following inoculation affected infection rates. To avoid the effect of native entomopathogenic fungi, a survey was carried out on soils from 12 localities within Guanajuato state using the Galleria mellonella baiting method (Zimmerman 1986) (data not shown). We used only sites where no G. mellonella infection was recorded. Soil samples from the following four localities were selected, Jerecuaro, Valle de Santiago, Penjamo and Comonfort. Soil samples from selected localities were taken at 30 cm depth using a shovel disinfected with 70% ethanol. Five samples were obtained from each site (a total of ~3 kg) and maintained at 4°C until required.
Five 80 g sub-samples were taken from each locality and baited with G. mellonella larvae to confirm no occurrence of native entomopathogenic fungi in the soil. This procedure was repeated twice. Within four days of collection, the natural microbial populations of bacteria, fungi and actinomycetes for each soil sample were estimated as previously described (Lorch et al. 1995). One g of soil was weighed using an analytical scale (OHAUS, Pine Brook, NY, USA), heated in a microwave oven for 20 min, and then weighed again to obtain the dry weight of each soil sample. Ten g of each soil sample were deposited in a 250 ml Erlenmeyer flask containing 90 ml of sterile distilled water and incubated using a rotary shaker at room temperature for 18 min at 60 rpm. Serial dilutions were prepared for bacteria (1 × 10−4–1 × 10−6), actinomycetes (1 × 10−2–1 × 10−4) and fungi (1 × 10−1–1 × 10−3). 100 μl samples were taken from each dilution and deposited in the centre of a Petri dish containing media specific for the microorganism being evaluated. The 100 μl sample was evenly distributed using a sterile “L” shape glass rod. Nutrient agar (NA) (J.T. Baker, Xalostoc, Mexico) plates were used to quantify bacteria, potato dextrose agar (PDA) (J.T. Baker, Xalostoc, Mexico) supplemented with rose bengal plates for fungi and Czapeck media plates (J.T. Baker, Xalostoc, Mexico) (pH 8) for actinomycetes. Three replicates were carried out for each dilution and microorganism evaluated. All plates were incubated at 28° C for four, five and 15 days for NA, PDA and Czapeck media, respectively. After the incubation period, the number of colony forming units (CFU) was estimated by selecting the dilution in each media which allowed an accurate enumeration. The CFU g−1 of dried soil was estimated by multiplying the average of CFUs per plate, by the dilution selected in each case. The resulting number was divided by the dried weight of soil estimated. Physical and chemical characteristics of soils were carried out by the Soil Physic Laboratory personnel at the Colegio de Postgraduados, Mexico.
Twelve third instar A. cincta larvae were inoculated with a 1 × 108 conidia ml−1 conidia concentration of M. anisopliae (GC01) using the wire cage method described previously. A different conidial suspension of the same conidia concentration (1 × 108 conidia ml−1) was used to inoculate groups of larvae to be incubated in different soil locations. Each soil location had its own control where larvae were treated with 0.03% Tween 80. Two positive controls were included, and were represented by inoculated larvae incubated in either cell culture plates (as described above) or in plastic cups containing sterile peat moss. Positive controls were used to assess whether the experimental method of incubation using soil and plastic cups was causing additional mortality. Following inoculation, each larva was placed in a transparent plastic cups containing ca. 80 g of soil and incubated at 25°C in complete darkness. Mortality was recorded every three days for 36 days. The seven treatments were all conducted on the same day, and the complete experiment was repeated on three different occasions.
A completely randomized experimental design was used. Logistic regression, with blocking structure for occasions was used. Firstly, the control treatment was compared with the combined isolate treatments. Secondly, the positive control versus the soil treatments was compared, and thirdly differences in infection amongst soil treatments were assessed.
Results
Susceptibility of P. polyphylla larvae against B. bassiana and M. anisopliae isolates
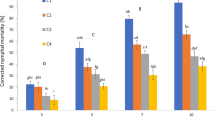
No significant differences were found between replicates (χ 22 = 4.12, P = 0.127). There was a significant difference between the observed mortality in the control treatment and the combined isolates treatments (χ 21 = 10.50, P = 0.001), as no mortality was recorded in the control treatment. However, there was no significant differences amongst isolate treatments (χ 26 = 3.44, P = 0.752). Overall, the observed mortality was small as the proportion of larvae dying from fungal infection never exceeded 0.1 (Fig. 1).
