Abstract
This study investigated the potential use of acid-fermented chicken silage on growth performance, digestive enzymes, liver and intestinal histology in Oreochromis mossambicus (Mozambique tilapia) (Peters 1852). Five silage-based diets were formulated by replacing fishmeal at 0% (control), 25%, 50%, 75% and 100%, thereafter denoted as D1, D2, D3, D4 and D5, respectively. The control diet (0%) contained 40% of fishmeal while D5 contained no fishmeal. The diets contained the same protein (30.06%), fat (15%) and energy levels (18 MJ kg−1). Silage (29.31% protein and 10.97% fat) fed alone and a commercial feed (35% protein and 2.97% fat) were designated as D6 and D7, respectively. Each diet was randomly assigned to triplicate groups of O. mossambicus (6.06 ± 0.27 g) stocked in 400-L fiberglass tanks (50 fish/tank). Fish were fed with the diets to apparent satiation twice daily for 45 days. Among the silage-based diets, weight gained was significantly higher (P < 0.05) in D4 (18.34 ± 1.51 g) and D5 (20.86 ± 1.95 g) than in D1 (14.28 ± 1.21 g), D2 (14.19 ± 1.51 g) and D3 (14.43 ± 1.34 g). The highest specific growth rates were found in D4 (1.10 ± 0.19%) and D5 (1.23 ± 0.04%) than in D1 (0.96 ± 0.22%), D2 (0.74 ± 0.06%) and D3 (0.92 ± 0.04%). The best feed conversion ratio was found in D4 (2.55 ± 0.27) and D5 (2.41 ± 0.32) compared to D1 (3.13 ± 0.38), D2 (3.50 ± 0.34) and D3 (3.26 ± 0.42). Apparent digestibility coefficient for protein values was higher in D4 (93.50 ± 2.96%) and D5 (92.55 ± 3.90%) than in the control (86.68 ± 2.80%). However, the best growth rates were recorded in the D6 and D7 diets. The highest protease, amylase and lipase activities were recorded in D6 and D7 followed by D5 and D4. No pathological alterations were observed in the liver and intestines of fish among dietary treatments. This study confirmed that acid-fermented chicken silage could serve as an alternative and cheaper protein source, as well as complete diet for O. mossambicus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tilapias are among the most cultured fish species in the world, with China, South-east Asia, Latin America and the USA dominating in terms of production (FAO 2016). In South Africa, the Mozambique tilapia (Oreochromis mossambicus) is popular for freshwater aquaculture and interest in farming this species has increased over the past decade (DAFF 2019). O. mossambicus is considered an ideal fish species for rural aquaculture due to its desirable characteristics such as its adaptability, tolerance of changing environmental conditions, fast growth rates, disease resistance, its capacity to feed on various food sources (omnivorous), high fecundity and its high flesh quality (El-Sayed 2006). In South Africa, the culture of O. mossambicus is dominated by the small-scale sector, which is mainly found in rural parts of the country.
One of the major problems facing small-scale tilapia farmers in South Africa is the rising cost of commercial feeds. The price of commercial feeds is high and many small-scale fish farmers cannot afford to buy them. As a result, many small-scale tilapia farmers in the country resort to using poor quality feeds, and this affects farm production and profitability. Therefore, attempts are underway to identify cheap, locally available ingredients that can be used in aqua-feeds. For example, on a global scale, there has been investigation of using plant ingredients with high protein content in aqua-feeds (Gatlin et al 2007; Hardy 2010; NRC 2011). However, the use of plant-based protein sources is often restricted because of the presence of anti-nutritional factors (ANFs), which are known to reduce the digestibility and bio-availability of nutrients (Francis et al. 2001). Additionally, another limiting factor associated with plant-based protein sources is the unbalanced amino acid profile, which can result in a deficiency in some of the essential amino acids. Although these limitations can be easily corrected by the use of exogenous enzymes (to inactivate ANFs) and synthetic amino acids (to improve the amino acid profile), such options may be too expensive for small-scale farmers, who often lack resources. Therefore, there is a need to continue the search for new, cost-effective and high quality protein ingredients in order to add more diversity to the selection of ingredients used to formulate aqua-feeds.
Cheap and promising sources of protein for inclusion in aqua-feeds can be obtained from processing wastes resulting from livestock and poultry production. Poultry by-product meal can be incorporated into tilapia feeds (El-Sayed 2006). They are readily available in most countries and they are rich in protein and have a balanced amino acid profile. In addition, they are a good source of minerals and vitamins, all of which are essential for enhancing growth in fish. Moreover, further processing of poultry meat through techniques such as fermentation can contribute to the overall nutritional quality of the product and make it more suitable as an ingredient with high nutritional value. Fermentation increases the digestibility and bioavailability of nutrients because of the presence of hydrolyzed proteins, carbohydrates and fats (Vidotti et al. 2002). The use of acid during the fermentation process is known to enhance the nutritional value of the product (Fagbenro et al. 1994). It also increases the safety and acceptability of the product by reducing microbial contaminants, which is one of the challenges associated with the utilization of animal wastes in fish feeds from a safety point of view (Samaddara et al. 2015). Fermentation also improves the bioavailability of minerals such as calcium, iron and zinc (Gupta et al. 2015). Furthermore, animal by-products can be mixed with other ingredients such as rice, maize, bread and vegetables during fermentation to enrich their nutritional profile (Zeineddine et al. 2021). This is an indication that it is possible to develop complete, high-quality aqua-feeds using locally available, cheap protein sources.
