Abstract
The multicolored Asian ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae), is considered an important generalist predator that can be used as a biological control agent against Hemiptera Sternorrhyncha, Thysanoptera, and the eggs and larvae of Lepidoptera, Coleoptera and Diptera. There are currently abundant natural resources of overwintering H. axyridis in Asia and North America. Given its potential as a biological control agent, methods can be developed to increase its effectiveness for pest control. The availability of an adequate cold storage method would enable the use of field-collected pre-wintering ladybirds for pest suppression in the following season. We studied the effect of cold storage (30, 60, 90, 120 and 150 days stored at −3, 0, 3 and 6°C) on survival, fecundity and predation in field-collected populations. The survival of both female and male ladybirds decreased significantly as storage duration increased at −3°C and 0°C. The ladybirds showed more than 80% survival when they were stored for 150 days at 3°C and 6°C. Long-term cold storage had different effects on the fecundity of H. axyridis at different temperatures. Prolonged cold storage at both 3°C and 6°C shortened pre-oviposition duration and had no adverse effect on reproductive capacity as compared to that of unstored individuals. The adults that experienced 90-day storage at 0°C had the shortest pre-oviposition duration and the largest reproductive capacity. The individuals that were stored for 150 days at 3°C consumed significantly more aphids than the unstored ones. Generally, 3–6°C is a suitable temperature for cold storage of the ladybird without any reduction in fitness. This study will help the exploitation and application of pre-wintering H. axyridis for the biological control of insect pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The multicolored Asian ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) is native to China, Japan, Korea, Mongolia and Siberia (Brown et al. 2008). Currently, the ladybird has gained much attention because it has become a threat to native biodiversity, a potential pest in certain fruit crops and a human nuisance in its invasive range in North America and Europe (Roy and Wajnberg 2008). However, the generalist ladybird has successfully been used for the biological control of some pests in greenhouses and outdoor crops. Several aphid species, such as Aphis gossypii Glover, Macrosiphum rosae (L.), Toxoptera piricola Matsumura, and Brachycorynella asparagi Mordvilko, were successfully suppressed by releasing H. axyridis in China (Li et al. 1995; Sun et al. 1996; Ma et al. 2005). In North America and Europe, the ladybird has been used in the biocontrol of A. gossypii (Wells et al. 2001; Gil et al. 2004), A. glycines (Landis et al. 2004; Mignault et al. 2006), M. rosae (Ferran et al. 1996), Acyrthosiphon pisum (Harris) (Cardinale et al. 2003), and Phorodon humuli (Schrank) (Trouve et al. 1997). Furthermore, this species has also exhibited effective biocontrol on scale insects, such as Matsucoccus massonianae Yang and Hu in China (Wang 1982), M. matsumurae (Kuwana) in Japan (McClure1986), and Pseudaulacaspis pentagona (Targioni) in Korea (Choi et al. 1995). The decline in the populations of the pseudococcid, Nesticoccus sinensis Tang and the eriococcid, Rhizococcus transversus (Green), both important pests of bamboo in Jiangsu, China (Xu and Wu 1989), and of the curculionid, Diaprepes abbreviatus L., a major pest of citrus in Florida (Stuart et al. 2002), were largely due to this predator.
There are abundant natural resources of H. axyridis in northeastern China. This ladybird is the most common aphidophagous predator in the local forest ecosystems and in orchards and agricultural fields (Yuan et al. 1994). Rising numbers of H. axyridis have been found in recent years in Jilin Province, which may be related to the occurrence of a large number of corn leaf aphids, Rhopalosiphum maidis (Fitch), with the expansion of maize planting areas (personal observation). In mid-October in northeastern China, massive swarms of ladybirds flew out of the cornfields toward the sunlit south-southwestern sides of buildings or rocks, searching for overwintering sites. The migrations were usually aggravated once the noontime temperature rose above 18°C following a cool period (Huelsman et al. 2002).