Susceptibility of P. polyphylla and A. cincta larvae to B. bassiana and M. anisopliae isolates
No significant differences were found amongst repetitions (F2, 48 = 0.21, P = 0.814). A significant difference was found between the control treatment and the combined isolates and larvae species treatments (F1, 48 = 71.97, P = 0.001). There was a significant effect of larval species on infection (F1,48 = 121.79, P < 0.001), with A. cincta larvae being more susceptible than P. polyphylla larvae. There was also a significant effect of isolate on infection (F3,48 = 45.11, P < 0.001), with the largest mortality due to fungal infection observed with M. anisopliae isolates GC01 and GC02 and the smallest with B. bassiana isolates GC15 and GC03. There was a significant interaction between isolate and larval species (F4,48 = 15.27, P < 0.001). Metarhizium anisopliae isolates caused greater infection rate to A. cincta larvae whilst B. bassiana isolates caused greater infection rate to P. polyphylla larvae (Fig. 2).
Effect of soil type on the susceptibility of A. cincta larvae against a Metarhizum anisopliae isolate
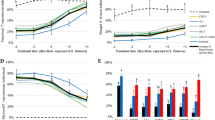
The G. mellonella baiting method confirmed that no native entomopathogenic fungi populations infectious for G. mellonella were present in any of the experimental soil samples. The largest actinomycetes population was present in soil from Valle de Santiago, and the smallest population in soil from Jerecuaro (Fig. 3a). The largest fungi population was obtained in the soil from Penjamo and the smallest population in soil from Jerecuaro (Fig. 3a). The largest bacterial population was obtained in soil collected from Comonfort, and the smallest population in soil collected from Jerecuaro (Fig. 3b). Physical, biological and chemical characteristics of each soil sample are show on Table 2.
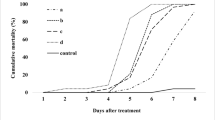
No differences were found amongst the experimental replicates (χ 22 = 1.0125, P = 0.603). There was a significant difference between mortality in the control treatment and mortality in the treatments containing M. anisopliae (GC01) conidia (χ 21 = 424.85, P < 0.001). No differences were found in mortalities due to fungal infection between the positive control treatments and the soil treatments (χ 21 = 0.81, P = 0.368), suggesting that inoculation method and further incubation in cell culture, cups or soil provided consistent infection rates, which results allowed for a valid conclusion on the effect of soil in the proportion of infection obtained in A. cincta larvae with M. anisopliae (GC01). Significant differences were found in fungal infection proportions amongst soil treatments (χ 23 = 20.53, P < 0.001). The largest proportion of infection (1.0) was obtained when larvae were incubated in soil from Penjamo whilst the smallest infection (0.65) was found when larvae were incubated in soil collected from Jerecuaro (Fig. 4).
Discussion
This study represents the first report of B. bassiana and M. anisopliae isolates infecting white grub larva from the species P. polyphylla and A. cincta. Infection rates in P. polyphylla larvae were lower than those reported on other white grub species (Rodríguez-del-Bosque et al. 2005; Berón and Díaz 2005). For example, infection of Phyllophaga crinita (Burmeister) was greater than 60% within ten days when inoculated with isolates of B. bassiana or M. anisopliae (Rodríguez-del-Bosque et al. 2005) whilst isolates of M. anisopliae isolates caused mortalities above 70% within 40 days in Cyclocephala signaticollis (Burmeister) (Berón and Díaz 2005).
Field insect populations that are continuously exposed to pathogens may produce offspring with increased resistance to infection (Moret 2006). Exposure to pathogens may be greater in soil dwelling insects (Tunaz and Stanley 2009), such as white grubs. It is possible that P. polyphylla populations at Guanajuato had been continuously challenged by B. bassiana and M. anisopliae isolates commonly found in Guanajuato soil samples (data not shown). Changes in the insect’s immune system which decreases susceptibility to pathogens will make it more difficult to establish microbial control strategies (Stanley and Miller 2006).