The poultry farming industry is the largest provider of animal protein in South Africa and it generates large quantities of carcasses and by-product wastes that are unsuitable for human consumption. These wastes could be used to make poultry by-product meal, or be subjected to acid fermentation to produce silage, for inclusion in aqua-feeds. However, the use of animal processing waste, such as poultry by-products, in the diets of farmed freshwater fish species in South Africa has not been investigated. Processed poultry by-products can be beneficial in areas where key ingredients used as main protein sources are expensive or scarce, as is often the case in the small-scale aquaculture industry in developing countries. It will be beneficial for farmers in this sector to explore the use of fermented poultry waste in fish diets. Therefore, the aim of the current study was to investigate the use of diets containing different inclusion levels of acid-fermented chicken waste on growth performance, digestive enzymes and histology (liver and gut) of juvenile O. mossambicus.
Materials and methods
Collection of chicken carcasses and fermentation procedure
The acid-fermented chicken waste silage was prepared as follows: chicken carcasses (not older than 24 h) from birds that had died naturally were collected daily in the morning and afternoon from an abattoir in the Western Cape, South Africa. These were on farm mortalities that were considered unsuitable for human consumption due to ethical and health reasons. No visibly decomposed carcasses that had been lying for more than 24 h in the slaughter house were used in this study. The carcasses were immediately taken to a cool place where feathers were plucked off. The carcasses were then dipped in Foodpro® organic acid blend (lactic acid E270 and Acetic acid E260, diluted to obtain carcass surface pH of below 4) for at least 1 min. The carcasses were then minced using an electric meat Grinder (REX MC3-1) to produce an even meat paste. After grinding, the meat paste was thoroughly mixed with fine maize meal at a ratio of 2/3 meat paste to 1/3 maize meal (50 kg carcasses with 25 kg maize meal). During the mixing, Foodpro® organic acid blend was sprayed over the mixture (1L 20 kg−1). The product was placed in airtight containers (10-L plastic buckets) and sealed with lids to prevent oxygen from coming into contact with mixture. The acid was added in order to lower the pH of the mixture to 3.5. The containers were then placed in a shade (> 20 °C) out of direct sunlight to ferment. The buckets were shaken daily to keep the sample slurry thoroughly mixed and pH was monitored daily to ensure that the acidity of the mixture was maintained at a pH − 4 by periodically adding more Foodpro® in case the pH increased during the fermentation course. The addition of the acid ensures that the silage can be appropriately preserved. The pH was monitored using a pH tester (Hanna HI981036) designed for meat pH measurements. This was inserted into the sample using a self-closing mounted rubber grommet in the lid of the bucket and sealed off from the external air environment. The additional Foodpro® was administered through the self-closing grommet when required. The mixture was allowed to ferment for a period of 42 days.
The fermented slurry was then spread on twin-wall polypropylene Correx sheets (1 250 mm × 2 500 mm × 3, 5 mm) for air-drying. The air-dried product tends to stick to the sheet surface; therefore, the surface of the sheets was firstly sprayed with a thin layer of a lecithin based agent (Spray and Cook®) to minimize the adhesiveness of the sheets. The layering of the slurry was done such that it was not thicker than 3 mm. The boards were placed on tables positioned in the middle of a 4 × 12 m greenhouse tunnel (50 mm uPVC pipe frame) with a UV deck plastic cover (3 m peak height). Sun radiation and the warm air in the tunnel were used to dry the slurry to a dough-like texture. After about 8 h (one daylight summer day), the dried product was collected and stored in sealable polythene bags, which were kept in a dry and cool place until needed.
Feed preparation
The dried silage was ground into fine powder using a grinder and mixed with the following dry ingredients: fish meal, maize meal, soybean meal, rapeseed meal, sunflower meal, maize gluten, wheat middlings, wheat bran, binder (gelatine) and vitamin/mineral premix (Batch No. 16017-M3817: Liberty Food Services, Gauteng, South Africa) (Table 1). Five silage-based diets were formulated by adding the silage powder to these ingredients to replace fishmeal at 0% (control), 25%, 50%, 75% and 100% (Table 1). The control contained 40% of fishmeal and no fermented silage while D5 contained 40% of the fermented silage and no fishmeal. The diets were designated as D1, D2, D3, D4 and D5 for the 0%, 25%, 50%, 75% and 100% inclusion levels, respectively (Table 1). The diets were formulated to contain the same amount of protein, fat and energy and to meet the protein level mainly used in commercial diet for O. mossambicus. All the ingredients were purchased from local suppliers. Chromic oxide was added to each diet as an inert marker. The binder was added to stabilize the pellets in order to minimize large losses due to break-up and dissolution. The silage-based diets were prepared as follows: the required individual amounts of each ingredient for each percentage diet were carefully measured and placed in containers corresponding to each diet. The measured ingredients were then transferred to a mixer (Hobart, Troy, OH, USA), which was set at low speed. During the mixing, water (10–20% v/w) was added occasionally until the mixture formed a homogenized dough. The dough was pushed through a meat mincer to produce extruded strings, which were placed on open trays and immediately taken outdoors for air-drying. Two additional diets were also formulated and denoted as D6 and D7. D6 was formulated to contain only the silage. For this diet, the silage powder was mixed with the binder and chromic oxide and transferred to the mixer and water added until a dough was obtained. D7 was a commercial diet that was first crushed, mixed with the binder and chromic oxide and then moistened until a dough was formed. These diets were also passed through the meat mincer and dried outdoors. The processing of D7 was done in order to ensure that digestibility analyses could be conducted and compared among all experimental diets. Once the strings were dry, they were broken into appropriate pellet sizes of 3–4 mm for the fish used in the experiment. Finally, the pellets were stored in air-tight plastic containers marked with a corresponding diet and kept in a dry and cool place until required.
