Abstract
The effect of two insect growth regulators and a neonicotinoid insecticide were tested on immature stages and adults of the parasitoid Aphtyis melinus DeBach (Hymenoptera: Aphelinidae), a key natural enemy of California red scale, Aonidiella aurantii (Maskell) (Hemiptera: Diaspididae), in California. No significant effects of the insect growth regulators on survival or development to the adult stage were found when the parasitoid was treated at any of the egg, larval, or pupal stages. The broad-spectrum neonicotinoid acetamiprid also showed no significant effect on the development of A. melinus to the pupal stage, probably because immature stages of this ectoparasitoid are protected under the cover of its armored scale host. However, 48 h exposure of adults to acetamiprid residues following emergence resulted in high levels of wasp mortality. Aphytis melinus adults treated with either of the two insect growth regulators as larvae survived 48 h exposure to pesticide residues as adults and showed levels of fecundity comparable with control insects. We conclude that the two insect growth regulators are compatible with augmentative releases of A. melinus but that treatments of acetamiprid should be avoided in situations where biological control by this parasitoid is important.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
California red scale, Aonidiella aurantii (Maskell), is a key pest of citrus in California (Flint et al. 1991). The main control agent for California red scale in the San Joaquin Valley region is insecticides and treatments are typically applied in each grove on an annual or alternate year basis (Grafton-Cardwell and Vehrs 1995). However, approximately 10% of growers release Aphytis melinus DeBach for control of California red scale, which is an ectoparasitoid that attacks large second instar males and females and pre-ovipositional third instar females (Forster et al. 1995; Murdoch et al. 1995). Aphytis melinus is less exposed to the environment or spray drift compared with most ectoparasitoids; although it feeds externally on the scale body, it develops under the armored scale cover which is cemented to the plant substrate (leaves, twigs, or fruit) except during molts.
Augmentative releases of A. melinus have been used for many years to suppress California red scale in southern California (Moreno and Luck 1992) but early attempts to manage California red scale with A. melinus in the San Joaquin Valley of California were unsuccessful (Riehl et al. 1980). In the mid 1980s, an additional attempt at biological control of California red scale in the San Joaquin Valley met with greater success due to the development of a more holistic, biologically-based integrated pest management program designed to minimize use of broad-spectrum pesticides, which interfered with predators and parasitoids of California red scale. This biological control program used releases of A. melinus at a rate of 241,000 per ha per year, with 20 annual releases made on an alternate week basis from mid February to mid November (Haney et al. 1992; Luck et al. 1997; Morse et al. 2007).
Use of the biologically-based integrated pest management program reached a peak of approximately 30% grower adoption in the San Joaquin Valley in the mid 1990s as California red scale resistance to organophosphates and carbamates became severe (Grafton-Cardwell et al. 2001; Grafton-Cardwell and Vehrs 1995). However, most growers preferred to continue to control California red scale chemically and as a result, in 1998–1999, two insect growth regulators, pyriproxyfen and buprofezin, were requested and granted emergency registration to control California red scale in citrus (Grafton-Cardwell 1999). Pyriproxyfen acts as a juvenile hormone mimic (Ishaaya et al. 1994) and buprofezin is a chitin synthesis inhibitor (Izawa et al. 1985). Both insecticides are effective in causing mortality of scales as they molt. These insecticides exhibit compatibility with several hymenopteran species in contrast to broad-spectrum organophosphate and carbamate insecticides (Ishaaya et al. 1992; Liu and Stansly 1997; Van Driesche et al. 2001; Rothwangl et al. 2004). However, in field evaluations of A. melinus, a pyriproxyfen-exposed field showed a lower percentage of yellow sticky cards containing A. melinus compared to a field exposed to buprofezin or the controls (Grafton-Cardwell et al. 2006). These data suggest that pyriproxyfen might be more toxic than buprofezin to A. melinus. However, in these studies, pyriproxyfen showed greater efficacy in control of California red scale compared with buprofezin. Thus, it could not be determined whether pyriproxyfen affected A. melinus levels directly or indirectly via reduction of host density, which affected parasitization levels (Grafton-Cardwell et al. 2006).