The majority of the P. polyphylla larvae that survived fungal challenge, especially when inoculated with M. anisopliae, showed small pigmented areas randomly distributed on the cuticle of the insect, which may be melanin deposited around encapsulated objects, hemocyte nodules or sites of fungal infection of the cuticle (Gillespie et al. 1997). These melanizated areas suggest that the infection process by M. anisopliae had started but apparently did not succeed as few infected larvae was recorded. Further research on the immune system of P. polyphylla larvae is in progress and will hopefully provide information on the mechanisms used by this species against entomopathogenic fungi. Anomala cincta larvae were very susceptible to infection by M. anisopliae, with infection rates of nearly 100%. It is possible that the two species have been exposed to the fungus for different amounts of time. Potentially P. polyphylla may have co-existed with the fungus for longer than A. cincta and, therefore, have developed greater resistance to local fungal pathogens. Unfortunately, historical data on the occurrence and population fluctuations of these two species of white grub in Guanajuato is not available. The identification of Phyllophaga adults showed the presence of other species in the larvae population used in the experiments, which is common in the field (Marin-Jarillo and Bujanos-Muñiz 2003; Morón 1986). However, we believe the presence of other Phyllophaga species, which were present at a low level (~10%), had no significant effect on our results. It was not possible to perform a species determination prior to experimentation as an accurate species determination must be carried out based on genitalia of adult male insects (Morón 1986).
The higher virulence of M. anisopliae compared to B. bassiana isolates against A. cincta found in this investigation is consistent with that reported by other authors (Ansari et al. 2004; Flores et al. 2002; Poprawski and Yule 1991; Rodríguez-del-Bosque et al. 2005). One hypothesis is that Metarhizium species are better adapted to infect soil dwelling insects as it has been more commonly found causing infection on soil pests (Klein et al. 2000). However, there are examples of isolates of B. bassiana being more virulent to soil pests than M. anisopliae (Berón and Díaz 2005). Therefore, pest-specific isolates should be developed when establishing microbial control strategies. The isolates used in this investigation were obtained from different geographical regions, which suggest all isolates may have been exposed to different biotic and abiotic conditions, and therefore their biological attributes modified, for example, differences of in vitro behaviour such as speed of growth and quantity of conidia production were observed but not quantified.
When comparing the levels of infection obtained in the first and second experiment, in P. polyphylla, the overall results were similar with proportions of infection of 0.1. Although some small differences were observed in the levels of infection using the same isolates and insect species, we do not consider this as a result of inconsistency in the methods used as all factors of variation were constant during the experiments and no additional mortality factors were detected in the control treatments. This difference may have been a result of the insects being collected from field. Indeed, previous studies have suggested that field collected and laboratory reared insects differ. For example, Dembilio et al. (2010) reported variation in the response of field collected adult Rhynchophorus ferruginensis (Olivier) (Coleoptera: Curculionidae) to an isolate of B. bassiana. Although the authors did not provide an explanation for this, it is likely that age variations amongst different groups of adults may have affected the infection process as described for other insect species (Bonduriansky and Brassil 2002; Dukas 2008; Kawasaky et al. 2008; Sherratt et al. 2010). We believe the same phenomenon occurred in our experiments, where a mixture of early and late third instars larvae were collected from the field for experiments. However, this effect was minimal as no statistical differences were found amongst replicates of all experiments.
