Abstract
Muscle aging is closely related to unhealthy late-life and organismal aging. Recently, the state of differentiated cells was shown to be critical to tissue homeostasis. Thus, understanding how fully differentiated muscle cells age is required for ensuring healthy aging. Adult Drosophila muscle is a useful model for exploring the aging process of fully differentiated cells. In this study, we investigated age-related changes of γH2AX, an indicator of DNA strand breaks, in adult Drosophila muscle to document whether its changes are correlated with muscle degeneration and lifespan. The results demonstrate that γH2AX accumulation increases in adult Drosophila thoracic and leg muscles with age. Analyses of short-, normal-, and long-lived strains indicate that the age-related increase of γH2AX is closely associated with the extent of muscle degeneration, cleaved caspase-3 and poly-ubiquitin aggregates, and longevity. Further analysis of muscle-specific knockdown of heterochromatin protein 1a revealed that the excessive γH2AX accumulation in thoracic and leg muscles induces accelerated degeneration and decreases longevity. These data suggest a strong correlation between age-related muscle damage and lifespan in Drosophila. Our findings indicate that γH2AX may be a reliable biomarker for assessing muscle aging in Drosophila.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tissue aging is linked with longevity. Particularly, muscle aging is closely related to the occurrence of age-related diseases and unhealthy late-life conditions (Nair 2005; Demontis et al. 2013). A cohort study revealed that decline in muscle function can elevate mortality (Newman et al. 2006). Skeletal muscle is a tissue with low cellular turnover and high regenerative potential (Rando 2006). Recently, the state of differentiated muscle cells was shown to be critical to muscle homeostasis (Gopinath and Rando 2008).
Drosophila muscle is a suitable model for studying aging of fully differentiated muscle cells. Adult Drosophila muscle is composed of highly differentiated muscle cells and contains no stem cells (Daczewska et al. 2010). Moreover, adult flies have a short lifespan, and longevity strains with different lifespans have revealed that climbing activity is dependent on lifespan (Piazza et al. 2009). Additionally, muscular degenerative markers such as poly-ubiquitin aggregates are available for studying muscle aging in Drosophila (Demontis and Perrimon 2010).
DNA damage is an important contributor to aging (Li et al. 2008; Moskalev et al. 2013). Progressive accumulation of DNA damage induces physiological changes that disrupt various regulatory mechanisms that maintain cellular or tissue integrity (Freitas and de Magalhães 2011). Among the various types of DNA damage, strand breaks are known to trigger apoptotic cell death (Kaina 2003). In fact, compared to other organs, muscle is especially sensitive to DNA damage (Garcia et al. 2010), and progressive loss of muscle mass and function accompanying apoptotic cell death is regarded as a key feature of muscle aging (Du et al. 2004; Zheng et al. 2005; Marzetti and Leeuwenburgh 2006; Augustin and Partridge 2009; Cruz-Jentoft et al. 2010). However, the correlation between muscle degeneration and nuclear DNA damage in differentiated muscle cells remains unclear.
Histone variant H2AX is phosphorylated on Serine 139 by ataxia telangiectasia mutated (ATM) or ataxia telangiectasia and Rad3-related (ATR) in response to DNA strand breaks to form γH2AX (Rogakou et al. 1998; Tanaka et al. 2006). Due to its role in sensing DNA damage, γH2AX formation is regarded as an early indicator of damage (Mah et al. 2010). Gamma-H2AX foci are reversibly generated a few minutes after application of a genotoxic agent and subsequently eliminated in 24–48 h in typical cells (Redon et al. 2011). Recent studies have reported that γH2AX is a marker for aging in various tissues and organisms (Wang et al. 2009; Mah et al. 2010; Park et al. 2012; Bopp et al. 2013).
DNA damage accumulation is dependent on chromatin state (Burgess et al. 2012). Loss of heterochromatin components induces genomic instability phenotypes and increases DNA double-strand breaks (DSBs) (Peng and Karpen 2009), and heterochromatin levels have been reported to decline with age (O’Sullivan and Karlseder 2012). Recent studies using flies to examine the knockdown of heterochromatin protein 1 (HP1) reported that loss of heterochromatin shortens lifespan (Larson et al. 2012). Furthermore, loss of heterochromatin-associated proteins, including HP1, makes DNA susceptible to damage (Falk et al. 2008) and can induce genomic instability due to their roles in chromatin compaction and genome protection (Yan et al. 2011; Papamichos-Chronakis and Peterson 2013). A study in Drosophila larval tissues reported that loss of HP1a induces the increase of γH2AX formation (Yan et al. 2011).
In this study, we investigated age-related changes in the levels of γH2AX in adult Drosophila muscle and their significance with respect to muscle aging and longevity.