In 2003, a third novel insecticide was registered for use in citrus, the neonicotinoid acetamiprid, which acts on insect nicotinic acetylcholine receptors (Ishaaya et al. 2005). Field evaluations demonstrated that acetamiprid controlled citricola scale (Coccus pseudomagnoliarum (Kuwana)); however, acetamiprid showed only a suppressive effect on California red scale (Grafton-Cardwell and Reagan 2003, 2004). In a 2004 field study, acetamiprid reduced several pest populations, but only 28% of A. melinus emerged safely (E.E.G., unpublished data). In studies comparing acetamiprid to other neonicotinoids such as imidacloprid, acetamiprid exhibited broad-spectrum insecticidal activity that included toxicity to predatory beetles, predaceous and omnivorous bugs, green lacewings, predatory flies, and several moth species (Iwasa et al. 2004; Naranjo and Akey 2005).
Pyriproxyfen, buprofezin, and acetamiprid are insecticides that are used in citrus to control several key citrus pests. In this study, we evaluated the compatibility of these three insecticides with A. melinus to determine if one or more could be used in conjunction with A. melinus augmentative releases. If compatibility were observed, then the insecticide might be safely used to reduce populations of California red scale (buprofezin or pyriproxyfen) or citricola scale (acetamiprid) while concurrently employing A. melinus releases to aid in suppression of California red scale. The following laboratory study was designed to determine the effects of buprofezin, pyriproxyfen, and acetamiprid on A. melinus development when treatments were applied to any of three developmental stages, the egg, larva, or pupa. Additionally, we examined the effects of the insect growth regulators on wasp reproduction when A. melinus was exposed to the pesticide during the larval stage.
Materials and methods
Rearing
The A. melinus used in this study were obtained from a colony reared on Aspidiotus nerii Bouche-infested lemons at the University of California, Riverside Insectary. The colony was collected in India/Pakistan in 1956–1957 and has been maintained at the University of California, Riverside Insectary since then on green lemons held at 28°C, 38% RH, and 24L: 0D (Yu and Luck 1988). California red scale were obtained from the University of California, Riverside Insectary colony that had been maintained for >20 years on green Lisbon lemons at 27.5 ± 2°C, 60 ± 10% RH, and 24L: 0D fluorescent lighting.
Insecticide Bioassays
Three stages of A. melinus (egg, larva, and pupa) were studied to determine if any of the stages and subsequent molts were sensitive to applications of pyriproxyfen, buprofezin, and acetamiprid. Experimental lemons were obtained by releasing 200 A. melinus for 24 h into a 30.4 cm × 30.4 cm × 45.7 cm cage that contained seven California red scale-infested lemons, each infested with approximately 200–300 early third instar female scales. Experimental lemons were held in a growth chamber at 26.5 ± 2°C, 40 ± 5% relative humidity, and 12L: 12D. Each week, seven experimental lemons per parasitoid life stage were placed in the growth chamber and parasitoids were allowed to develop to the egg stage (1 day), larval stage (5 days), or pupal stage (10 days) before randomly selecting a lemon to be treated with pyriproxyfen (1 lemon), buprofezin (1 lemon), acetamiprid (1 lemon at each of two rates), or a water control (3 lemons). The experiment was a randomized block design over a five week period with three trials per week (each life stage) completed over each of five consecutive weeks (n = 5 lemons with 200–300 red scale, one treated per week with each treatment).
Pyriproxyfen and buprofezin were used at the recommended field rates whereas acetamiprid was used at the field rate and at 10% of the field rate. Experimental lemons were dipped in concentrations of 17.0 ppm pyriproxyfen (0.86 EC Esteem [103.1 g AI per liter emulsifiable concentrate], Valent USA Corp., Walnut Creek, CA), 320.0 ppm buprofezin (70 DF Applaud [70% AI dry flowable], Nichino America, Inc., Wilmington, DE), and 2.8 or 28.0 ppm acetamiprid (70 WP Assail [70% AI wettable powder], Cerexagri, Inc, King of Prussia, PA). With each pesticide and the water control, 0.02% Triton X-100 (Sigma Aldrich, St. Louis, MO) was added to the dip solution in deionized water and the lemons were dipped in the solution for three seconds. After air-drying for 15 min, each lemon was individually contained in a 710 ml tall, 10.2 cm diameter, round plastic container covered with a 95 cm2 area fabric mesh (MONO135 fabric, McLogan Supply Co., Anaheim, CA). Each container was labeled with the date of parasitism by A. melinus, parasitoid life stage treated, treatment date, pesticide, and concentration. Once a week for each life stage, two lemons, one treated with pyriproxyfen and the other with buprofezin, were placed in a growth chamber along with two control lemons while the two lemons treated with the high and low rates of acetamiprid and one control lemon were placed in a separate growth chamber. Each of these seven lemons was placed in a separate container. The containers with insect growth regulator-treated lemons were separated from the neonicotinoid-treated lemons and its control due to possible fumigation by acetamiprid, which had been observed in lab studies with another neonicotinoid, imidacloprid (J.G.M., unpublished data). Both growth chambers were maintained at 26.5 ± 2°C and 40 ± 5% RH.