Overwintering adults of H. axyridis are very resistant to winter conditions and may survive for short periods, even at −30°C (Pervez and Omkar 2006). They overwinter in cracks and crevices of rocks and buildings or caves, which offer them a degree of protection from low temperatures (Sakurai et al. 1992). In addition, they acquire cold hardiness by myo-inositol accumulation (Watanabe 2002) and protect themselves against low temperatures by supercooling (Koch et al. 2004; Berkvens et al. 2010). The adults of a Japanese strain may survive up to 200 days at −5°C (Watanabe 2002).
Cold storage is a valuable tool for insects used in biological control programs. It not only provides a steady supply of insects for research but also yields flexibility and efficiency in mass production, allows for the synchronization of a desired developmental stage for release, and lengthens their availability to consumers (Leopold 1998). Long-term cold storage of some parasitoids has been successfully achieved by inducing diapause or quiescence without decreasing their fitness (López and Botto 2005; Chen et al. 2008). Recently, Riddick and Wu (2010) tested long-term storage of the predatory mite Phytoseiulus persimilis Athias-Henriot with cryoprotectant and carbohydrate chemicals. The possibility of cold storage has been studied for many coccinellids including Coccinella septempunctata L., Adalia bipunctata L. (Hamalainen 1977), C. undecimpunctata L. (Abdel-Salam and Abdel-Baky 2000) and Coleomegilla maculata lengi Timberlake (Gagné and Coderre 2001). Several studies have investigated the cold hardiness of H. axyridis (Ma et al. 1997; Watanabe 2002; Koch et al. 2004; Berkvens et al. 2010), but the effect of cold storage on fitness of the ladybird has rarely been documented. Ma et al. (1997) examined the effect of temperature and relative humidity (RH) on the survival of the pre-wintering adults of H. axyridis from northeastern China and found that 0–4°C and 70–80% RH were the most favorable conditions. However, the reproductive parameters of H. axyridis after long-term cold storage have not been evaluated until now.
With the swarming and gathering behavior in pre-wintering adults of H. axyridis in autumn, massive natural supplies are easily collected manually or mechanically from the walls of buildings. Developing effective methods to store biocontrol agents without compromising their fitness is crucial to releasing them in augmentative biological control programs (Leopold 1998). We hypothesized that an optimal duration of cold storage would not influence the fitness of pre-wintering H. axyridis in terms of survival, predation capacity and fecundity. Storing pre-wintering adults of H. axyridis may be useful for the biological control of insect pests in the following season.
Materials and methods
Insects
Pre-wintering adults of Harmonia axyridis were collected at Jilin Agricultural University (43°48′N, 125°23′E) on October 10, 2009 while they swarmed on and around the walls of buildings. The adults were separated into two groups of females and males according to labrum pigmentation (McCornack et al. 2007). The two groups were fed a 20% honey solution for two days at 11°C. Then approximately 200 females or males were put into plastic Ziploc bags (length 16 cm, width 13 cm) with pinholes on each side for ventilation and filled with folded filter papers. These adults were immediately used for the following long-term cold storage experiments or as unstored controls.
Survival of adult H. axyridis during storage
The female or male ladybirds in plastic Ziploc bags as described above were transferred to four different incubators set at different temperatures (−3, 0, 3, 6 ± 1°C) for five different durations (30, 60, 90, 120, 150 days) under full darkness and 60–70% RH. A total of fifteen plastic Ziploc bags containing about 200 females (or males) were prepared for each storage temperature. Considering the adverse impacts of sudden temperature changes, the adults that were taken out of the incubators could not be returned to continue cold storage once survival was determined. Three plastic Ziploc bags containing females (or males) at various storage durations were randomly removed from the incubator and initially placed at 10 ± 1°C for 24 h, then transferred to standard conditions (25 ± 1°C, 60 ± 5% RH and 16:8 L:D). The adults were considered alive if they were still mobile after 24 h at standard conditions.
