Abstract
This study determined the effects of heat shock protein 70 (Hsp70) overexpression on disuse muscle atrophy in senescent rats. Solei of young and senescent rats were co-injected with Hsp70 plus a nuclear factor kappa B (NF-κΒ) reporter plasmid. After 4 days, the hind limbs of half the young and senescent rats were immobilized for 6 days with the remainder serving as weight bearing controls. Hsp70 protein levels and cross-sectional area decreased in both groups (~20%) after immobilization. Atrophy was prevented in those fibers overexpressing Hsp70. NF-κΒ activity increased in the soleus of both young (three-fold) and senescent (five-fold) animals after immobilization and was prevented by Hsp70 overexpression. Inhibitor of κΒ decreased in young (~30%) and senescent (~10%) animals with immobilization and returned to normal with Hsp70. Heat shock protein 70 overexpression prevents disuse atrophy in senescent rats, possibly through suppression of the NF-κB pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The loss of skeletal muscle mass with age (sarcopenia) is characterized by a reduction in muscle fiber cross-sectional area (CSA) (Lexell et al. 1983; Porter et al. 1995), reduced force production and shortening velocity (Larsson et al. 1997), and a preferential loss and atrophy of type II muscle fibers (Larsson et al. 1979). These losses in mass and function with aging have been implicated in many of the injuries seen in the elderly (Marks et al. 2003). Further complicating the aging-induced loss in mass is the fact that the elderly are more susceptible to periods of muscle disuse due to injuries and illness that require immobilization or bed rest. It is well accepted that these conditions result in significant muscle wasting and dysfunction (Boonyarom and Inui 2006).
Interestingly, aging and disuse may have a synergistic effect on muscle wasting in that, during periods of muscle disuse, older individuals may lose greater muscle volume compared to younger individuals (Urso et al. 2006). However, despite knowing the characteristics of sarcopenia and disuse-induced muscle wasting, our understanding of the signaling molecules that regulate muscle mass during age and disuse with age are poorly defined. Until they are better identified specific interventions are not likely.
Heat shock proteins (HSP) have been implicated as playing a significant role in the muscle wasting associated with immobilization (Selsby and Dodd 2005). In an investigation of the effects of heating on immobilization-induced atrophy, we have previously shown that heating significantly increases muscle Hsp25 and Hsp70 protein levels, and attenuates atrophy (Selsby and Dodd 2005). Further, in more recent work we used plasmid injection and electrotransfer to overexpress only the Hsp70 protein in young rats (to a physiologically attainable level) and showed that Hsp70 prevented muscle atrophy (Senf et al. 2008).
Although there is comprehensive evidence that NF-κΒ activity is increased during disuse and is required for the disuse atrophy (Cai et al. 2004; Judge et al. 2007), there are few studies that have assessed NF-κB activity following disuse in senescent muscle. The only available evidence suggests that NF-κΒ activation is actually decreased during the first 1–2 weeks of immobilization (Bar-Shai et al. 2005a, b). However, these studies also showed that NF-κΒ activity, acting through the classic pathway, is decreased during immobilization in young animals. However, other studies, using genetic manipulations, have clearly shown that the classic NF-κΒ pathway is increased and required for muscle atrophy during disuse in young rats (Cai et al. 2004; Judge et al. 2007).
Thus, the NF-κΒ response to muscle disuse in senescent animals has not been clearly delineated. Furthermore, it is unclear whether NF-κΒ is changed in weight bearing senescent animals. It has been shown to be elevated in the skeletal muscle of (weight bearing) senescent mice (Broome et al. 2006), whereas others have shown it to be unchanged or decreased (Phillips and Leeuwenburgh 2005). Therefore, NF-κΒ activation in weight bearing senescent skeletal muscle also warrants further investigation in order to determine its possible role in sarcopenia.
Thus, the purpose of this study was to determine if; (1) Hsp70 overexpression in senescent muscle could attenuate disuse-induced atrophy; (2) NF-κΒ activity is increased in senescent muscle and increased further following disuse in senescent muscle and; (3) Hsp70 overexpression inhibits activation of NF-κΒ during disuse.
Materials and methods
Animals
Male, Fischer 344 rats (5-month-old (n = 6) and 26-month-old (n = 6)) were obtained from the National Institute of Aging colony through a gift from Dr. Robert Yezierski (University of Florida). All animal procedures were approved by the University of Florida institutional animal care and use committee.