The effect of soil type on the mortality of A. cincta larvae when inoculated with M. anisopliae was significant. Larvae incubated in soil from Jerecuaro had the lowest infection rate, although only in soil from Penjamo was infection significantly higher. Organic matter content, pH and texture may affect survival of entomopathogenic fungi in the field (Quesada-Moraga et al. 2007). The samples assessed here had different physical and chemical properties, and it is difficult to determine which affected infection. Clearly this may be a combination of many factors, and our experiment was not designed specifically to isolate these factors. Further experiments need to be carried out to assess the effect of different components of soil on the susceptibility of A. cincta larvae to infection with entomopathogenic fungi. Although different authors have suggested pH as one of the main factors affecting entomopathogenic fungi in soil, isolates may have different optimum pH ranges regardless their species (Quesada-Moraga et al. 2007; Rath et al. 1992). Organic content has also been cited as an important factor affecting entomopathogenic fungi, especially its abundance (Mietkiewski et al. 1997; Quesada-Moraga et al. 2007). Organic matter may therefore affect germination and infectivity of conidia. In our study, there was no relationship between this component and infected insects. It is difficult to identify components modifying virulence of M. anisopliae against A. cincta larvae. However, inoculated larvae incubated in soil from Jerecuaro had the lowest proportion of infected larvae. The soil sample from Jerecuaro also showed low bacterial, fungal and actinomycetes populations. The largest actinomycetes population was found in soil from Valle de Santiago. These microorganisms are considered as antagonists of entomopathogenic fungi (Vänninen et al. 2000). This would suggest lower germination and infection rates from M. anisopliae in that soil, but the results were the opposite. The effect of soil on entomopathogenic fungi needs additional study, since biotic elements are the main factor affecting entomopathogenic fungi (Lingg and Donaldson 1981; Rath et al. 1992). The fungus Penicillium urticae (Bainier), a common soil microorganism, produces metabolites that may inhibit B. bassiana conidia germination (Shields et al. 1981). This suggests a more detailed identification of microorganisms in soil is needed before entomapathogenic fungi should be introduced. A study of soil microbial interactions, including culturable, slow growing and non-culturable microorganisms is indeed important, as more information of the complex interactions of soil microbial ecology can be obtained (Widmer et al. 2001; Bloem et al. 2006). For example, the effect to natural microbial population on entomopathogenic fungi may be studied, but also how the introduction of entomopathogenic fungi may modify the population structure of soil indigenous fungi (Schwarzenbach et al. 2009). The study of the interactions, including culturable and non-culturable microorganisms is important, but represents a research area where molecular biology plays an important role (Anderson and Cairney 2004; Prosser 2002).
In conclusion, the relationship between entomopathogenic fungi and white grub larvae is very complex. Using the same isolates, the proportion of P. polyphylla-infected was small compared to A. cincta. Components of the soil samples also affected infection. Although the information obtained did not allow us to draw definite conclusions, the information produced by our experiments increases the knowledge and ecology of these microorganisms, and confirms the necessity to continue investigating this system to allow us to efficiently use entomopathogenic fungi against these insect species.
References
Anderson IC, Cairney JW (2004) Diversity and ecology of soil fungal communities: increased understanding through the application of molecular techniques. Environ Microbiol 6:769–779
Ansari MA, Vestergaars S, Tirry L, Moens M (2004) Selection of a highly virulent isolate, Metarhzium anisopliae CLO 53, for controlling Hoplia philanthus. J Invertebr Pathol 85:89–96
Berón CM, Díaz BM (2005) Pathogenicity of hyphomycetous fungi against Cyclocephala signaticollis. BioControl 50:143–150