The impacts of egg and larval treatments were evaluated in two ways: (1) by destructively assessing parasitoid status at the pupal stage and (2) by determining how many adult parasitoids emerged from the remaining scale. Nine days after egg treatment and four days after larval treatment, when parasitoids would be in the pupal stage (based on Yu and Luck’s 1988 degree-day model for rearing red scale at our rearing temperature of 26.5°C), 50 scale covers were randomly chosen and turned over with a small probe. Percentage survivorship was calculated from the number of live A. melinus divided by the total number of A. melinus (dead + alive) found under the 50 scales.
For trials done during the first four weeks, all adult A. melinus were counted 48 h after they began to emerge. Emergence began ca. 14 days after egg treatment, ca. 10 days after larval treatment, and ca. 5 days after pupal treatment. Undiluted honey was placed within the containers two days before emergence as a food source for adult A. melinus. For trials completed during the fifth week, emergence was assessed in the same way but in addition, the remaining scale covers were turned over to examine an average of 250 scales per lemon. The stage and number of dead and live A. melinus as well as the number of host-fed (due to initial exposure to A. melinus parental females or the newly emerged parasitoid progeny) and unparasitized scales were recorded. The average number of emerged A. melinus for the first four weeks showed similar results to the fifth week and thus, the data were combined for further analysis.
Impact of insect growth regulators on wasp fecundity
The fecundity of parasitoids treated as larvae with the insect growth regulators was evaluated by placing five similarly sized A. melinus females that had successfully emerged from each treatment (including controls) into individual vials with 0.1 ml pure honey for 24 h along with several untreated males. The five A. melinus females per larval treatment were then transferred into a 710 ml tall, 10.2 cm diameter, round plastic container with a 95 cm2 area mesh lid that contained a California red scale-infested lemon with approximately 200–300 third instar female scales. This procedure was replicated three times per week in weeks 3, 4, and 5 (n = 9 replicates with 45 Aphytis females in total). The adults were removed after five days and the A. melinus immatures were allowed to develop for 16 days to the adult stage. At this time, all scale covers were turned over and the number of emerged Aphytis (i.e., those with a hole in the scale cover), unemerged parasitoids (i.e., parasitoid pupae), non-parasitized scale, and host-fed scale was recorded. Wasp fecundity (average number of eggs per female) was calculated as the total number of emerged A. melinus per lemon divided by five (i.e. five parental A. melinus). Due to low survivorship of the A. melinus in the control treatment in the growth chamber with the acetamiprid-exposed lemons, fecundity was not evaluated for the acetamiprid treatment or its control.
Statistical analysis
A two-way ANOVA was used to test the effects of A. melinus life stage and insecticide on survival of A. melinus with least significant difference (LSD) mean separation (PROC GLM; SAS Institute 2004). If no significant differences were found with parasitoid life stage but significant differences were observed with insecticidal treatment, then the stages were combined and a one-way ANOVA with LSD mean separation was used to determine which insecticidal treatments were significantly different (PROC GLM; SAS Institute 2004). The two insect growth regulators were analyzed separately from the neonicotinoid because they were maintained in different growth chambers. Normality and heterogeneity of variance were tested, and data were transformed using arcsine (square root [x]) for percentage data and square root (x) for count data as suggested by Zar (1984). The Kruskal–Wallis non-parametric test was used to test the effects of the insect growth regulators on A. melinus fecundity because non-normality was observed (PROC NPAR1WAY; SAS Institute 2004).
Results
With pyriproxyfen and buprofezin, the percentage of A. melinus that survived to the pupal stage was not significantly different for the wasp life stage when the lemon was treated (eggs or larvae) (F = 1.73; df = 1, 27; P = 0.954) or among insecticide treatments compared to the control (F = 0.09; df = 1, 27; P = 0.773) (Table 1). With acetamiprid treatment and fruit held in a separate incubator, the percentage of A. melinus pupae that survived exposure was also not significantly different between the stage of A. melinus exposure (F = 0.46; df = 1, 26; P = 0.503) or among insecticide treatments (F = 2.97; df = 2, 26; P = 0.069) (Table 2). No effects of pyriproxyfen, buprofezin, or acetamiprid were demonstrated on the development of A. melinus to the pupal stage.