Materials and methods
Fly stocks
Fly stocks were maintained at 23–25 °C on standard food [79.2 % water, 1 % agar, 7 % cornmeal, 2 % yeast, 10 % sucrose, 0.3 % bokinin (butylparaben, anti-bacterial and anti-fungal additive), and 0.5 % propionic acid] under a 12/12 h light/dark cycle. Female flies were used in the experiments, and Oregon-R (OR) flies were used as wild-type. w 1118, long-lived (La) and normal-lived (Ra) strains were carefully maintained as previously reported (Arking 1987). w 1118 flies were used as control for La and Ra. Mef2-Gal4 (#27390), Mhc-Gal4 (#38464), and UAS-GFP (#4775) strains were obtained from the Bloomington Drosophila Stock Center (Indiana University, Bloomington, IN, USA). UAS-HP1a-RNAi (#31994), UAS-ATM-RNAi (#22502), and UAS-ATR-RNAi (#103624) strains were obtained from the Vienna Drosophila RNAi Center (VDRC, Vienna, Austria).
Immunochemistry
To obtain thorax muscles, thorax tissues were fixed in 4 % paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) for 2 h at room temperature and infiltrated with 20 % sucrose overnight at 4 °C. After freezing in Tissue-Tec OCT medium (SAKURA, West Chester, PA, USA), 12-μm thick sections were obtained by Leica CM1850 cryostat (Leica Microsystems, Wetzlar, Germany). Sections were blocked in 1 % bovine serum albumin (BSA) for 30 min and incubated overnight with primary antibodies, washed with phosphate-buffered saline (PBS), and then incubated for 1 h with secondary antibodies. After washing with PBS, samples were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) and analyzed using a Zeiss AxioSkop 2plus microscope (Carl Zeiss, Gottingen, Germany).
Dissected mid-leg muscles were fixed for 30 min in 4 % paraformaldehyde at room temperature then washed with PBS supplemented with 0.1 % Triton X-100 (PBST). The samples were incubated overnight with primary antibodies at 4 °C. After washing with PBST, the samples were incubated for 1 h with secondary antibodies at room temperature, washed in PBST, mounted with Vectashield, and analyzed using a Zeiss AxioSkop 2plus microscope.
Antisera
Antibodies for immunostaining were diluted as follows: rabbit anti-γH2AvD (the Drosophila ortholog of γH2AX; Rockland Immunochemicals, Limerick, PA, USA, 1:1000), mouse anti-mono and poly-ubiquitin (Enzo Life Sciences, Farmingdale, NY, USA, 1:1000), rabbit anti-cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA, 1:400), mouse anti-HP1 (DSHB, Iowa City, IA, USA, 1:100), rabbit anti-4-hydroxynonenal (Abcam, Cambridge, UK, 1:400), and rhodamine-phalloidin (Invitrogen, Grand Island, NY, USA, 1:400). Secondary antibodies included Cy3-conjugated goat anti-rabbit, FITC-conjugated goat anti-rabbit, and FITC-conjugated goat anti-mouse (Jackson ImmunoResearch, West Grove, PA, USA, 1:400). DAPI (Molecular Probes, Grand Island, NY, USA, 1:400) was used to detect DNA.
Ex vivo paraquat treatment
Dissected leg muscles were treated with 10 mM paraquat (PQ) in Shields and Sang M3 Insect Medium (WelGene, Daegu, Korea) for 1 h. Samples were subsequently fixed and immunostained as described above. Control samples were treated only with M3 media.
γ-Irradiation
Adult flies were irradiated with a γ-irradiation machine (137CS, 21.275tBq [575 Ci], MDS Nordion International, Kanata, Canada) at a rate of 2.55 Gy/min.
Climbing assay
Analysis of climbing activity was performed as previously described (Gargano et al. 2005). Adult flies were anesthetized, and approximately 30 flies were loaded into plastic vials. After 12 h, flies were tapped to the bottoms of the vials, and climbing flies were recorded by Sony NEX-F3 digital camera (Sony, Tokyo, Japan). The heights of flies 4 s after initiation of climbing were measured by analyzing the recordings.
Measurement of lifespan
To measure lifespans, 25–30 adult flies were cultured in a vial and transferred into new vials containing fresh food every 2–3 days. Dead flies were counted every 2–3 days.
Measurement of mean fluorescence
To measure the mean fluorescence of γH2AvD signals, 15–20 nuclei per sample were selected and analyzed by Adobe Photoshop CS3 extended software (Adobe Systems, San Jose, CA, USA). The value was normalized to randomly selected background fluorescence intensity.
Quantitative analysis of foci number
To quantitatively analyze the number of poly-ubiquitin and cleaved caspase-3 foci, images were analyzed by ImageJ (NIH, Bethesda, MD, USA). The number of foci was counted automatically by the Analyze Particle tool.
Statistical analysis
Quantified data are expressed as the mean ± SEM values. Significance testing was conducted using Student’s t test (unpaired, two-sided t test). All data analyses and plotting were conducted by GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA).