Chemical composition of diets
The fermented silage and the silage-based diets were separately analyzed for proximate composition using standard procedures published by AOAC (2012) for moisture, crude protein, crude lipid, fibre, gross energy and ash (Table 1). Each sample was analyzed in triplicates. The manufacturer of the reference diet provided data for proximate composition (Table 1). The fermented silage and the experimental diets were analyzed for gross energy using DDS isothermal CP 500 bomb calorimeter. The Soxhlet extraction apparatus (Tecator Soxtech HT 1043 Extraction unit) was used to determine crude lipid content. Ash content was determined by the combustion method by placing samples in a muffle furnace at 550 °C for 4 − 5 h. The Van Soest et al (1991)’s protocol was used to determine acid detergent fibre. Moisture was determined by weighing a wet silage sample and drying it in an oven at 105 °C overnight. Moisture content was determined as the difference between the initial weight and loss in weight. Crude protein (CP) was calculated from the nitrogen content of sample (N × 6.25), which was determined using the LECO FP2000 Nitrogen Analyser. The method quantifies the nitrogen fraction using the Dumas combustion method. Crude fibre was analyzed as loss in weight after ignition of dried lipid-free residues following digestion using 1.25% H2SO4 (sulphuric acid solution) and 1.25% NaOH (sodium hydroxide solution). The remaining residue was then ashed in a muffle furnace at 550 °C for 2 h. The difference in weight on ashing represented crude fibre (hydrolysis resistant organic matter), which was expressed as a percentage of the original un-defatted sample.
Chromic oxide in diets and faeces was analyzed using Furukawa and Tsukahara (1966)’s method. Briefly, a concentrated nitric acid was added to the samples followed by the oxidation of chromic oxide with 70% perchloric acid. The oxidation of chromium III to chromium VI results in an orange colour. Once this was reached, the solution was then read on a spectrophotometer (Uvikon 810; NorthStar Scientific, Bedfordshire, UK) at 350 nm and distilled water was used as a blank. Chromic oxide was then calculated as: Weight of chromic oxide in sample = (Absorbance − 0.0032/0.2089).
Chromic oxide (%) = weight of chromic oxide/sample weight (mg) × 100%.
Amino and fatty acids determination
The amino acid compositions of the fermented silage were determined using an automatic Biochrome30 Amino acid analyzer chromatography system after samples were hydrolyzed with methanesulphonic acid at 115 °C for 22 h (Peace and Gilani 2005). The amino acids analyzed from the fermented silage are presented in Table 2. Essential amino acids (EAAs) from fishmeal, reference diet and the amino acids (AA) requirement for tilapia are also listed in Table 2 for comparisons. The manufacturer of the reference diet provided data on its AA composition (Table 2). The fatty acid methyl esters (FAMEs) analyzed from the fermented silage are presented in Table 3. Lipids were extracted for fatty acid (FA) analysis using chloroform: methanol solution (2:1, v/v) (Folch et al. 1957), containing 0.01% butylated hydroxytoluene (BHT) as an antioxidant. Samples were homogenized in the extraction solvent for 30 s using a polytron mixer (Wiggen Hauser D-500 Homogeniser, fitted with a standard shaft 1, speed setting D). Heptadecanoic acid (C17:0) was used as an internal standard (catalogue number H3500, Sigma-Aldrich, Gauteng, South Africa) to quantify the individual fatty acids in the samples. Subsequently, 250 μL of the extracted lipids was transmethylated for 2 h at 70 °C using 2 mL of a methanol: sulphuric acid solution (19:1, v/v) as the transmethylating agent. Thereafter, the mixture was left to cool at room temperature. After the mixture was allowed to cool down, distilled water and hexane were then used to extract the fatty acid methyl esters (FAMEs). After the separation of the distilled water and FAMEs-containing hexane fluids, the top layer (FAMEs-containing hexane) was transferred into designated individual tubes and dried under nitrogen until further analysis. One hundred microliters of hexane was then added to each dried FAMEs sample, after which 1 μL from each sample was injected into the gas chromatograph.