Post-storage fitness of adult H. axyridis
After the survival investigation mentioned above, the fecundity of adult H. axyridis was immediately determined after 60, 90, 120 or 150 days of storage, as was the fecundity of the unstored adults. In this experiment, pairs of female and male adults that had experienced various storage durations at each storage temperature were introduced in individual Petri dishes (diameter 9 cm, height 2 cm) with about 400–500 cowpea aphids Aphis craccivora Koch on the detached leaves of horsebean, Vicia faba L. The cowpea aphids were cultured for more than ten generations on horsebean plants in the laboratory. Every 24 h, the total number of eggs laid was recorded, and the live females and males were transferred to a new Petri dish containing a similar number of aphids. During the experiment, only a single male was used to mate with each female. This process lasted for 20 days after the ladybird couples were introduced. Twenty-five pairs of adults for each treatment were initially used, and the data from 18 to 23 replicates were analyzed because several adults escaped during manipulation or were parasitized by a Perilitus species.
At the end of long-term cold storage, we investigated the predation capacity on aphids by the adults. In this experiment, pairs of female and male adults that had experienced a 150-day storage at 3°C were introduced in individual Petri dishes with 400 older-stage aphids (third-fourth instar nymphs and wingless adults) on the detached leaves of horsebean. To prevent aphids from escaping, the bottom of the dish was covered with a moist filter disk and inverted. Every 24 h, the total number of aphids consumed by the adult couple was recorded, and the live female and male were transferred to a new Petri dish containing a similar number of aphids. This process lasted for 15 days. Pairs of unstored adults were used as control in this study. A total of twenty replicates were used for both treatments, but only the data from 15 replicates of each treatment were analyzed because several adults escaped or were parasitized. All experiments were conducted under standard conditions (25 ± 1°C, 60 ± 5% R.H. and 16:8 L:D).
Statistical analysis
For the comparison of the survival, pre-oviposition period and fecundity of adult H. axyridis under different cold storage conditions, a two-factor analysis of variance (ANOVA) was performed. The two factors were storage temperature (four levels) and storage duration (survival: five levels; fecundity: four levels). Before analysis, data on the percentage of survival were arcsine square-root transformed. When an overall ANOVA indicated significant effects of the factors at P < 0.05, the means were compared using Tukey’s honestly significantly difference (HSD) test. A paired t-test was used to compare the number of aphids consumed by adult pairs that experienced 150-day storage at 3°C and those that were unstored. All statistical analyses were done using the statistical software package SAS (SAS Institute 2006).
Results
Survival of adult H. axyridis during storage
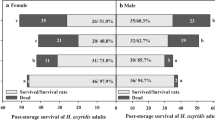
The survival of adult H. axyridis after storage at −3, 0, 3 and 6°C is shown in Fig. 1. Adult survival varied significantly among storage temperatures (Female: F 3, 40 = 13.68, P < 0.0001; Male: F 3, 40 = 12.66, P < 0.0001) and storage duration (Female: F 4, 40 = 6.78, P = 0.0003; Male: F 4, 40 = 7.21, P = 0.0002), and a significant storage temperature × duration interaction was detected (Female: F 12, 40 = 63.38, P < 0.0001; Male: F 12, 40 = 98.75, P < 0.0001). A similar dynamic tendency existed for both females and males at various storage conditions (Fig. 1 a, b). The survival of females and males decreased clearly at −3°C with 38.3–44.7% of survival after 90-day storage. All adults had died after a 150-day storage. More than 87% of female and male adults survived after 90-day storage at 0°C. After that, the ladybird showed a relatively rapid decrease in survival. At 150 days, survival had dropped to near 50%. Female and male adults showed more than 82% survival after 150-day storage at both 3°C and 6°C. Specifically, female adult survival was the highest (near 90%) at the end of the 3°C storage.
Post-storage fitness of adult H. axyridis
Fecundity Both storage temperature and storage duration had no significant effect on the fecundity of H. axyridis (Storage temperature: F 3,304 = 1.78, P = 0.1506; Storage duration: F 3, 304 = 0.88, P = 0.4517), but a significant storage temperature × duration interaction was detected (F 9,304 = 85.77, P < 0.0001). Similarly, neither storage temperature nor storage duration had a significant effect on the pre-oviposition duration of H. axyridis (Storage temperature: F 3,304 = 0.76, P = 0.5173; Storage duration: F 3,304 = 1.66, P = 0.1757), but a significant storage temperature × duration interaction was detected (F 9,304 = 165.98, P < 0.0001).