Results
Increased γH2AX in aged Drosophila muscles
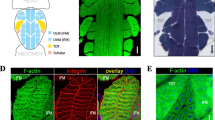
To determine whether γH2AX accumulates in Drosophila muscle with age, we stained the thoracic and leg muscles of OR flies with an anti-γH2AvD antibody. To confirm the γH2AvD signal in muscle, we treated flies with γ-irradiation, a DSB inducer. After 20 Gy irradiation, we detected increased γH2AvD foci in leg muscles of OR young (5-days-old) flies (Fig. S1). The level of γH2AvD also increased after ex vivo treatment with PQ, an oxidative stress inducer (Fig. S2a). Then, we stained the thoracic and leg muscles of OR young and aged flies with an anti-γH2AvD antibody. The level of γH2AvD in aged (30-days-old) flies was increased compared to young (5-days-old) flies in thoracic muscle (Fig. 1a, b). Similarly, we observed a gradual increase of γH2AvD in 30- and 60-days-old leg muscles compared to 5-days-old leg muscles (Fig. 1c–e). To compare γH2AvD levels accurately, we quantified the mean γH2AvD fluorescence in each DAPI region (Fig. 1f). The age-related increase of γH2AvD was only absent in muscle with ATM knockdown (Fig. S3a), indicating an ATM-dependent increase of γH2AvD in muscle but no dependence on ATR. Leg muscles of aged (40-days-old) flies showed much stronger level of γH2AvD than young (5-days-old) flies after 20 Gy irradiation (Fig. S1) indicating increased sensitivity to DNA damage in aged muscle. These results indicate that γH2AX levels increase in adult thoracic and leg muscles during aging.
γH2AvD accumulation in thoracic and leg muscles with age. a, b Immunostaining with anti-γH2AvD antibody (red) was performed in thoracic muscles of OR adult females. The level of γH2AvD in thoracic muscle nuclei was increased in 30-days-old compared to 5-days-old adult. c–e The age-related increase of γH2AvD was also detected in leg muscle. The γH2AvD level increased gradually from 5- to 60-days-old tissue. f The γH2AvD mean fluorescence was measured in selected nuclei of thoracic and leg muscles (5- and 30-days-old thorax, n = 64 and 80, respectively; 5-, 30-, and 60-days-old leg, n = 150, 150, and 150, respectively). Data are given as mean ± SEM, ***p < 0.001, two-tailed unpaired t test. Asterisks indicate significance compared to the 5-days-old control. (Color figure online)
Age-related degenerative markers in Drosophila muscle
To understand the significance of increased γH2AX in muscle, we set up muscular degenerative markers. We first measured the activity of caspase-3, the key protease in apoptosis (Nicholson and Thornberry 1997), as a muscular degenerative marker. OR thoracic muscles were stained with an anti-cleaved caspase-3 antibody, which detects the active form of caspase-3 (Kumar 2007). As expected, the number of cleaved caspase-3 foci showed an age-dependent increase. A robust increase in the number of foci was detected in muscle of 50-days-old flies, exceeding that in 5- and 30-days-old flies (Fig. 2a–c, g). This indicates that caspase-3 activity increases in aged thoracic muscle.
Age-related increase of degenerative markers in thoracic muscle. Immunostaining was performed on cryosections in 5-, 30-, and 50-days-old OR adult female thoracic muscle. Thoracic muscle was stained for nuclei (DAPI, blue), F-actin (rhodamine-phalloidin, red), a–c cleaved caspase-3 (green), and d–f poly-ubiquitin (green). Abundant foci were observed in aged thoracic muscle. g, h The foci were counted in a selected region of thoracic muscle (5-, 30-, and 50-days-old thorax, n = 8, 6, and 8, respectively). i Co-localization of cleaved caspase-3 (red) and poly-ubiquitin (green) is shown. Arrows indicate co-localization. All images display transverse views. Data are given as the mean ± SEM, *p < 0.05, ***p < 0.001, two-tailed unpaired t test. Asterisks indicate significance compared to the 5-days-old control. (Color figure online)
We also assessed poly-ubiquitin aggregates for damaged protein accumulation. As previously reported (Demontis and Perrimon 2010), we observed an age-dependent increase of poly-ubiquitin aggregates in OR thoracic muscle (Fig. 2d–f, h). Because caspase-3 affects muscular protein degradation through its protease activity associated with the ubiquitin–proteasome degradation pathway (Du et al. 2004; Wang et al. 2010), we co-immunostained cleaved caspase-3 and poly-ubiquitin in aged thoracic muscle. Interestingly, cleaved caspase-3 foci co-localized with poly-ubiquitin aggregates (Fig. 2i). Four-hydroxynonenal (4-HNE) foci, a marker of oxidative damage (Dalleau et al. 2013), also showed an age-related increase and co-localized with poly-ubiquitin aggregates (Fig. S2b). These results indicate that the levels of cleaved caspase-3 and poly-ubiquitin aggregates are useful as muscular degenerative markers in this study.