Fatty acid methyl esters (FAMEs) were separated and quantified using a Thermo TRACE 1300 series gas-chromatograph (Thermo Electron Corporation, Milan, Italy) equipped with a flame-ionization detector. A 30-m TR-FAME capillary column with an internal diameter of 0.25 mm and a 0.25-μm film (Cat. No. HY260M142P, Anatech, Cape Town, South Africa) was used to analyze the FAMEs. The oven temperature was initially set at 50 °C (for 1 min) and gradually increased to 240 °C as follows: from 50 °C, it was increased to 175 °C (at a rate of 25 °C min−1), then increased to 200 °C (at a rate of 1.5 °C min−1) and finally increased to 240 °C (at a rate of 10 °C min−1). This temperature was maintained at this level for a minimum of 2 min. The GC was equipped with an injector, maintained at 240 °C and detector, maintained at 250 °C. Flow rate in the column was set at 40 mL min−1, using hydrogen as a carrier gas. Qualitative identification of the FAMEs was performed by comparing their retention times with those of a standard FAME mixture (Supelco™ 37 Component FAME mix, Cat no. CRM47885, Supelco, USA). The concentrations of the FAMEs are expressed as mg fatty acid g−1 diet.
Fish maintenance
The University of Limpopo Animal Research Ethics Committee of the Faculty of Science and Agriculture approved the execution of the experiments. All efforts were made to avoid and minimize unnecessary pain during handling of fish. The feeding experiment was performed at the Aquaculture Research Unit (ARU), University of Limpopo, South Africa. Juvenile O. mossambicus used in this study were sourced from fish produced at ARU. The fish were collected from nursery holding tanks in the hatchery, placed in containers containing water mixed with an anaesthetic (2-phenoxyethanol (1 mL 5 L−1)) and then transferred to 21 fiberglass tanks (500-L capacity filled to a 400-L mark) in a recirculating system for acclimatization for 2 weeks. The system was supplied with aerated water from an air compressor at a continuous flow rate of 10 L min−1. Water temperature ranged from 26.2 to 28.4 °C and was maintained using submersible aquarium type heaters. Fish were stocked in each tank at a density of 60 fish per tank and left to acclimatize to experimental conditions for 2 weeks. During this this time, fish were fed with their normal diet (Aqua-Plus, Avi-Products (Pty) (Ltd)) twice a day, at 10:00 h and 16:00 h.
Experimental design
A completely randomized design was used in this feeding experiment. After the acclimatization period, fish were placed in 25-L buckets containing water that had been mixed with an anaesthetic (2-phenoxyethanol (1 mL 5 L−1) to be weighed prior to the commencement of the feeding trial. The fish were weighed (6.06 ± 0.27 g) individually before they were randomly transferred back into the 21 fiberglass tanks at a stocking density of 50 fish per tank or 1 fish 7 L−1. The tanks were divided into 7 groups, namely A, B, C, D, E, F and G. Group A, B, C, D and E corresponded with the five silage inclusion levels, namely D1, D2, D3, D4 and D5. Group F and G were randomly assigned to the fermented silage (D6) (not mixed with any ingredient) and a reference diet (D7) (Aqua-Plus, Avi-Products (Pty). This was done in order to compare the growth performance of fish fed with the fermented silage diet in relation to the silage inclusion levels (D1–D5) and the commercial diet. The silage was tested against the other diets out of interest as its protein and carbohydrate content showed that it could be used as a standalone diet. Each diet was then randomly assigned to triplicate tanks of each group. All fish groups were fed ad libitum twice daily, in the morning at 09:00 h and in the afternoon at 15:00 h for 45 days. Fish in a particular tank were regarded as satiated when one pellet was left uneaten for 5 min. In each group, the amount of feed eaten was recorded after every feeding. After 4 h of each feeding activity, faeces were retrieved from each tank using a fine net and placed in capped 500-mL plastic bottles in order to determine digestibility of the diets. The faecal samples from each replicate treatment tanks were pooled and the samples were stored at − 20 °C until analyzed. The pooled samples for each diet were analyzed separately. During the course of the experiment, optimal rearing culture conditions for O. mossambicus were maintained as follows: water temperature ranged from 26.2 to 28.4 °C, dissolved oxygen was 6.82 ± 0.31 mg L−1 and the average salinity was 14 ± 3.5 ppt. Conductivity and pH were maintained at 605 ± 5 μS and 7.4 ± 0.4, respectively. These conditions were monitored daily using a digital handheld YSI 556 MPS multiparameter instrument (YSI, Yellow Springs, OH, USA). The photoperiod cycle was 12 h light and 12 dark for the entire duration of the feeding regime.