Bloem J, Schoutern AJ, Sørensen SJ, Rutgers M, van der Werf A, Breure A (2006) Monitoring and evaluating soil quality. In: Bloem J, Hopkins DW, Benedetti A (eds) Microbiological methods for assessing soil quality. CABI, International, Wallingford, pp 23–49
Bonduriansky R, Brassil CE (2002) Rapid and costly ageing in wild male flies. Nature 420:377
Dembilio O, Quesada-Moraga E, Santiago-Álvarez C, Jacas JA (2010) Potential of an indigenous strain of the entomopathogenic fungus Beauveria bassiana as a biological control agent against the Red Palm Weevil, Rhynchophorus ferrugineus. J Invertebr Pathol 104:214–221
Dolci P, Guglielmo F, Secchi F, Ozino OI (2006) Persistence and efficacy of Beauveria brongniartii strains applied as biocontrol agents against Melolontha melolontha in the Valley of Aosta (northwest Italy). J Appl Microbiol 100:1063–1072
Dukas R (2008) Mortality rates of honey bees in the wild. Insectes Soc 55:252–255
Enkerli J, Widmer F, Keller S (2004) Long-term field persistence of Beauveria brongniartii strains applied as biocontrol agents against European cockchafer larvae in Switzerland. Biol Control 29:115–123
Flores AG, de la Rosa W, Rojas JC, Castro-Ramírez AE (2002) Evaluation of Beauveria bassiana and Metarhizium anisopliae (Mitosporic) against species of the “white grub complex” in the South of Mexico. Southw Entomol 27:73–83
García GL, Ortega-Arenas L, Hernández HG, García AA, Nápoles JR, Cortés RR (2009) Descripción de las Larvas de Tercer Instar de Melolonthidae (Coleoptera) Asociadas al Cultivo de Agave tequilana var. Azul y su Fluctuación. Neotrop Entomol 38:769–780
Gillespie JP, Kanost MR, Trenczek T (1997) Biological mediators of insect immunity. Annu Rev Entomol 42:611–643
Jackson TA, Saville DJ (2000) Bioassays of replicating bacteria against soil-dwelling insect pests. In: Navon A, Ascher KRS (eds) Bioassays of entomopathogenic microbes and nematodes. CABI Publishing, Wallingford, UK, pp 73–93
Kawasaky N, Brassil CE, Brooks RC, Bonduriansky R (2008) Environmental effects on the expression of life span and aging: an extreme contrast between wild and captive cohorts of Telostylinus angusticollis (Diptera: Neriidae). Am Nat 172:346–367
Keller S, Schweizer C, Shah P (1999) Differential susceptibility of two Melolontha populations to infections by the fungus Beauveria brongniartii. BioControl Sci Technol 9:441–446
Kessler P, Matzke H, Keller S (2003) The effect of application time and soil factors on the occurrence of Beauveria brongniartii applied as a biological control agent in soil. J Invertebr Pathol 84:15–23
Kessler P, Enkerli J, Schweizer C, Keller S (2004) Survival of Beauveria brongniartii in the soil after application as a biocontrol agent against the European cockchafer Melolontha melolontha. BioControl 49:563–581
Klein MG, Grewal PS, Jackson TA, Koppenhöfer AM (2000) Lawn, turf and grassland pests. In: Lacey LA, Kaya HK (eds) Field manual of techniques in invertebrate pathology. Springer, Dordrecht, The Netherlands, pp 655–675
Lingg AJ, Donaldson MD (1981) Biotic and abiotic factors affecting stability of Beauveria bassiana conidia in soil. J Invertebr Pathol 38:191–200
Loera-Gallardo J, Pérez-Domínguez JF, Rodríguez-del-Bosque LA (2010) Control Qúmico. In: Plagas del Suelo LA, Rodríguez-del-Bosque LA, Morón MA (eds) Mundi Prensa, México, pp 197–214
Lorch HJ, Benckieser G, Ottow JCG (1995) Basic methods for counting microorganisms in soil and water. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic Press, New York, pp 146–160
Marin-Jarillo A, Bujanos-Muñiz R (2003) Especies del complejo “Gallina Ciega” del género Phyllophaga en Guanajuato, México. Agricultura Técnica en México 34:349–355
Mietkiewski RT, Pell JK, Clark SJ (1997) Influence of pesticide use on the natural occurrence of entomopathogenic fungi in arable soils in the UK: field and laboratory comparisons. BioControl Sci Technol 7:565–575
Moret Y (2006) “Trans-generational immune priming”: specific enhancement of the antimicrobial immune response in the mealworm beetle, Tenebrio molitor. Proc R Soc B 273:1399–1405
Morón MA (1986) El género Phyllophaga en México: morfología, distribución y sistemática supraespecifica (Insecta: Coleoptera) Publ. 20, Instituto de Ecología, México