Correlation among γH2AX levels, muscle degenerative markers, and organismal aging in longevity strains
We investigated whether the γH2AX level is correlated with muscular degenerative markers and organismal aging. For this purpose, we used finely selected longevity strains with short- (w 1118), normal- (Ra), or long-lived (La) lifespans (Arking 1987; Soh et al. 2007).
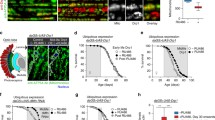
The age-related changes of γH2AX levels were examined in 5-, 30-, and 50-days-old thoracic and leg muscles of longevity strains by using the anti-γH2AvD antibody. In immunohistochemical images and quantified data, La shows no increase in γH2AvD, whereas w 1118 and Ra show age-related increases of γH2AvD in thoracic muscle (Fig. 3a–c, g, S4). In leg muscle, all three strains showed age-related increases of γH2AvD; however, the lowest and highest rates of increase were observed for La and w 1118, respectively (Fig. 3d–f, h, S5). These results indicate that the age-related increase of γH2AvD in muscle is related to organismal aging.
γH2AvD accumulation in thoracic and leg muscles of longevity strains. Immunostaining with anti-γH2AvD antibody (red) was performed in a–c thoracic and d–f leg muscles of three longevity strains: w 1118, Ra, and La. The level of γH2AvD in 50-days-old leg muscle is lowest in La and highest in w 1118. g, h The exact comparison was achieved by measuring the γH2AvD fluorescence in thoracic (w 1118, Ra, and La: 5-days-old, n = 50, 40, and 40; 30-days-old, n = 50, 40, and 40; 50-days-old, n = 50, 60, and 60, respectively) and leg muscle (w 1118, Ra, and La: 5-days-old, n = 75, 60, and 60; 30-days-old, n = 60, 60, and 60; 50-days-old, n = 60, 45, and 45, respectively). Nuclei (DAPI, blue) were visualized. Data are given as the mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed unpaired t test. (Color figure online)
Then, we examined the age-related changes of caspase-3 activity and ubiquitin aggregates in longevity strains. In w 1118 and Ra, age-related increases of the number of cleaved caspase-3 foci were observed in thoracic muscle. w 1118 showed the most rapid increase in the number of foci, but the La strain showed no significant increase in aged muscle (Fig. 4a–c, g, S6). Age-related increases in poly-ubiquitin aggregates were detected in all three longevity strains. Whereas w 1118 has the most abundant foci in aged muscle, La showed only a slightly increased level of poly-ubiquitin aggregates (Fig. 4d–f, h, S7). These levels of cleaved caspase-3 and poly-ubiquitin aggregates in the three longevity strains indicate that age-related increases of caspase-3 and poly-ubiquitin aggregates in muscle are related to organismal aging. Altogether, these results indicate that the γH2AX level is correlated with muscular degenerative markers as well as organismal aging.
Increase of degenerative markers in thoracic muscle of longevity strains. Immunostaining was performed on cryosections from w 1118, Ra, and La thoracic muscle. Nuclei (DAPI, blue) and F-actin (rhodamine-phalloidin, red) were visualized. a–c Cleaved caspase-3 (green) was stained in 50-days-old thoracic muscle. w 1118 tissue exhibits the most abundant foci while La tissue has few foci. d–f Poly-ubiquitin (green) was stained in 50-days-old thoracic muscle. La tissue has remarkably fewer foci than w 1118 and Ra. For quantification, g cleaved caspase-3 (w 1118, Ra, and La: 5-days-old, n = 15, 11, and 12; 30-days-old, n = 12, 11, and 12; 50-days-old, n = 7, 6, and 8, respectively) and h poly-ubiquitin foci (w 1118, Ra, and La: 5-days-old, n = 14, 12, and 12; 30-days-old, n = 13, 12, and 11; 50-days-old, n = 7, 6, and 7, respectively) were counted in selected regions of thoracic muscle. Data are given as the mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed unpaired t test. (Color figure online)
Correlation among γH2AX level, muscle degenerative markers, and organismal aging in muscle-specific HP1a-knockdown flies
To test whether γH2AX level is correlated with muscular degenerative markers and organismal aging, we also examined flies with muscle-specific knockdown of HP1a by using muscle-specific Gal4 drivers (Fig. S8). Flies with muscle-specific HP1a knockdown showed significant loss of HP1 in thoracic and leg muscles (Fig. 5, S9a). Moreover, we observed that muscle-specific knockdown of HP1a shortens lifespan compared to control flies (Fig. S10a). We observed that flies with muscle-specific HP1a knockdown have a rapid, age-related increase of the γH2AvD signal in thoracic and leg muscles compared to control flies (Fig. 5). This indicates that HP1 depletion in muscle induces increased γH2AvD levels.