Growth performance indices
At the termination of the feeding regime, fish were starved for 24 h before being processed for data collection. After the 24 h fasting period, fish were netted, placed in holding containers and sedated using 2-phenoxyethanol (1 mL 5 L−1). Fish were weighed and the data used to determine growth performance indices. The growth performance indices were calculated as follows: specific growth rate (SGR) was calculated according Winberg (1956) as: \(\mathrm{SGR}=\frac{\mathrm{In }{\mathrm{W}}_{\mathrm{f}}-\mathrm{In }{\mathrm{W}}_{0}}{\mathrm{t}}\times 100\); where: Wf = final body weight (g), W0 = initial body weight (g), In = natural Logarithm (log)−10, t = feeding period (days). Feed utilization was determined using feed conversion ratio (FCR) = \(\frac{\text{food consumed (g) }}{\text{weight gained (g)}}\), which represents the ratio between the food consumed and weight gained. Feed Intake (FI) (FI, g fish−1 day−1) was calculated as follows: FI = dry feed intake (g)/number fish/days. Protein efficiency ratio (PER) was also determined by looking at the weight gained against the protein intake. Apparent digestibility coefficient (ADC) for protein was determined as follows:
\(\mathrm{Protein ADC}\left(\mathrm{\%}\right)=100\left(1-\left(\frac{\mathrm{\% }{\mathrm{Cr}}_{2}{\mathrm{O}}_{3}\mathrm{ diet}}{\mathrm{\% }{\mathrm{Cr}}_{2}{\mathrm{O}}_{3}\mathrm{ in faeces}}\right)\times \left(\frac{\mathrm{\% protein in faeces}}{\mathrm{\% protein in diet}}\right)\right).\)
Digestive enzyme activity
After weighing, five fish from each dietary replicate were euthanized with 1 ml L−1 2-phenoxyethanol, dissected out and the whole intestines removed for the digestive enzyme analyses. The samples were placed in clearly marked sterile plastic sample bottles and temporarily stored at − 80 °C until analyzed. For the analyses, samples were defrosted and the proximal part of the intestines was cut and homogenized (1:2 w/v) with 50 mM Tris–HCl buffer (pH 7.5) in an ice water bath, using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY). The homogenate was centrifuged at 4200 × g for 60 min at 4 °C. Following centrifugation, the supernatant was gently pipetted and transferred into small aliquots (100–200 μL) and kept at − 20 °C until required for use in spectrophotometric assays to determine the activities of digestive enzymes. Analyses for the different enzymes were carried out as follows: total protease activity was characterized according to the method described by Bezerra et al. (2005). Briefly, 125 μL of 1% (w/v) Azocasein, prepared in 0.1 M phosphate buffer (pH = 7), was mixed with 5 μL of crude enzyme extract and 40 μL of the buffer solution. The mixture was incubated at 30 °C for 30 min. After incubation, the reaction was stopped by adding 100 μL of Trichloroacetic acid (TCA). The mixture was then centrifuged at 2800 × g for 10 min. After centrifugation, 100 μL of the supernatant was added to 100 μL of 1 M sodium hydroxide. The absorbance of the mixture was read at an optical density of 540 nm. Amylase activity was quantified following the methods outlined by Bernfeld (1951) using starch as a substrate. A solution containing 1% starch (w/v) was prepared in 0.1 M phosphate buffer (pH = 7). Three microliters of the crude enzyme extract and 27 μL of the buffer solution were added to 33 μL of the starch substrate. The resultant mixture was then incubated at 30 °C for 10 min. After the 10-min incubation, 100 μL of 3,5-dinitrosalicylic acid (DNS) solution was added and the mixture was then heated and boiled for 5 min; thereafter, it was allowed to cool down at 4 °C. Absorbance of the solution was then measured at 540 nm. To characterize cellulase activity, the same method used for amylase was followed, except that starch was substituted and replaced with carboxyl-methyl-cellulose (1% w/v). The determination of lipase activity was carried according to Markweg et al. (1995)’s method using 0.01 M para-nitrophenyl palmitate (pNNP) dissolved in iso-propanol as a substrate. Briefly, 10 μL of the substrate was mixed with 12.5 μL of crude enzyme extract and 215 μL of 0.1 M phosphate buffer (pH 7). The absorbance of the solution was then read at 410 nm. All samples for the digestive enzyme activity were carried out in triplicates using appropriate blanks consisting of substrate only and homogenates only (in buffers).
Histological analysis
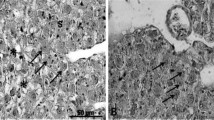
Histological analyses were undertaken in order to evaluate the effect of the experimental diets on the health of the fish. The liver and intestines were excised from five fish per replicate (15 fish per dietary treatment) just after they were euthanized with 2-phenoxyethanol (1 mL L−1). The samples (approximately 1 cm) were fixed in 10% buffered formalin solution which was replaced after 24 h. The fixed samples were sliced into appropriate sizes and dehydrated in increasing graded ethanol series. For gut histology, 1 cm segments were obtained from the mid intestine of the fish. Tissues were then passed through xylene before being embedded in molten paraffin wax. Each tissue sample was sectioned (3–5 μm) using a microtome, mounted on slides and afterwards stained with haematoxylin and eosin (H&E rapid stain). Five slides or sections per replicate (15 per treatment) were prepared for the histological analyses of the liver and gut samples. The slides were examined using a light trinocular microscopy at 400 × magnification (Leica Microsystems model DM750, Leica, Bannockburn, IL, USA). Pictures were taken with the aid of a digital camera (Digital Video Camera Company, Austin, TX, USA) mounted on the light microscope. The Image J (1.46) software was used to take measurements of intestinal villi height (μm) and width (μm) from at least 10 measurements from each slide. The method described by Baeverfjord and Krogdahl (1996) was used for counting of goblet cells in each segment of the intestines. Liver histology was evaluated based on alterations in hepatocyte nuclei, vacuolization and cytoplasm. McFadzen et al. (1997)’s method was used to scores the degree of changes in the liver with specimens scored 1–3, 1 = healthy specimen, 2 = moderate and 3 = degraded liver condition.