Harmonia axyridis that were stored for 90 days at 0°C laid more eggs than those of the unstored control but laid similar egg numbers than those stored for 90–150 days at 3°C and 120–150 days at 6°C. The beetles that were stored for 90–150 days at 3°C laid more eggs than those stored for 60 days at the same temperature. The number of eggs laid was increased with prolonged storage duration at 6°C until the storage duration reached 120 days. However, the number of eggs laid was decreased with a prolonged storage duration at −3°C until the storage duration reached 150 days and all individuals were dead (Fig. 2a).
The pre-oviposition duration of H. axyridis that were stored for 120 days was longer than for those stored for 60–90 days at −3°C. In contrast, the pre-oviposition duration tended to shorten with a prolonged storage duration at 0–6°C, with the exception of the 0°C 120-day treatment (Fig. 2b).
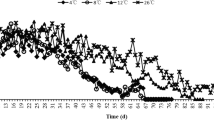
Predation There was a tendency that the individuals that were stored for 150 days at 3°C consumed more aphids per day than the unstored controls from the second day onward (t = 1.2601–9.1698, df = 28, P-value between 0.0001 and 0.2180) with the exception of 12th day (t = 3.2246, df = 28, P = 0.0032) (Fig. 3). Over a period of 15 days, cold-stored H. axyridis couples consumed significantly more aphids than the unstored controls (3534.6 ± 26.7 vs. 3184.6 ± 33.3; t = 8.1898, df = 28, P < 0.0001).
Discussion
The results of this study indicate that long-term storage at or below 0°C had negative effects on the survival of adult H. axyridis. More than 50% of adults died when they were stored for 90 days at −3°C, and no individuals survived 150-day storage at −3°C. In contrast, the ladybirds showed more than 80% survival when they were stored for 150 days at both moderately low temperatures (3°C and 6°C). Harmonia axyridis, a ‘chill intolerant’ insect, can survive at freezing temperatures by moving to physically protected areas or by acquiring cold hardiness by cold acclimation and supercooling (Pervez and Omkar 2006). A temperature of −3°C would result in more cold damage, especially in swarming adults (collected in early October), which have not acquired adequate cold tolerance yet. In addition, the cold tolerance of adult H. axyridis changes seasonally. The supercooling point of adults collected in Japan between December and February reached −18°C, far lower than the supercooling point of those collected in October (−7°C) (Watanabe 2002). All adults kept for 150 days at −3°C died in the current study. Whereas in the study of Labrie et al. (2008), about 45% of adults collected from the field in Canada in November were still alive when they were stored for at least 150 days at −5°C. These differences were probably related to different quantities of accumulated myo-inositol between the two collections (Watanabe 2002). Here, female and male adults exhibited a similar dynamic tendency in survival at various storage conditions, which is in accordance with previous studies of Koch et al. (2004) and Berkvens et al. (2010).
The effects of cold storage on the fitness of numerous mass-reared parasitoids (López and Botto 2005; Pandey and Johnson 2005; Chen et al. 2008) and predators (Abdel-Salam and Abdel-Baky 2000; Gagné and Coderre 2001; Riddick and Wu 2010) have been assessed. However, the effect of cold storage on the fitness of pre-wintering H. axyridis have rarely been documented, except for some studies focusing on survival (Koch et al. 2004; Berkvens et al. 2010). Our results indicated that long-term cold storage had different effects on the fecundity of H. axyridis at different temperatures. Prolonged cold storage at both 3°C and 6°C shortened pre-oviposition duration and had no adverse effect on reproductive capacity. Compared with unstored individuals, there was a significantly shorter pre-oviposition duration at shorter storage durations for those kept at −3°C. Subsequently, prolonged cold storage exhibited deleterious effects that occurred in the form of extended pre-oviposition duration and reduced fecundity. Interestingly, the individuals that experienced 90-day storage at 0°C had the shortest pre-oviposition duration and the largest reproductive capacity. Nevertheless, 3–6°C appear to be suitable storage temperatures for the swarming adult H. axyridis that were collected in October. These temperatures resulted in higher survival and fecundity. The ladybirds that were stored for 150 days at 3°C consumed more aphids than the unstored controls, which further demonstrated that there was no adverse effect of long-term cold storage on their fitness. The higher predation capacity of adults post-storage could be explained in part by the fact that H. axyridis is a temperate insect with a facultative winter diapause (Koch 2003). Although these individuals survive much longer at a moderately low temperature (3°C), they must consume a certain amount of energy reserves to maintain their metabolism and/or produce cryoprotectant compounds, such as myo-inositol (Watanabe 2002). The ladybirds emerging from hibernation are in a status of starvation. Thus, they have the potential to consume more prey to replenish energy and promote oogenesis.