Morón MA (1988) Las especies de Phyllophaga (Coleoptera: Melolonthidae) con mayor importancia agrícola en México. En: Tercer Mesa Redonda sobre Plagas del suelo. Soc. Mex. Entomología e ICI, México, pp 81–102
Morón MA (1994) Fauna de coleóptera Lamellicornia en las montañas del noreste de Hidalgo, México. Acta Zool Mex 63:7–59
Morón MA (2010) Diversidad y Distribución del Complejo “Gallina Ciega” (Coleoptera: Scarabediae). In: Plagas del Suelo LA, Rodríguez-del-Bosque LA y Morón MA (eds) Mundi Prensa, México, pp 41–63
Morón MA, Aragón A, Tapia-Rojas AM, Rojas-García R (2000) Coleóptera Lamellicornia de la sierra del Tentzo, Puebla, México. Acta Zool Mex 79:77–102
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2005) GenStat for Windows (8th edn), introduction. VSN International, Hemel Hempstead
Poprawski TJ, Yule WN (1991) Incidence of fungi in natural populations of Phyllophaga spp. and susceptibility of Phyllophaga anxia (Leconte) (Col, Scarabeidae) to Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina). J App Entomol 112:359–365
Prosser JI (2002) Molecular and functional diversity in soil micro-organisms. Plant Soil 244:9–17
Quesada-Moraga E, Navas-Cortes JA, Maranhao EAA, Ortiz-Urquiza A, Santiago-Alvarez C (2007) Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol Res 111:947–966
Rath AC, Koen TB, Yip H (1992) The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae en Tasmanian pasture soils. Mycol Res 96:378–384
Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF-1α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97:84–98
Ritcher PO (1966) White grubs and their allies. A study of North American scarabaeoid larvae. Studies in entomology no. 4. Oregon State University Press, Corvallis, USA, p 219
Rodríguez-del-Bosque LA, Silvestre F, Hernández VM, Quiroz H, Throne JE (2005) Pathogenicity of Metarhizium anisopliae and Beauveria bassiana against Phyllophaga crinita and Anomala flavipennis (Coleoptera: Scarabeidae). J Entomol Sci 40:67–73
Schwarzenbach K, Enkerli J, Widmer F (2009) Effects of biological and chemical insect control agents on fungal community structures in soil microcosms. App Soil Ecol 42:54–62
Sevim A, Demir I, Höfte M, Humber RA, Demibarg Z (2010) Isolation and characterization of entomopathogenic fungi from hazelnut-growing region of Turkey. BioControl 55:279–297
Sherratt TN, Laird RA, Hassall C, Lowe CD, Harvey IF, Watts PC, Cordero-Rivera A, Thompson DJ (2010) Empirical evidence of senescence in adult damselflies (Odonota: Zygoptera). J Anim Ecol 79:1034–1044
Shields MS, Lingg AJ, Heimsch RC (1981) Identification of a Penicillium urticae metabolite which inhibits Beauveria bassiana. J Invertebr Pathol 38:374–377
Stanley DW, Miller JS (2006) Eicosanoid actions in insect cellular immune functions. Entomol Exp Appl 119:1–13
Tunaz H, Stanley D (2009) An immunological axis of biocontrol: infections in field-trapped insects. Naturwissenschaften 96:1115–1119
Vänninen I, Tyni-Juslin J, Hokkanen H (2000) Persistence of augmented Metarhizium anisopliae and Beauveria bassiana in Finnish agricultural soils. BioControl 45:201–222
Widmer F, Fliessbach A, Laczko A, Schulze-Aurich J, Zeyer J (2001) Assessing soil biological characteristics: a comparison of bulk soil community DNA-, PLFA-, and Biolog™-analyses. Soil Biol Biochem 33:1029–1036
Wilcox DB, Dove B, McDavid D, Greer D (2002) UTHSCSA Image Tool for Windows ver. 3.0. The University of Texas Health Science Center in San Antonio, USA
Zimmerman G (1986) The Galleria bait method for detection of entomopathogenic fungi in soil. J App Entomol 102:213–215
Acknowledgments
This research was supported by Fundación Guanajuato Produce A.C. Project FGP521/09. We are grateful to Dr. Judith K. Pell and Dr. Jason Baverstock for their valuable comments and correcting the English on an earlier version of this manuscript. We wish to thank Suzanne Clark for her valuable statistical advice.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Martin Erlandson.
Rights and permissions
About this article
Cite this article
Guzmán-Franco, A.W., Hernández-López, J., Enríquez-Vara, J.N. et al. Susceptibility of Phyllophaga polyphylla and Anomala cincta larvae to Beauveria bassiana and Metarhizium anisopliae isolates, and the interaction with soil properties. BioControl 57, 553–563 (2012). https://doi.org/10.1007/s10526-011-9421-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9421-3