Statistical analysis
Prior to statistical analyses, the Shapiro–Wilk normality and Levene tests were performed to test for normality and homogeneity of variance, respectively. This was done to ensure that the data met all the requirements for parametric analyses. Statistical comparisons of WG, SGR, FCR, ADC and FI values among the diets were executed with one-way analysis of variance (ANOVA) followed by the Tukey’s HSD post hoc test. All statistical analyses were run using SPSS (Statistical Package and Service Solutions software program, IBM SPSS version 20, Chicago, IL, USA) statistical package. The confidence interval was set at P < 0.05. The numerical data for the growth parameters and enzyme activities is presented as mean plus standard deviation.
Results
Growth parameters
The fish readily accepted the diets and there was no sign of rejection such as spitting of the pellets. The highest WG, SGR, ADC and FI values were found in fish fed with D6 and D7 compared to the silage inclusion levels (D2–D5) and the control (Table 4). The best FCR values were also recorded in fish fed with D6 (1.92 ± 0.10) and D7 (1.78 ± 0.19) than in the control (D1 = 3.13 ± 0.38) and the silage-based inclusion levels, where values decreased from 3.50 ± 0.34 in D2 to 2.41 ± 0.32 in D5 (Table 4). Among the fish fed with the fermented silage inclusion levels, WG, SGR and ADC values increased with increasing fermented silage in the diets (Table 4). In these dietary groups, WG was significantly higher (P < 0.05) in D4 (18.34 ± 1.51 g) and D5 (20.86 ± 1.95 g) than in D1 (14.28 ± 1.21 g), D2 (14.19 ± 1.51 g) and D3 (14.43 ± 1.34 g). Specific growth rates were higher in D4 (1.10 ± 0.19%) and D5 (1.23 ± 0.04%) than in D1 (0.96 ± 0.22%), D2 (0.74 ± 0.06%) and D3 (0.92 ± 0.04%). Feed conversion ratio was significantly (P < 0.05) lower in D4 (2.55 ± 0.27) and D5 (2.41 ± 0.32) compared to D1 (3.13 ± 0.38), D2 (3.50 ± 0.34) and D3 (3.26 ± 0.42). The highest ADC values were observed in D4 (93.50 ± 2.96%) and D5 (92.55 ± 3.90%) compared to the control (D1) (86.68 ± 2.80%). The highest FI values was recorded in D4 (0.93 ± 0.07) and D5 (0.97 ± 0.02) than in D1 (0.71 ± 0.05), D2 (0.69 ± 0.11) and D3 (0.75 ± 0.06) (Table 4).
Digestive enzymes
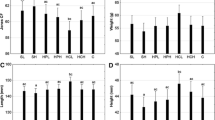
Fish fed with D6 (145 ± 6.05) had the highest protease activity compared to the silage inclusion levels (D1–D5) and D7 (142 ± 4.21) (Fig. 1A). Among the silage inclusion levels, fish fed with D4 (138 ± 4.21) and D5 (142 ± 3.99) had significantly higher (P < 0.05) protease activity than fish fed with the control (92 ± 3.72) and the lowest inclusion levels (D2 = 99 ± 4.61 and D3 = 125 ± 5.68) (Fig. 1A). The highest amylase activity was also recorded in fish fed with D6 (66 ± 3.29) followed by fish fed with D5 (63 ± 2.79) and D7 (62 ± 3.94) (Fig. 1B). Among the silage-based diets, amylase activity was higher in fish fed with the highest silage inclusion levels (D4 = 60 ± 3.31 and D5 = 63 ± 4.01) while the lowest amylase activity was recorded in fish fed with the control diet (D1) (35 ± 2.98) and the lowest silage inclusion levels (D2 = 47 ± 3.01 and D3 = 52 ± 3.47) (Fig. 1B). The highest lipase activity was also found in D6 (32 ± 2.61) followed by D7 (26 ± 2.76), D5 (25 ± 2.53) and D4 (23 ± 2.31), while the lowest levels were found in D1 (10 ± 1.82), D2 (13 ± 1.99) and D3 (15 ± 1.89) (Fig. 1C). Among the silage-based diets, lipase activity showed an increasing trend from the lowest silage inclusion levels (D2 = 13 ± 1.99) to the highest inclusion levels (D5 = 25 ± 2.53) (Fig. 1C). The lowest cellulase activity was detected in fish fed with D6 (0.29 ± 0.05) followed by fish fed with D7 (0.57 ± 0.08) when compared with fish fed with the control (D1 = 0.72) and silage inclusion levels (D2–D5) (Fig. 1D). Among the silage inclusion levels, cellulase activity was very low and it was not significantly affected among the diets (Fig. 1D). However, cellulase activity decreased with increasing silage levels in the diets (Fig. 1D).
Histology
There were no marked histological alterations observed in the intestines of fish across diets (Fig. 2). The histological measurements did not reveal any significant differences (P < 0.05) in villous length and width as well as intestinal muscle thickness of fish among diets (Table 5). The number of goblet cells also did not vary among dietary treatments (Table 5). Similarly, there were no discernible pathological changes in the liver of fish across the diets (Fig. 3). The hepatocyte condition across diets was awarded a score of 1, representing a normal liver condition (Table 5).