Expression and reporter plasmids
The EGFP-c1 plasmid was obtained from clontech and the Hsp70-EGFP plasmid was created as previously described (Senf et al. 2008). The NF-κΒ-GL3 reporter plasmid was a gift from Dr. Steffan Ho, and has been previously used and described (Hunter et al. 2002; Judge et al. 2007). Plasmid DNA was prepared and isolated using an Endotoxin-free maxi or Mega prep kit (Qiagen).
Plasmid injection and electroporation
Plasmid injection and sequential transfection of skeletal muscle has been detailed previously (Mitchell-Felton and Kandarian 1999; Senf et al. 2008). Briefly, 10 μg of EGFP or Hsp70-EGFP and 40 μg of the NF-κΒ reporter plasmid were injected into the soleus muscle in a total volume of 50 μl 1× PBS. We have previously demonstrated that Hsp70 function is not impaired by the EGFP fusion (Senf et al. 2008).
Following injection, electric pulses were delivered using an electric pulse generator (Electro square porator ECM 830; BTX) by placing two paddle-like electrodes on each side of the muscle. Five pulses were delivered in 200 ms interpulse intervals, each with an effective intensity of 125 V/cm and 20 ms duration. Electrotransfer of a mixture containing two vectors in skeletal muscle shows co-transduction of a given fiber nearly 100% of the time (Alzghoul et al. 2004; Rana et al. 2004), thus a fiber that takes up one vector will also take up the other.
Immobilization
About 4 days following plasmid injection, animals were immobilized bilaterally with the ankle joint in the plantarflexed position to induce maximal atrophy of the soleus muscle, as described previously (Booth and Kelso 1973; Selsby and Dodd 2005). Surfaces to be casted were first wrapped in a thin layer of protective padding (Medipore Dress-it, 3M, St. Paul, MN, USA) to prevent abrasions due to the plaster cast. The padding began just below the ribs and continued down to encompass the lower abdomen and hind limbs, leaving adequate space so that urination would not be affected. A layer of plaster was then applied and allowed to dry (Specialist, Johnson & Johnson). Muscles of immobilized and weight bearing animals were extracted 6 days later (10 days following plasmid injection).
Muscle preparation and analysis
Soleus muscles were removed and either rapidly frozen in liquid nitrogen and stored at −80°C for subsequent biochemical analyses, or fixed in tissue-freezing medium and frozen for fiber sectioning and subsequent immunohistochemical analysis.
NF-κΒ Activity
Following homogenization in a passive lysis buffer (Promega) and centrifugation for 2 min at 5,000g, 20 μl of the supernatant was added to 100 μl of luciferase reagent (Promega) for determination of total muscle luciferase activity, using an LMax II microplate luminometer (molecular devices corp).
Immunohistochemistry
Cross sections (10 μm) from the midbelly of the soleus muscle were cut with a cryostat microtome (Microm HM 550, Microm International) and fixed in 4% paraformaldehyde. For visualization of muscle fibers under fluorescence microscopy muscle sections were incubated with wheat germ agglutinin Texas Red-X conjugate (Invitrogen). Images were captured with an Olympus I×50 camara and the muscle fiber area of ~200 fibers from each muscle was traced and measured using Image Pro Discovery software.
Western blot
A detergent-compatible assay (Bio-Rad) was used to determine protein concentration of muscle homogenates. Samples were diluted in loading buffer (Bio-Rad) containing 5% mercaptoethanol to achieve a protein concentration of 2 mg/ml and heat denatured. Equal amounts of protein were loaded onto 4–15% linear gradient gels and separated using SDS–polyacrylamide gel electrophoresis. Proteins were transferred for 90 min at 100 V onto an immobilon-FL polyvinylidene fluoride membrane (Millipore), blocked in PBS containing 5% milk and 0.05% Tween for 1 h and incubated overnight with primary antibody diluted in blocking buffer. The following primary antibodies were used: Anti-Hsp70 (ab6535, abcam), Anti-GFP (sc-8334, Santa Cruz) and Anti-IκΒα (sc-371, Santa Cruz). Following a series of washes, the membranes were incubated with fluorescent-dye conjugated secondary antibodies and visualized using odyssey infrared imaging system (LI-COR Biosciences). Relative quantification of proteins was determined by measuring the fluorescence of each lane at the appropriate molecular weight.