Increased γH2AvD in flies with muscle-specific HP1a knockdown. Immunostaining with anti-γH2AvD (red) and anti-HP1 (green) antibodies was performed for flies with muscle-specific knockdown of HP1a knockdown (+/+; Mef2-Gal4/UAS-HP1a-RNAi). a, b In 7-days-old thoracic muscle of HP1a-knockdown flies, HP1 was successfully depleted, and the γH2AvD level was higher than in the control (+/+; Mef2-Gal4/+). c, d Increased γH2AvD was also detected in 5-days-old leg muscle of HP1a-knockdown flies with depletion of HP1 in nuclei. e The γH2AvD fluorescence in leg muscle nuclei was measured (5- and 30-days-old: control, n = 90 and 90, respectively; HP1a knockdown, n = 90 and 90, respectively). Data are given as the mean ± SEM, ***p < 0.001, two-tailed unpaired t test. (Color figure online)
We next examined muscular degeneration by observing levels of cleaved caspase-3 and ubiquitin aggregates. The number of cleaved caspase-3 foci in 30-days-old muscle with HP1a knockdown strikingly increased compared to the control, whereas 5-days-old thoracic muscle with HP1a knockdown was similar to control (Fig. 6a–c). Similarly, 30-days-old flies with muscle-specific knockdown of HP1a showed a great increase in the number of ubiquitin aggregate foci in thoracic muscle compared to the control, although there was no significant difference between 5-days-old control and HP1a-knockdown flies (Fig. 6d–f, S9b, S10b). This further supports the correlation between high levels of γH2AvD and increased degenerative markers in muscle.
Increase of degenerative markers in flies with muscle-specific HP1a knockdown. Degenerative markers, nuclei (DAPI, blue), and F-actin (rhodamine-phalloidin, red) were stained in 30-days-old thoracic muscles of flies with muscle-specific knockdown of HP1a. a, b 30-days-old flies had a higher number of cleaved caspase-3 foci (green) in HP1a-knockdown muscle than in control muscle. c Cleaved caspase-3 foci were counted in a selected region of the transverse view (5- and 30-days-old: control, n = 15 and 13, respectively; HP1a knockdown, n = 14 and 15, respectively). d, e The number of poly-ubiquitin aggregate foci (green) was also higher in HP1a knockdown muscle than in control muscle. f The poly-ubiquitin foci were counted in a selected region of the transverse view (5- and 30-days-old: control, n = 15 and 13, respectively; HP1a knockdown, n = 14 and 15, respectively). Data are given as the mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed unpaired t test. g Decreased climbing activity was detected in 1- to 5-week-old flies with muscle-specific HP1a knockdown (control and HP1a knockdown: 1-week-old, n = 10 and 5; 2-week-old, n = 14 and 10; 3-week-old, n = 15 and 10; 4-week-old, n = 10 and 6; 5-week-old, n = 5 and 5, respectively). Data are given as the mean ± SD, *p < 0.05, ***p < 0.001, two-tailed unpaired t test. (Color figure online)
In addition, we assessed the effects of HP1a depletion on climbing activity. Control flies showed decreases in climbing activity between 1- and 5-week-old female adults. However, the climbing activity of HP1a-knockdown flies decreased more sharply than that of the controls (Fig. 6g). These results support our previous findings that increased γH2AX is correlated with muscular aging as well as organismal aging.
Discussion
In the present study, we demonstrated an age-related increase of DNA damage in fully differentiated Drosophila muscle cells as well as a robust correlation between muscle damage and longevity.
The age-related increase of γH2AX in muscle is likely to be proximately due to oxidative stress. Oxidative stress is regarded as a significant cause of DNA damage and physiological decline in function during aging (Finkel and Holbrook 2000), and muscle tissue shows spontaneous production of oxidative stress (Powers et al. 2010). Our finding that 4-HNE shows an age-dependent increase in muscle further supports the role of endogenous oxidative damage in muscle tissue (Fig. S2b). The La strain exhibits a significantly lower level of γH2AX in aged muscle and has higher expression of genes involved in antioxidant defense than the normal-lived Ra strain, which accounts for the extended longevity phenotype of the La strain (Fig. 3, S4, S5) (Arking 2001). Mitochondria from the La strain produce significantly lower levels of ROS in vitro relative to Ra mitochondria (Ross 2000). Cybrid flies with Ra nuclear genomes and La mitochondrial genomes showed an extended longevity phenotype, suggesting mitochondria as a key determinant of longevity (Soh et al. 2007). Additionally, our data showed that ex vivo treatment with PQ induces a significant increase of γH2AX levels in muscle (Fig. S2a). Therefore, age-related mitochondrial dysfunction may be a factor underlying the age-related increase of γH2AX in muscles.