Many factors might have affected the survival of the predatory natural enemies in the cold storage experiments, including the food given prior to cold storage (Coudron et al. 2009) or during storage (Tauber et al. 1997; Thorpe and Aldrich 2004), cryoprotectants and carbohydrates sprayed prior to cold storage (Riddick and Wu 2010), and populations tested in storage (Berkvens et al. 2010). For the assessment of the cold tolerance of H. axyridis, it would be more practical to use individuals from laboratory populations than those collected from the field (Bazzocchi et al. 2004; van Lenteren et al. 2008). However, the cold tolerance of individuals collected from the field was much higher than that of laboratory-reared individuals (Berkvens et al. 2010). Therefore, it appears that freshly collected field populations are more suitable for long-term cold storage. A honey solution was offered to adult ladybirds for two days prior to storage in the current study. Further research is necessary to investigate the effects of other foods prior to storage, including natural and unnatural foods (Dong et al. 2001) on the survival, reproduction and predation capacity of H. axyridis.
In summary, we conclude that swarming H. axyridis adults collected in October can be safely stored with a high survival rate at 3°C for 150 days without any reduction in reproductive and predatory capacity. The cold storage methods show the potential of using field-collected pre-wintering ladybirds for pest suppression in the following season. As adult ladybirds tend to fly away from the crop soon after release, their effectiveness is constrained (Adachi-Hagimori et al. 2011). Nevertheless, these stored adults could be used to mass-produce eggs or larvae in the laboratory prior to inundative release. Furthermore, these results can be used to maintain laboratory and mass-reared colonies of H. axyridis. Although there was no adverse effect of long-term cold storage on the fecundity and predation of adult H. axyridis in the laboratory, further field studies are needed to investigate the success of stored ladybirds under natural conditions, particularly in dispersal, predation capacity and prey location.
References
Abdel-Salam AH, Abdel-Baky NF (2000) Possible storage of Coccinella undecimpunctata (Col., Coccinellidae) under low temperature and its effect on some biological characteristics. J Appl Entomol 124:169–176
Adachi-Hagimori T, Shibao M, Tanaka H, Seko T, Miura K (2011) Control of Myzus persicae and Lipaphis erysimi (Hemiptera: Aphididae) by adults and larvae of a flightless strain of Harmonia axyridis (Coleoptera: Coccinellidae) on non-heading Brassica cultivars in the greenhouse. BioControl 56:207–213
Bazzocchi GG, Lanzoni A, Accinelli G, Burgio G (2004) Overwintering, phenology and fecundity of Harmonia axyridis in comparison with native coccinellid species in Italy. BioControl 49:245–260
Berkvens N, Bale JS, Berkvens D, Tirry L, De Clercq P (2010) Cold tolerance of the harlequin ladybird Harmonia axyridis in Europe. J Insect Physiol 56:438–444
Brown PMJ, Adriaens T, Bathon H, Cuppen J, Goldarazena A, Hagg T, Kenis M, Klausnitzer BEM, Kovar I, Loomans AJM, Majerus MEN, Nedved O, Pedersen J, Rabitsch W, Roy HE, Ternois V, Zakharov IA, Roy DB (2008) Harmonia axyridis in Europe: spread and distribution of a non-native coccinellid. BioControl 53:5–21
Cardinale BJ, Harvey CT, Gross K, Ives AR (2003) Biodiversity and biocontrol: emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol Lett 6:857–865