Statistical analysis
Hsp70 protein expression was analyzed using a one tailed, student’s t-test, with all other data analyzed using a two-way ANOVA followed by Bonferroni corrections for multiple comparisons when appropriate (GraphPad Software, San Diego, CA, USA). All data are expressed as means ± SEM, and significance was established at the P < 0.05 level.
Results
As expected, and in agreement with our previous findings (Senf et al. 2008), endogenous Hsp70 protein levels were significantly decreased after immobilization in young rats (Fig. 1). In addition, Hsp70 levels were significantly decreased in the soleus muscles of senescent rats compared to young rats, and further decreased in the muscles of senescent rats following immobilization.
To overexpress Hsp70 we injected an Hsp70-EGFP expression plasmid into the soleus muscle of rats. This led to a 2.5-fold increase in Hsp70 protein expression over endogenous levels (Fig. 2). The presence of the EGFP-tag on the N-terminus of Hsp70 permits visualization of Hsp70 (Fig. 3). This allows for the cross sectional area comparison of those fibers overexpressing Hsp70 to those fibers that do not overexpress Hsp70. As shown in Fig. 3, the transfection efficiency (i.e., the number of fibers expressing the Hsp70-EGFP plasmid) is ~60–70%.
Representative cross sections taken from the soleus muscle of weight bearing 5 month (a) and 26 month (b) animals illustrating the reduced fiber cross sectional area seen with senescence. c and d illustrate cross sections of 26 month weight bearing (c) and 26 month immobilized (d) soleus injected with Hsp70 plasmid as indicated by EGFP fluorescence. The fiber cross sectional area was visualized by incubating sections with wheat germ agglutinin Texas Red-X conjugate, imaged using fluorescent microscopy, and measured using image pro discovery software. With this technique, cross sectional areas of fibers overexpressing Hsp70 (green fluorescent fibers) can be compared to those fibers not overexpressing Hsp70 (non-fluorescent fibers)
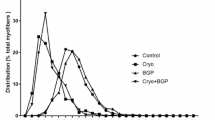
As shown in Fig. 4, although the fiber cross sectional area is slightly decreased in senescent animals compared to young, the immobilization-induced atrophy in the young and senescent animals is approximately the same (~22%). Importantly, however, the overexpression of Hsp70 prevented the muscle fiber atrophy in both young and senescent animals.
In order to test the hypotheses that NF-κΒ activity is (1) increased in senescent rats compared to young, (2) further increased after immobilization, and (3) inhibited by overexpression of Hsp70, we used a NF-κΒ reporter plasmid. Figure 5 shows that NF-κΒ activity is unchanged in the soleus muscle of senescent rats compared to young rats, suggesting there is no chronic upregulation of NF-κΒ activity. However, immobilization caused NF-κΒ activity to increase significantly in both young and senescent animals, 3 and 5-fold, respectively. However, in immobilized muscles overexpressing Hsp70, NF-κΒ activity was not different from control animals in either the young and senescent groups. Thus, a physiologically attainable increase in Hsp70 in vivo prevents the immobilization-induced increase in NF-κΒ activity.
Since a decrease in the endogenous inhibitor of κΒ (IκΒ) is required for NF-κΒ activation during skeletal muscle disuse (Judge et al. 2007), we sought to determine whether the inhibition of NF-κΒ by Hsp70 was through IκΒα. As shown in Fig. 6, IκΒα is significantly reduced following immobilization in young but not in senescent animals. This may be due to the lower basal levels of IκΒα in senescent solei muscles compared to young. Furthermore, overexpression of Hsp70 increased IκΒα levels in the weight bearing and immobilized muscles in both young and senescent animals. These findings suggest that Hsp70 may inhibit NF-κΒ activity through increasing the levels of IκΒα that are available to bind, and retain, NF-κΒ proteins in the cytosol.
Discussion
The findings of this study show, in both young and senescent rats, that (1) Hsp70 overexpression prevents disuse muscle atrophy; (2) NF-κΒ activity is increased during disuse and; (3) Hsp70 overexpression abolishes the disuse-induced NF-κΒ activity.
The finding that overexpression of Hsp70 prevented immobilization-induced muscle atrophy in both young and senescent animals was the most interesting finding of this study and suggests that Hsp70 has a major influence on the pathways controlling protein degradation. While our previous studies had shown that heating skeletal muscle could increase Hsp70 and attenuate atrophy by 30–50% (Selsby and Dodd 2005; Selsby et al. 2007), this study confirms that the increase in Hsp70 likely played a direct role in mediating the atrophy process.