Table 3 shows the number of adult A. melinus that survived 48 h exposure to buprofezin or pyriproxyfen residues following adult emergence from the scale. Numbers of live A. melinus were not significantly different among insecticide treatments or wasp treatment stages (F = 0.01; df = 2, 54; P = 0.994). When data from treatment of all three wasp life stages were combined and reanalyzed, the impact of insecticide treatment remained non-significant (F = 0.62; df = 2, 54; P = 0.542). Thus, there appeared to be no residual effect of either insect growth regulator on adult emergence or survival for 48 h after emergence.
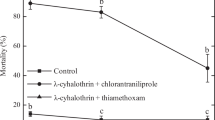
Table 4 shows that the total number of live A. melinus was significantly lower after 48 h exposure to both rates of acetamiprid compared to results with water treated lemons (F = 7.33; df = 2, 40; P = 0.002). No significant difference was found between results observed with acetamiprid treatment of the three wasp life stages (F = 0.50; df = 2, 40; P = 0.608). Thus, data for all three life stages were combined and reanalyzed in aggregate (bottom row, Table 4). Acetamiprid demonstrated a residual impact that significantly reduced survival of adult A. melinus at both the 1 × and 0.1 × field rate concentrations (F = 7.51; df = 2, 40; P = 0.002).
The number of A. melinus alive 48 h after emergence from the water-treated control treatments in the chamber with acetamiprid treated fruit was on average 3.5 ± 0.69 fold lower than that observed with water-treated controls in the chamber containing the insect growth regulator treated fruit (Table 3, 4). Because fruit containing parasitized scale were randomly assigned to treatments, we conclude that acetamiprid can have a significant fumigant effect on A. melinus adults in an enclosed system. The fumigant effect in our study is most likely greater than would be observed in field situations due to field pesticide degradation after exposure to sunlight, wind, and rain in addition to the differences between an enclosed growth chamber and a field setting. Thus, additional studies might examine to what degree fumigation impacts are observed under field conditions.
Impact of insect growth regulators on wasp fecundity
Averaging over the nine replicates, the mean number of offspring per wasp female was 3.93 ± 0.60 (mean ± SEM) for the control, 3.09 ± 0.09 for buprofezin, and 2.93 ± 0.15 for the pyriproxyfen treatment, respectively. Wasp fecundity with treated lemons was not significantly different from that observed with control fruit (χ2 = 5.281, df = 2, P = 0.071). Fecundity following acetamiprid exposure could not be evaluated due to insufficient numbers of live adults surviving 48 h of exposure to this material.
Discussion
Several previous studies have shown that armored scale parasitoids that develop underneath a scale cover are protected from foliar insecticide applications (Rosenheim and Hoy 1988; Raupp et al. 2001). The insect growth regulators, buprofezin and pyriproxyfen, demonstrated no noticeable effects when A. melinus were treated at the egg, larval, or pupal stages, no effect on adult survival 48 h after adult emergence, and no effect on fecundity. In contrast, whereas acetamiprid was nontoxic to immature stages of A. melinus, significant toxicity was documented for emerging adults. The residues may have been absorbed by the adult parasitoid through contact as it walked on the treated surface, through preening, or due to consuming pesticide residues when the parasitoid chewed its way through the scale cover during emergence. This A. melinus colony has been reared for over forty years and may be more susceptible to insecticides than field colonies. Because the insect growth regulators did not affect our laboratory reared A. melinus, field populations are unlikely to be effected. Acetamiprid caused significant mortality of lab reared A. melinus adults in our study, but high acetamiprid toxicity was also observed in field populations of adult A. melinus in a 2004 field study (E.E.G., unpublished data).
Based on our study, releases of A. melinus are compatible with the insect growth regulators pyriproxyfen and buprofezin, but much less so with the neonicotinoid acetamiprid. Although these two insect growth regulators were determined to be safe for several hymenopteran species (Mendel et al. 1994), both pyriproxyfen and buprofezin are highly toxic to Coccinellidae including the vedalia beetle, Rodolia cardinalis (Mulsant), a predator relied on to control cottony cushion scale in citrus in the San Joaquin Valley (Grafton-Cardwell and Gu 2003); Chilocorus nigritus (Fabricius), a major predator of California red scale in South Africa (Magagula and Samways 2000); Chilocorus bipustulatus Linnaeus, a predator of armored and soft scale insects (Mendel et al. 1994); and several other Coccinellidae (Wakgari and Giliomee 2003; Liu and Stansly 2004; Naranjo and Akey 2005). Acetamiprid was also found to be toxic to several Coccinellidae including Stethorus japonicus Kamiya, Harmonia axyridis (Pallas), and R. cardinalis (Grafton-Cardwell and Gu 2003; Naranjo and Akey 2005).