One of our major questions we were asking is whether the γH2AX levels can be used as a biomarker of muscle function in Drosophila. Our data showed an age-related increase of γH2AX accompanying the increase of degenerative markers. As shown in the La strain, when the γH2AX level did not increase significantly, the number of foci indicating degenerative markers also did not increase (Fig. 4, S6, S7). Conversely, excessive γH2AX formation induced by muscle-specific HP1a knockdown leads to increased accumulation of muscle degenerative markers (Fig. 6, S9). These results are similar to previous work showing that loss of heterochromatin with excessive DNA damage accumulation and genomic instability is a crucial cause of neurodegenerative disease in Drosophila brain tissues, which is also composed of fully differentiated cells (Frost et al. 2014). In murine study, it was reported that DNA damage in muscle occurs prior to muscle degeneration (Sandri et al. 1995). And decreased DNA damage accumulation in myostatin knockout mice showed decreased muscle degeneration (Sriram et al. 2014). Therefore, our data suggest that nuclear DNA damage, specifically DSBs represented by γH2AX is closely linked to age-related macromolecular changes represented by degenerative markers.
The correlation between γH2AX and muscle degeneration can be explained in terms of the DNA damage repair mechanism in differentiated cells. Age-related phenotypes differ between proliferative midgut stem cells and differentiated muscle cells with increased γH2AX formation, and this difference may be associated with the DNA repair capacity and the outcome of the DNA damage response. Formation of γH2AX is an early response of DSB repair (Tanaka et al. 2006), after which cells repair damage and then re-enter either the cell cycle or senescence and death (Bartek and Lukas 2007). Pyo et al. (2014) showed that γH2AX disappeared within 24 h after irradiation of highly proliferative Drosophila intestinal stem cells (ISCs), indicating a rapid repair process that is consistent with other observations (Redon et al. 2011). Moreover, aged Drosophila ISCs show increased proliferative activity despite increased γH2AX formation (Park et al. 2012; Na et al. 2013). In differentiated muscle cells, however, factors involved in DNA damage repair are downregulated compared to their counterparts in proliferative cells (Fortini and Dogliotti 2010; Szczesny et al. 2010) and muscle cells have slow kinetics of γH2AX formation after DNA damage (Fortini et al. 2012). Our finding that irradiation-induced γH2AX remained present for 168 h in Drosophila muscle (Fig. S3) further indicates a slow and inactive repair process. Generally, DNA repair ends 48 h after inducing damage, and persistent damage repair foci can promote senescence-associated gene expression changes (Fortini et al. 2012; Rodier et al. 2011). Despite cellular senescence is generally termed in proliferating cells, it was reported that fully differentiated neuron cell showed senescence-like phenotype with age (Jurk et al. 2012). Therefore, although it needs more precise and diverse approaches for the possibility of senescence phenotypes in differentiated muscle cell, tissue degeneration may result from distinct DNA damage repair in fully differentiated muscle cells.
We observed that flies with muscle specific ATM knockdown are defective in γH2AX formation in muscle. These flies show more rapid increase of degenerative markers in muscle with age than control or ATR knockdown (Fig. S3b–e). The phenotypes of flies with muscle specific ATM knockdown may indicate that γH2AX formation is required in muscle during aging. Increased degenerative markers in muscle with ATM knockdown may be also due to γH2AX independent role of ATM, since it was reported that extranuclear localized ATM negatively regulates cellular oxidative stress level (Reichenbach et al. 2002; Watters et al. 1999).
It is well known that tissue interactions seem to coordinate and regulate systemic aging via endocrine signals (Russell and Kahn 2007). Recent work in Drosophila has shown that muscle has the capacity to significantly alter organismal longevity by either autonomous or non-autonomous processes involving other types of signals (Demontis et al. 2014; Bai et al. 2013; Owusu-Ansah et al. 2013). It seems as if muscle-autonomous signal can result in preserving muscle functions, but that muscle non-autonomous signals can result in extended longevity of the organism. In the present study, we demonstrated a close association between the γH2AX level in muscle and longevity: longer-lived strains experience a much slower increase of γH2AX in muscle tissue (Fig. 3, S4, S5). Furthermore, DNA damage accumulation in muscle by muscle-specific HP1a knockdown supports the association between γH2AX and longevity, implying accelerated organismal aging (Fig. S10). Our findings are in line with previous evidence that excessive DNA damage accumulation can shorten organismal longevity (reviewed in Moskalev et al. 2013). The γH2AX level provides a cross-sectional molecular biomarker which can be usefully combined with the longitudinal climbing assay to deliver an integrated view of flight and/or leg muscle function in Drosophila. Its use also should allow the specific identification of the signaling pathways involved in the experimental modulation of the DNA damage response in Drosophila muscle and other tissues.
Notably, cleaved caspase-3 was detected in thoracic muscle but not in leg muscle (data not shown). Drosophila thoracic and leg muscles are similar to mammalian slow-twitch and fast-twitch muscles, respectively (Taylor 2006). Our results may indicate a difference in the caspase-3 pathways of thoracic and leg muscle, and further investigation is required to determine the source of these differences.