Our finding that endogenous Hsp70 is significantly decreased in the young, adult animals after 6 days of disuse is in agreement with previous studies using an immobilization model (Stevenson et al. 2003; Selsby and Dodd 2005; Lawler et al. 2006; Selsby et al. 2007; Senf et al. 2008). In addition, the immobilization-induced decreased Hsp70 in the senescent animals has not been demonstrated but is not surprising. The mechanism causing a reduction in Hsp70 with immobilization is unknown.
The decrease in muscle fiber CSA with immobilization (~22%) was similar to the decrease in muscle mass seen in both young and senescent animals after immobilization (Chakravarthy et al. 2000; Selsby and Dodd 2005; Selsby et al. 2007) and was prevented by Hsp70 overexpression in both groups. The decrease in fiber CSA during skeletal muscle disuse is due mostly to a decrease in muscle protein content that is dependent upon both reduced protein synthesis and increased protein degradation (Thomason et al. 1989; Lecker et al. 1999; Krawiec et al. 2005). However, the latter plays the larger role and is primarily mediated by the ubiquitin proteasome pathway. In fact, the rate of protein synthesis has been shown to be unaltered following 5-days of immobilization, despite significant muscle atrophy (Krawiec et al. 2005).
One of the major signaling pathways that regulates muscle mass, possibly through regulation of components of the ubiquitin proteasome pathway, is NF-κΒ and there is convincing evidence that NF-κΒ is increased during skeletal muscle disuse in young rats, and required for the muscle atrophy (Cai et al. 2004; Hunter and Kandarian 2004; Judge et al. 2007). Thus, we examined NF-κΒ activity and its inhibitor, IκΒα, to determine their level of association with the immobilization-induced muscle atrophy in senescent animals.
Our finding that NF-κΒ reporter activity is increased during disuse in the soleus of young rats was expected. However, this is the first work to show that NF-κΒ activity is also increased in senescent rats during disuse. Although this latter finding was also expected, it is in disagreement with the findings of Bar-Shai et al. (2005a, b), who found a decrease in NF-κΒ activity during the first 2 weeks of disuse in both young and senescent rats. Based on these results the authors suggested that NF-κΒ activation follows a biphasic pattern during disuse in both young and senescent rats. This purported pattern shows a decrease in the classic NF-κΒ pathway during the first 2 weeks of disuse, but an increase in NF-κΒ activation through an alternative pathway involving p50 and Bcl-3, followed by an increase in activation of the classic NF-κΒ pathway after 4 weeks of disuse. However, this hypothesis ignores the findings of others showing that the classic NF-κΒ pathway is required for disuse-induced NF-κΒ activation and muscle atrophy following 1–2 weeks of disuse in young rodents (Cai et al. 2004; Judge et al. 2007).
IκΒα is the inhibitor of NF-κΒ, preventing its translocation to the nucleus to initiate transcription. To activate the classic NF-κΒ pathway, IκΒα is phosphorylated, then ubiquitinated, which leads to its degradation through the proteasome. Indeed, it is now accepted that IκΒα degradation is required for disuse-induced NF-κΒ activation and muscle atrophy in young rodents (Cai et al. 2004; Judge et al. 2007). Thus, our finding of a decrease in IκΒα expression with immobilization in both the young and senescent animals was expected and suggests activation of the classic NF-κΒ pathway.
The fact that Hsp70 prevented the increase in NF-κΒ and the decrease in IκΒα in both young and senescent animals after immobilization is striking and agrees with our previous findings in young rats (Senf et al. 2008). Since inhibition of NF-κΒ activation during disuse attenuates a significant portion of atrophy in the fibers overexpressing Hsp70 (Cai et al. 2004; Judge et al. 2007), it would follow that Hsp70 overexpression in this study is responsible for a significant portion of the attenuation of atrophy through the NF-κΒ pathway.
Although this study did not focus on the mechanisms by which Hsp70 inhibits NF-κΒ activity, it is interesting that Hsp70 increased IκΒα levels. Since the prevention of IκΒα degradation during disuse inhibits NF-κΒ activity, the increased levels of IκΒα found in this study could potentially explain the inhibition of NF-κΒ activity. However, much further investigation is necessary.
In summary, the finding that overexpression of Hsp70 within the normal physiological range in vivo can prevent immobilization-induced muscle atrophy is unique, especially in the senescent animals. The results suggest that Hsp70 has a major influence on the NF-κΒ signaling pathway in the senescent animals and therefore, may provide a focal point for future experimentation in attempting to elucidate the mechanisms controlling disuse atrophy in senescent muscle.