Overall, caution needs to be taken in integrated pest management programs that rely on both pesticide applications and natural enemies for integrated pest control. If possible, the impacts of insecticides on important predator and parasitoid populations should be documented for each insecticide that is used in such a program. Selecting a pesticide with minimal impacts on natural enemies allows biological control to assist in key pest reduction and reduces the likelihood of secondary pest outbreaks. Based on our results, pyriproxyfen and buprofezin appear to be relatively innocuous to A. melinus and may be used to lower California red scale populations prior to the use of augmentative releases of A. melinus. Acetamiprid was found to be toxic to emerging adults and should not be recommended for use in groves where A. melinus plays a key role in management of California red scale.
References
Flint ML, Kobbe B, Clark JK, Dreistadt SH, Pehrson JE Jr, Flaherty DL, O’Connell NV, Phillips PA, Morse JG (1991) Integrated pest management for citrus. 2nd edn. University of California, Division of Agriculture and Natural Resources Publ. 3303, Oakland, CA
Forster LD, Luck RF, Grafton-Cardwell EE (1995) Life stages of California red scale and its parasitoid. University of California, Division of Agriculture and Natural Resources Publ. 21529, Oakland, CA
Grafton-Cardwell EE (1999) Section 18s for California red scale control. Citrograph 84:8–10
Grafton-Cardwell EE, Gu P (2003) Conserving vedalia beetle, Rodolia cardinalis (Mulsant) (Coleoptera: Coccinellidae), in citrus: A continuing challenge as new insecticides gain registration. J Econ Entomol 96:1388–1398
Grafton-Cardwell EE, Reagan CA (2003) California red scale foliar insecticide efficacy trial, 2001. Arth Manag Tests 28 (D4). Web-based
Grafton-Cardwell EE, Reagan CA (2004) Citricola scale insecticide efficacy trial, 2002. Arth. Manag. Tests 29 (D4). Web-based
Grafton-Cardwell EE, Vehrs SLC (1995) Monitoring for organophosphate- and carbamate-resistant armored scale (Homoptera: Diaspididae) in San Joaquin Valley citrus. J Econ Entomol 88:495–504
Grafton-Cardwell EE, Lee JE, Stewart JR, Olsen KD (2006) Role of two insect growth regulators in integrated pest management of citrus scales. J Econ Entomol 99:733–744
Grafton-Cardwell EE, Ouyang Y, Striggow R, Vehrs S (2001) Armored scale insecticide resistance challenges San Joaquin Valley citrus growers. Calif Agric 55:20–25
Haney PB, Morse JG, Luck RF, Griffiths HJ, Grafton-Cardwell EE, O’Connell NV (1992) Reducing insecticide use and energy costs in citrus pest management. University of California Statewide IPM Project Publ. 15, Davis, CA
Ishaaya I, Decock A, Degheele D (1994) Pyriproxyfen, a potent suppressor of egg hatch and adult formation of the greenhouse-whitefly (Homoptera, Aleyrodidae). J Econ Entomol 87:1185–1189
Ishaaya I, Kontsedalov S, Horowitz AR (2005) Biorational insecticides: mechanism and cross-resistance. Arch Insect Biochem Physiol 58:192–199
Ishaaya I, Mendel Z, Blumberg D (1992) Effect of buprofezin on California red scale; Aonidiella aurantii (Maskell); in a citrus orchard. Israel J Entomol 25–26:67–71
Iwasa T, Motoyama N, Ambrose JT, Roe RM (2004) Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee, Apis mellifera. Crop Prot 23:371–378
Izawa Y, Uchida M, Sugimoto T, Asai T (1985) Inhibition of chitin biosynthesis by buprofezin analogs in relation to their activity controlling Nilaparvata lugens Stal. Pestic Biochem Physiol 24:343–347
Liu TX, Stansly PA (1997) Effects of pyriproxyfen on three species of Encarsia (Hymenoptera: Aphelinidae), endoparasitoids of Bemisia argentifolii (Homoptera: Aleyrodidae). J Econ Entomol 90:404–411
Liu TX, Stansly PA (2004) Lethal and sublethal effects of two insect growth regulators on adult Delphastus catalinae (Coleoptera: Coccinellidae), a predator of whiteflies (Homoptera: Aleyrodidae). Biol Control 30:298–305