Chen WL, Leopold RA, Harris MO (2008) Cold storage effects on maternal and progeny quality of Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae). Biol Control 46:122–132
Choi K, Lee S, Kim J, Park J (1995) Role of the coccinellid beetle, Harmonia axyridis in the biological control of the black pine bast scale insect, Matsucoccus thunbergianae. J Forest Sci 51:115–118
Coudron TA, Popham HJR, Ellersieck MR (2009) Influence of diet on cold storage of the predator Perillus bioculatus (F.). BioControl 54:773–783
Dong H, Ellington JJ, Remmenga MD (2001) An artificial diet for the lady beetle Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Southwest Entomol 26:205–213
Ferran A, Niknam H, Kabiri F, Picart JL, De Herce C, Brun J, Iperti G, Lapchin L (1996) The use of Harmonia axyridis larvae (Coleoptera: Coccinellidae) against Macrosiphum rosae (Hemiptera: Sternorrhyncha: Aphididae) on rose bushes. Eur J Entomol 93:59–67
Gagné I, Coderre D (2001) Cold storage of Coleomegilla maculata larvae. Biocontrol Sci Technol 11:361–369
Gil L, Ferran A, Gambier J, Pichat S, Boll R, Salles M (2004) Dispersion of flightless adults of the Asian lady beetle, Harmonia axyridis in greenhouses containing cucumbers infested with the aphid Aphis gossypii: effect of the presence of conspecific larvae. Entomol Exp Appl 112:1–6
Hamalainen M (1977) Storing dormant Coccinella septempunctata and Adalia bipunctata (Col., Coccinellidae) adults in the laboratory. Ann Agric Fenn 16:184–187
Huelsman MF, Kovach J, Jasinski J, Young C, Eisley B (2002) Multicolored Asian lady beetle (Harmonia axyridis) as a nuisance pest in households in Ohio. In: Jones SC, Zhai J, Robinson WH (eds), Proceedings of 4th International Conference on Urban Pests, pp 243–250
Koch RL (2003) The multicolored Asian lady beetle, Harmonia axyridis: a review of its biology, uses in biological control, and non-target impacts. J insect Sci 3:1–16
Koch RL, Carrillo MA, Venette RC, Cannon CA, Hutchison WD (2004) Cold hardiness of the multicolored Asian lady beetle (Coleoptera: Coccinellidae). Environ Entomol 33:815–822
Labrie G, Coderre D, Lucas E (2008) Overwintering strategy of multicolored Asian lady beetle (Coleoptera: Coccinellidae): cold-free space as a factor of invasive success. Ann Entomol Soc Am 101:860–866
Landis DA, Fox TB, Costamagna AC (2004) Impact of multicolored Asian lady beetle as a biological control agent. Am Nat 50:153–154
Leopold RA (1998) Cold storage of insects for integrated pest management. In: Hallman GJ, Denlinger DL (eds) Temperature sensitivity in insects and application in integrated pest management. Westview Press, Boulder, pp 235–267
Li XL, Zhu XS, Yuan F (1995) Studies on natural enemies of asparagus aphid, Braechycorynella asparagi. Acta Univ Agric Boreali-Occidentalis 23:39–43
López SN, Botto E (2005) Effect of cold storage on some biological parameters of Eretmocerus corni and Encarsia formosa (Hymenoptera: Aphelinidae). Biol Control 33:123–130
Ma CS, He YR, Zhang GH, Chen YW (1997) Effects of temperature and relative humidity on survival of the overwintering Asian coccinellid, Harmonia axyridis. Acta Ecol Sinica 17:23–28
Ma F, Yang RS, Gao DS (2005) Occurrence of aphid in orchard and control of aphid using Harmonia axyridis. Liaoning Agric Sci 2:37–39
McClure MS (1986) Role of predators in regulation of endemic populations of Matsucoccus matsumarae (Homoptera: Margarodidae) in Japan. Environ Entomol 15:976–983
McCornack BP, Koch RL, Ragsdale DW (2007) A simple method for in-field sex determination of the multicolored Asian lady beetle Harmonia axyridis. J Insect Sci 7:1–12