Discussion
In this study, the best growth performance (e.g. WG, FCR and SGR) and feed utilization were recorded in O. mossambicus fed with the highest silage inclusion levels (D4 and D5), fermented silage only (D6) and the reference diet (D7) when compared to fish fed with the control (D1) and the lowest silage inclusion levels (D2 and D3). The increase in growth performance with increasing fermented silage inclusion levels in the diets demonstrates the nutritive value of the fermented chicken waste as a suitable alternative protein source in the diet of O. mossambicus. It is of interests to note that the reference diet is formulated as a high-quality commercial diet for tilapia. However, this study showed that the growth performance of fish fed with the silage diet alone was not significantly different from the fish fed with the reference diet. The improvements in growth performance of fish fed with the highest silage inclusion levels as well as the silage diet alone (D6) could be attributed to improved bioavailability of hydrolyzed protein, carbohydrates and fat in the silage after acid fermentation.
Although there is limited information on the use of fermented chicken waste in fish diets with which to compare the present findings, available literature on other fermented animal waste products indicate that such products improve growth rates in fish. For example, Haider et al. (2016) found that inclusion of acid fish silage up to 75% in the diets of Labeo rohita (Rohu) Hamilton, 1822 improved growth performance. Rangacharyulu et al. (2003) also reported that the total replacement of fishmeal in the diets of cyprinids, Catla catla (Catla) Hamilton, 1822, Cirrhinus mrigala (Mrigal carp) Hamilton, 1822, L. rohita and Hypophthalmichthys molitrix (Silver carp) (Valenciennes, 1844), with acid-fermented silkworm pupae silage resulted in better growth performance compared to fish fed with the control. An improvement in growth performance was also reported in common carp, Cyprinus carpio Linnaeus, 1758, fed with silage produced from fish (Manikandavelu et al. 1992) and silage prepared from fish viscera and poultry intestines (Rao et al. 1996).
The increase in the growth performance of fish fed with the silage-based diets was also demonstrated by the higher ADC values (> 86%) in fish fed with the highest silage inclusion levels (D4 and D5) and D6. The determination of digestibility coefficients provides an indication about nutrient utilization in fish (Lee 2002). In this study, the fermented silage in D2–D5 was mixed with higher amounts of raw ingredients such as maize, soya bean, canola meal and sunflower, which naturally contain certain amounts of different ANFs or fibre and complex proteins/lipids. The control diet contained no silage, but also contained the same raw ingredients. The presence of different inhibitory factors such as ANFs or fibre in these raw ingredients could have reduced digestibility and bioavailability of nutrients (Makkar et al. 2007). On the other hand, fermentation has been shown to enhance the quality of raw food components by removing antinutrients (Sudarmadji and Markakis 1977; Kirk 1979; Fardiaz and Markakis 1981; Sutardi and Buckle 1985; Teutino and Knorr 1985; Mukhopadhyay and Ray 1999). This may explain the reduced growth performance observed in fish fed with the control and D2–D5 when compared to fish fed with D6, indicating that the D1–D5 diets may have contained low levels of hydrolyzed nutrients. It must be kept in mind that the silage was mixed with maize, which contains ANFs or fibre. However, the higher ADC values in fish fed with the silage diet indicate that the acid fermentation may have reduced ANFs or fibre in the maize and improved its digestibility and nutrients availability. A number of previous studies have shown that the inclusion of hydrolyzed protein in the diet of fish enhanced digestibility (Ramírez-Ramírez et al. 2008; Kaviraj et al. 2013; Samaddara et al. 2015). Samaddara et al. (2015) observed that fermented animal by-product in the diets of L. rohita increased protein digestibility and they reported that the fermented by product was characterized by higher level of EAAs. This is consistent with the growth performance observed in fish fed with the highest silage inclusion levels (D5 and D5), which was significantly higher than in fish fed with the control and the lowest silage inclusion levels (D2 and D3).
The current study showed that the fermented silage had the appropriate nutritional value for the fish. For example, the proximate composition showed that the carbohydrate and fat content of the fermented silage was significantly higher than in the reference diet. This is evident in the higher growth rates recorded in fish fed with the fermented silage only when compared to D1–D5. Therefore, the reduced growth performance in fish fed with the control diet, which contained the highest fishmeal inclusion level, may be due to high levels of complex protein (less digestible) normally found in fishmeal and the other ingredients used. Although the fermented silage had a lower protein content (29.31%) than the reference diet (35%), it has been shown that the importance of a protein source does not only depend on the amount of protein it possesses, but also on the composition of the amino acids and their bio-availability (El-Sayed 2006). In aqua-feeds, sufficient quantities of EAAs are considered important basic requirements for key protein sources. The chemical analyses conducted on the silage showed that the levels of EAAs were within acceptable ranges for O. mossambicus, despite being lower than that found in fishmeal. Wilson (2002) stated that protein digestibility is positively correlated with the availability of EAAs and FAAs in the diet. Therefore, the high fat content in the silage could also be indicative of increased bio-availability of free fatty acids, which may have contributed to improved growth performance.