Luck RF, Forster LD, Morse JG (1997) An ecologically based IPM program for citrus in California’s San Joaquin Valley using augmentative biological control. Proc Intern Soc Citriculture 1996 1:499–503
Magagula CN, Samways MJ (2000) Effects of insect growth regulators on Chilocorus nigritus (Fabricius) (Coleoptera: Coccinellidae), a non-target natural enemy of citrus red scale, Aonidiella aurantii (Maskell) (Homoptera: Diaspididae), in southern Africa: Evidence from laboratory and field trials. Afr Entomol 8:47–56
Mendel Z, Blumberg D, Ishaaya I (1994) Effects of some insect growth-regulators on natural enemies of scale insects (Hom, Coccoidea). Entomophaga 39:199–209
Moreno DS, Luck RF (1992) Augmentative releases of Aphytis melinus (Hymenoptera: Aphelinidae) to suppress California red scale (Homoptera: Diaspidiae) in southern California lemon orchards. J Econ Entomol 85:1112–1119
Morse JG, Luck RF, Grafton-Cardwell EE (2007) The evolution of biologically-based integrated pest management on citrus in California. University of California Plant Protection Quarterly 16(4)/17(1):1–11
Murdoch WW, Luck RF, Swarbrick SL, Walde S, Yu DS, Reeve JD (1995) Regulation of an insect population under biological control. Ecology 76: 206–217
Naranjo SE, Akey DH (2005) Conservation of natural enemies in cotton: Comparative selectivity of acetamiprid in the management of Bemisia tabaci. Pest Manag Sci 61:555–566
Raupp MJ, Homes JJ, Sadof C, Shrewsbury P, Davidson JA (2001) Effects of cover sprays and residual pesticides on scale insects and natural enemies in urban forests. J Arboriculture 27:203–213
Riehl LA, Brooks RF, McCoy CW, Fisher TW, Dean HA (1980) Accomplishments toward improving integrated pest management for citrus. In: Huffaker CB (ed) New technology of pest control. Wiley, New York, NY pp 319–363
Rosenheim JA, Hoy MA (1988) Genetic improvement of a parasitoid biological control agent: Artificial selection for insecticide resistance in Aphytis melinus (Hymenoptera: Aphelinidae). J Econ Entomol 81: 1539–1550
Rothwangl KB, Cloyd RA, Wiedenmann RN (2004) Effects of insect growth regulators on citrus mealybug parasitoid Leptomastix dactylopii (Hymenoptera: Encyrtidae). J Econ Entomol 97:1239–1244
SAS Institute (2004) SAS 9.1.2 Qualification Tools User’s Guide, Version 9 1 2. SAS Institute Inc, Cary, NC
Van Driesche RG, Hoddle MS, Lyon S, Sanderson JP (2001) Compatibility of insect growth regulators with Eretmocerus eremicus (Hymenoptera: Aphelinidae) for whitefly (Homoptera: Alyerodidae) control on poinsettias II. Trials in commercial poinsettia crops. Biol Control 20:132–146
Wakgari WM, Giliomee JH (2003) Natural enemies of three mealybug species (Hemiptera: Pseudococcidae) found on citrus and effects of some insecticides on the mealybug parasitoid Coccidoxenoides peregrinus (Hymenoptera: Encyrtidae) in South Afr. Bull Entomol Res 93:243–254
Yu DS, Luck RF (1988) Temperature-dependent size and development of California red scale (Homoptera: Diaspididae) and its effect on host availability for the ectoparasitoid, Aphytis melinus DeBach (Hymenoptera: Aphelinidae). Environ Entomol 17:154–161
Zar JH (1984) Biostatistical Analysis. Prentice-Hall, Inc, Englewood Cliffs, NJ
Acknowledgements
We thank Lisa Forster, Jan Hare, Lindsay Robinson, Alan Urena, and Pamela Watkins at the University of California, Riverside for technical assistance. This research was supported in part by Citrus Research Board and forms part of the M.S. Thesis of S.M.R.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rill, S.M., Grafton-Cardwell, E.E. & Morse, J.G. Effects of two insect growth regulators and a neonicotinoid on various life stages of Aphytis melinus (Hymenoptera: Aphelinidae). BioControl 53, 579–587 (2008). https://doi.org/10.1007/s10526-007-9097-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-007-9097-x