In summary, we showed an age-related increase of γH2AX in fully differentiated Drosophila muscle and its correlation with degenerative markers, cleaved caspase-3 and poly-ubiquitin aggregates, and longevity. We suggest that aging in differentiated muscle cell is a crucial determinant of organismal aging. Our data indicates that γH2AX can be a biomarker of muscle aging in Drosophila differentiated muscle cells in further studies for interventions of muscle aging and healthy aging.
References
Arking R (1987) Successful selection for increased longevity in Drosophila: analysis of the survival data and presentation of a hypothesis on the genetic regulation of longevity. Exp Gerontol 22:199–220
Arking R (2001) Gene expression and regulation in the extended longevity phenotypes of Drosophila. Ann NY Acad Sci 928:157–167
Augustin H, Partridge L (2009) Invertebrate models of age-related muscle degeneration. Biochim Biophys Acta 1790:1084–1094
Bai H, Kang P, Hernandez A, Tatar M (2013) Activin signaling targeted by Insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet 9:e1003941
Bartek J, Lukas J (2007) DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 19:238–245
Bopp A, Wartlick F, Henninger C, Kaina B, Fritz G (2013) Rac1 modulates acute and subacute genotoxin-induced hepatic stress responses, fibrosis and liver aging. Cell Death Dis 4:e558
Burgess RC, Misteli T, Oberdoerffer P (2012) DNA damage, chromatin, and transcription: the trinity of aging. Curr Opin Cell Biol 24:724–730
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Daczewska M, Picchio L, Jagla T, Figeac N, Jagla K (2010) Muscle development and regeneration in normal and pathological conditions: learning from Drosophila. Curr Pharm Des 16:929–941
Dalleau S, Baradat M, Gueraud F, Huc L (2013) Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell Death Differ 20:1615–1630
Demontis F, Perrimon N (2010) FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143:813–825
Demontis F, Piccirillo R, Goldberg AL, Perrimon N (2013) The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12:943–949
Demontis F, Patel V, Swindell W, Perrimon N (2014) Intertissue control of the nucleolus via a myokine-dependent longevity pathway. Cell Rep 12:1481–1494
Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113:115–123
Falk M, Lukasova E, Kozubek S (2008) Chromatin structure influences the sensitivity of DNA to gamma-radiation. Biochim Biophys Acta 1783:2398–2414
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Fortini P, Dogliotti E (2010) Mechanisms of dealing with DNA damage in terminally differentiated cells. Mutat Res 685:38–44
Fortini P, Ferretti C, Pascucci B, Narciso L, Pajalunga D, Puggioni E, Castino R, Isidoro C, Crescenzi M, Dogliotti E (2012) DNA damage response by single-strand breaks in terminally differentiated muscle cells and the control of muscle integrity. Cell Death Differ 19:1741–1749
Freitas AA, De Magalhães JP (2011) A review and appraisal of the DNA damage theory of ageing. Mutat Res 728:12–22
Frost B, Hemberg M, Lewis J, Feany M (2014) Tau promotes neurodegeneration through global chromatin relaxation. Nat Neurosci 17:357–366
Garcia A, Calder R, Dollé M, Lundell M, Kapahi P, Vijg J (2010) Age- and temperature-dependent somatic mutation accumulation in Drosophila melanogaster. PLoS Genet 6:e1000950
Gargano JW, Martin I, Bhandari P, Grotewiel MS (2005) Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 40:386–395
Gopinath S, Rando T (2008) Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell 7:590–598
Jurk D, Wang C, Miwa S, Maddick M, Korolchuk V, Tsolou A, Gonos ES, Thrasivoulou C, Saffrey MJ, Cameron K, von Zglinicki T (2012) Postmitotic neurons develop a p21-dependent senescence-like phenotype driven by a DNA damage response. Aging Cell 11:996–1004
Kaina B (2003) DNA damage-triggered apoptosis: critical role of DNA repair, double-strand breaks, cell proliferation and signaling. Biochem Pharmacol 66:1547–1554
Kumar S (2007) Caspase function in programmed cell death. Cell Death Differ 14:32–43
Larson K, Yan SJ, Tsurumi A, Liu J, Zhou J, Gaur K, Guo D, Eickbush TH, Li WX (2012) Heterochromatin formation promotes longevity and represses ribosomal RNA synthesis. PLoS Genet 8:e1002473
Li H, Mitchell JR, Hasty P (2008) DNA double-strand breaks: a potential causative factor for mammalian aging? Mech Ageing Dev 129:416–424
Mah LJ, El-Osta A, Karagiannis T (2010) GammaH2AX as a molecular marker of aging and disease. Epigenetics 5:129–136
Marzetti E, Leeuwenburgh C (2006) Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol 41:1234–1238
Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE (2013) The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 12:661–684
Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, Yoo MA (2013) Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev 134:381–390