References
Alzghoul MB, Gerrard D, Watkins BA, Hannon K (2004) Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J 18:221–223
Bar-Shai M et al (2005a) The effect of hindlimb immobilization on acid phosphatase, metalloproteinases and nuclear factor-kappaB in muscles of young and old rats. Mech Ageing Dev 126:289–297. doi:10.1016/j.mad.2004.08.030
Bar-Shai M, Carmeli E, Reznick AZ (2005b) The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Ann N Y Acad Sci 1057:431–447. doi:10.1196/annals.1356.034
Boonyarom O, Inui K (2006) Atrophy and hypertrophy of skeletal muscles: structural and functional aspects. Acta Physiol (Oxf) 188:77–89. doi:10.1111/j.1748-1716.2006.01613.x
Booth FW, Kelso JR (1973) Production of rat muscle atrophy by cast fixation. J Appl Physiol 34:404–406
Broome CS et al (2006) Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity. FASEB J 20:1549–1551. doi:10.1096/fj.05-4935fje
Cai D et al (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119:285–298. doi:10.1016/j.cell.2004.09.027
Chakravarthy MV, Davis BradleyS, Booth FrankW (2000) IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J Appl Physiol 89:1365–1379
Hunter RB, Kandarian SC (2004) Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest 114:1504–1511
Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC (2002) Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J 16:529–538. doi:10.1096/fj.01-0866com
Judge AR, Koncarevic A, Hunter RB, Liou HC, Jackman RW, Kandarian SC (2007) Role for IkappaBalpha, but not c-Rel, in skeletal muscle atrophy. Am J Physiol Cell Physiol 292:C372–C382. doi:10.1152/ajpcell.00293.2006
Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH (2005) Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab 289:E969–E980. doi:10.1152/ajpendo.00126.2005
Larsson L, Grimby G, Karlsson J (1979) Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol 46:451–456
Larsson L, Li X, Frontera WR (1997) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol 272:C638–C649
Lawler J, Song W, Kwak H (2006) Differential response of heat shock proteins to hindlimb unloading and reloading in the soleus. Muscle Nerve 33:200–207. doi:10.1002/mus.20454
Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr 129:227S–237S
Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M (1983) Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6:588–595. doi:10.1002/mus.880060809
Marks R, Allegrante JP, Ronald MacKenzie C, Lane JM (2003) Hip fractures among the elderly: causes, consequences and control. Ageing Res Rev 2:57–93. doi:10.1016/S1568-1637(02)00045-4
Mitchell-Felton H, Kandarian SC (1999) Normalization of muscle plasmid uptake by Southern blot: application to SERCA1 promoter analysis. Am J Physiol 277:C1269–C1276
Phillips T, Leeuwenburgh C (2005) Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19:668–670
Porter MM, Vandervoort AA, Lexell J (1995) Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5:129–142
Rana ZA, Ekmark M, Gundersen K (2004) Coexpression after electroporation of plasmid mixtures into muscle in vivo. Acta Physiol Scand 181:233–238. doi:10.1111/j.1365-201X.2004.01282.x
Selsby JT, Dodd SL (2005) Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289:R134–R139. doi:10.1152/ajpregu.00497.2004
Selsby JT, Rother S, Tsuda S, Pracash O, Quindry J, Dodd SL (2007) Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J Appl Physiol 102:1702–1707. doi:10.1152/japplphysiol.00722.2006
Senf S, Dodd S, McClung J, Judge A (2008) Hsp70 overexpression inhibits NF-kB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy. FASEB J 22:3836–3845. doi:10.1096/fj.08-110163
Stevenson EJ, Giresi PG, Koncarevic A, Kandarian SC (2003) Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J Physiol 551:33–48. doi:10.1113/jphysiol.2003.044701
Thomason DB, Biggs RB, Booth FW (1989) Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol 257:R300–R305
Urso ML, Clarkson PM, Price TB (2006) Immobilization effects in young and older adults. Eur J Appl Physiol 96:564–571. doi:10.1007/s00421-005-0109-1
Acknowledgments
We thank Sarah Senf for critical reading and editorial comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dodd, S., Hain, B. & Judge, A. Hsp70 prevents disuse muscle atrophy in senescent rats. Biogerontology 10, 605–611 (2009). https://doi.org/10.1007/s10522-008-9203-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10522-008-9203-1