Mignault MP, Roy M, Brodeur J (2006) Soybean aphid predators in Québec and the suitability of Aphis glycines as prey for three Coccinellidae. BioControl 51:89–106
Pandey RR, Johnson MW (2005) Effect of cold storage on Anagyrus ananatis Gahan (Hymenoptera: Encyrtidae). Biol Control 35:9–16
Pervez A, Omkar GAK (2006) Ecology and biological control application of multicoloured Asian ladybird, Harmonia axyridis: a review. Biocontrol Sci Tech 16:111–128
Riddick EW, Wu Z (2010) Potential long-term storage of the predatory mite Phytoseiulus persimilis. BioControl 55:639–644
Roy H, Wajnberg E (2008) From biological control to invasion: the ladybird Harmonia axyridis as a model species. BioControl 53:1–4
Sakurai H, Kawai T, Takeda S (1992) Physiological changes related to diapause of the lady beetle, Harmonia axyridis (Coleoptera, Coccinellidae). Appl Entomol Zool 27:479–487
SAS Institute (2006) SAS/STAT version 9.1 for Windows. SAS Institute. Cary
Stuart RJ, Michaud JP, Olsen L, McCoy CW (2002) Lady beetles as potential predtors of the root weevil Diaprepes abbreviatus (Coleoptera: curculionidae) in Florida citrus. Florida Entomol 85:409–416
Sun XQ, Chen WL, Chen ZB, He JL, Ye WJ (1996) A preliminary study on the artificial diet of an aphidophagous coccinellid, Harmonia axyridis (Pallas) and its use to control strawberry aphids under plastic covering. J Shanghai Agric College 14:133–137
Tauber MJ, Albuquerque GS, Tauber CA (1997) Storage of nondiapausing Chrysoperla externa adults: influence on survival and reproduction. Biol Control 10:69–72
Thorpe KW, Aldrich JR (2004) Conditions for short-term storage of field-collected spined soldier bug, Podisus maculiventris (Say) (Heteroptera: Pentatomidae), adults prior to augmentative release. J Entomol Sci 39:483–489
Trouve C, Ledee S, Ferran A, Brun J (1997) Biological control of the damson-hop aphid, Phorodon humuli (Hom.: Aphididae), using the ladybeetle Harmonia axyridis (Col.: Coccinellidae). Entomophaga 42:57–62
van Lenteren JC, Loomans AJM, Babendreier D, Bigler F (2008) Harmonia axyridis: an environmental risk assessment for Northwest Europe. BioControl 53:37–54
Wang LY (1982) Control of Matsucoccus massonianae Young et Hu (Hom.: Margarodidae) by Leis axyridis (Pallas). Nat Enemies Insects 14:37–39
Watanabe M (2002) Cold tolerance and myo-inositol accumulation in overwintering adults of a lady beetle, Harmonia axyridis (Coleoptera: Coccinellidae). Eur J Entomol 99:5–9
Wells ML, McPherson RM, Ruberson JR, Herzog GA (2001) Coccinellids in cotton: population response to pesticide application and feeding response to cotton aphids (Homoptera: Aphididae). Environ Entomol 30:785–793
Xu FY, Wu DX (1989) Control of bamboo scale insects by intercropping rape in the bamboo forest to attract coccinellid beetles. Chinese J Biol Control 5:117–119
Yuan RC, Zhang FM, Wen GZ, Yu M, Wang XQ, Ma FC, Cao ZA (1994) Color pattern of Harmonia axyridis in Changbai Mountain. Jilin Agric Sci 4:45–54
Acknowlegments
We thank Wei Chen, Li-Long Qin and Zhen Guo for collecting the insects in this study. We also thank three anonymous reviewers and Eric Wajnberg for their invaluable comments on this manuscript. This research was funded by grants from the Science and Technology Bureau of Changchun, China (08YJ23).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Patrick De Clercq
Rights and permissions
About this article
Cite this article
Ruan, CC., Du, WM., Wang, XM. et al. Effect of long-term cold storage on the fitness of pre-wintering Harmonia axyridis (Pallas). BioControl 57, 95–102 (2012). https://doi.org/10.1007/s10526-011-9414-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-011-9414-2