Nair KS (2005) Aging muscle. Am J Clin Nutr 81:953–963
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61:72–77
Nicholson DW, Thornberry NA (1997) Caspases: killer proteases. Trends Biochem Sci 22:299–306
O’Sullivan RJ, Karlseder J (2012) The great unravelling: chromatin as a modulator of the aging process. Trends Biochem Sci 37:466–476
Owusu-Ansah E, Song W, Perrimon N (2013) Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155:699–712
Papamichos-Chronakis M, Peterson C (2013) Chromatin and the genome integrity network. Nat Rev Genet 14:62–75
Park JS, Lee SH, Na HJ, Pyo JH, Kim YS, Yoo MA (2012) Age- and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp Gerontol 47:401–405
Peng JC, Karpen GH (2009) Heterochromatic genome stability requires regulators of histone H3 K9 methylation. PLoS Genet 5:e1000435
Piazza N, Gosangi B, Devilla S, Arking R, Wessells R (2009) Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One 4:e5886
Powers SK, Duarte J, Kavazis AN, Talbert EE (2010) Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol 95:1–9
Pyo JH, Park JS, Na HJ, Jeon HJ, Lee SH, Kim JG, Park SY, Jin YW, Kim YS, Yoo MA (2014) Functional modification of Drosophila intestinal stem cells by ionizing radiation. Radiat Res 181:376–386
Rando T (2006) Stem cells, ageing and the quest for immortality. Nature 441:1080–1086
Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, Bonner WM (2011) Recent developments in the use of gamma-H2AX as a quantitative DNA double-strand break biomarker. Aging 3:168–174
Reichenbach J, Schubert R, Schindler D, Müller K, Böhles H, Zielen S (2002) Elevated oxidative stress in patients with ataxia telangiectasia. Antioxid Redox Signal 4:465–469
Rodier F, Muñoz D, Teachenor R, Chu V, Le O, Bhaumik D, Coppé JP, Campeau E, Beauséjour CM, Kim SH, Davalos AR, Campisi J (2011) DNA-SCARS: distinct nuclear structures that sustain damage-induced senescence growth arrest and inflammatory cytokine secretion. J Cell Sci 124:68–81
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273:5858–5868
Ross RE (2000) Age-specific decrease in aerobic efficiency associated with increase in oxygen free radical production in Drosophila melanogaster. J Insect Physiol 46:1477–1480
Russell S, Kahn C (2007) Endocrine regulation of ageing. Nat Rev Mol Cell Biol 8:681–691
Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, Franceschi C (1995) Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett 373:291–295
Soh JW, Hotic S, Arking R (2007) Dietary restriction in Drosophila is dependent on mitochondrial efficiency and constrained by pre-existing extended longevity. Mech Ageing Dev 128:581–593
Sriram S, Subramanian S, Juvvuna P, McFarlane C, Salerno M, Kambadur R, Sharma M (2014) Myostatin induces DNA damage in skeletal muscle of Streptozotocin-induced type 1 diabetic mice. J Biol Chem 289:5784–5798
Szczesny B, Tann A, Mitra S (2010) Age- and tissue-specific changes in mitochondrial and nuclear DNA base excision repair activity in mice: susceptibility of skeletal muscles to oxidative injury. Mech Ageing Dev 131:330–337
Tanaka T, Halicka H, Huang X, Traganos F, Darzynkiewicz Z (2006) Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle 5:1940–1945
Taylor MV (2006) Comparison of muscle development in Drosophila and vertebrates. In: Sink H (ed) Muscle development in Drosophila. Landes Bioscience, Springer, New York, pp 169–203
Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, Von Zglinicki T (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8:311–323
Wang XH, Zhang L, Mitch WE, LeDoux JM, Hu J, Du J (2010) Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J Biol Chem 285:21249–21257
Watters D, Kedar P, Spring K, Bjorkman J, Chen P, Gatei M, Birrell G, Garrone B, Srinivasa P, Crane D, Lavin M (1999) Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem 274:34277–34282
Yan SJ, Lim SJ, Shi S, Dutta P, Li WX (2011) Unphosphorylated STAT and heterochromatin protect genome stability. FASEB J 25:232–241
Zheng J, Edelman SW, Tharmarajah G, Walker DW, Pletcher SD, Seroude L (2005) Differential patterns of apoptosis in response to aging in Drosophila. Proc Natl Acad Sci USA 102:12083–12088
Acknowledgments
We thank Prof. Byung P. Yu (University of Texas Health Science Center) for invaluable comments on the manuscript. This work was supported by the R&D program of MOTIE/KEIT (10040391, Development of Functional Food Materials and Device for Prevention of Aging-associated Muscle Function Decrease).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jeon, HJ., Kim, YS., Park, JS. et al. Age-related change in γH2AX of Drosophila muscle: its significance as a marker for muscle damage and longevity. Biogerontology 16, 503–516 (2015). https://doi.org/10.1007/s10522-015-9573-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-015-9573-0