The higher growth rates and digestibility values obtained in fish fed with the highest silage inclusion levels and the silage diet was supported by the activities of the digestive enzymes. Fermented food products are known to enhance activities of a range of digestive enzymes such as protease, lipase and amylase (Samtiya et al. 2021), thus leading to increased availability and uptake of nutrients by the fish. For instance, protease activity in our study was higher in fish fed with the highest silage inclusion levels (D4 and D5) and the fermented silage diet. This is indicative of increased digestibility of protein and increased availability of EAAs. Generally, protease activity in fish is affected by the feed and its constituents, pH, fish size and feeding habits of the fish (Hidalgo et al. 1999; Lazzari et al. 2010). The activities of amylase were also higher in fish fed with diets containing the highest fermented silage and D6. Activities of amylase are known to increase with increasing carbohydrate content in the diet (German et al. 2004). In this study, amylase may have increased due to an increase in the availability of hydrolysable carbohydrate in the silage. This shows that O. mossambicus handled the fermented silage better and this resulted in improved growth rates. Lipase activity showed an increasing trend as the level of the silage increased in the diets and D6 had the highest lipase activities. Lipase activity provides an indication about the hydrolyses of fat in the diet of the fish. For example, De Almeida et al. (2006) reported that lipase activity in Colossoma macropomum (Cuvier 1818) increased with an increase in dietary lipids. The higher lipase activity observed with increasing fermented silage in the diets may indicate an increase in the availability of free fatty acids, with increased bio-availability. It may also indicate the capacity of O. mossambicus to efficiently digest and utilize the lipids from the fermented silage.
Cellulase activity was very low across silage inclusion levels, but the lowest levels were recorded in fish fed with the silage only. The low cellulase activity in fish fed with the fermented silage only was not unexpected, as fermentation may have reduced the presence of indigestible material in the silage diet. On the other hand, the slightly higher cellulase activity in D1–D5 may have been elicited by the presence of cellulose content, as the ingredients used are naturally rich in cellulose. Generally, cellulose is poorly digested by many fish species (Sinha et al. 2011; Rahimnejad et al. 2019). However, previous studies have shown that O. mossambicus has the capacity to handle diets with cellulose content, albeit at low capacity (Hlophe and Moyo 2013). Additionally, it has been reported that various herbivorous fish can also digest cellulose in their diets by using microbes in the gastrointestinal tract exerting cellulolytic activities with the ability to reduce crude fibre from plant proteins (Saha et al. 2006; Lee et al. 2008; Mondal et al. 2008; Ghosh et al. 2010).
The histological evaluation of the liver and gut did not reveal any marked pathological changes among diets. This indicates that the diets were not harmful to the health of the fish. The pH of the silage was maintained below < 4 to ensure that no pathogenic bacteria could thrive and subsequently harm the fish. On the other hand, Nasser et al. (2019) reported up to 100% mortalities when fish were fed diets containing unfermented or unprocessed food waste. The findings from Nasser et al. (2019) demonstrated that the use of food waste in fish diets could poison the fish due to the presence of harmful bacteria. It is thus important to process food waste to eliminate potentially harmful pathogenic microorganisms before it can be used in aqua-feeds. Furthermore, the low levels of ANFs in fermented meals reduce their toxic effect on gut health (Makkar et al. 2007).
Conclusion
This study has shown that the fermented silage can be used in aqua-feeds without compromising growth or affecting the health of the fish. It was also shown that it could be used as a complete diet for O. mossambicus. Local chicken farmers indicated that unwanted mortalities could be as high as 2% during production on farms, thus creating an opportunity for silage processing into cheaper fish feeds. Tilapia farmers in the small-scale sector can benefit from using chicken waste since it is readily available and cheaper to source. It can be easily processed on the farm and requires little training. All that is needed is for farmers to be trained to learn the basic techniques used during fermentation so that they can produce their own feed. Although the current findings are promising, there is a need to test the fermented chicken waste silage at production level. There is also a need to test it on Clarias gariepinus (African sharptooth catfish), which is another freshwater fish species widely cultured in South Africa.
Data availability
The data that supports the findings of this study is available on request from the corresponding author.
Code availability
Not applicable.
References
AOAC (Association of Official Analytical Chemists) International (2012) Official Methods of Analysis of AOAC International, 19th edn. Association of Analytical Communities, Gaithersburg
Baeverfjord G, Krogdahl A (1996) Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: a comparison with the intestines of fasted fish. J Fish Dis 19:375–387. https://doi.org/10.1046/j.1365-2761.1996.d01-92.x|
Bernfeld P (1951) Enzymes of starch degradation and synthesis. In: Nord FF (ed) Advances in Enzymology and Related Areas of Molecular Biology. John Wiley and Sons, Inc., Hoboken, NJ, USA
Bezerra RS, Lins EJF, Alencar RB, Paiva PMG, Chaves MEC, Luana CBB, Carvalho LB Jr (2005) Alkaline proteinase from intestines of Nile tilapia (Oreochromis niloticus). Process Biochem 40:1829–1834. https://doi.org/10.1016/j.procbio.2004.06.066
DAFF (Department of Agriculture, Forestry and Fisheries) (2019) Aquaculture yearbook. Department of Agriculture, Forestry and Fisheries, South Africa, pp 214
De Almeida LC, Lundstedt LM, Mores G (2006) Digestive enzyme response of Tambaqui (Colossoma macropomum) fed on different levels of protein and lipid. Aquac Nutr 12:443–450. https://doi.org/10.1111/j.1365-2095.2006.00446.x
El-Sayed AFM (2006) Tilapia Culture. CABI Publishers, Oxfordshire, UK
Fagbenro O, Jauncey K, Haylor G (1994) Nutritive value of diets containing dried lactic acid fermented fish silage and soybean meal for juvenile Oreochromis niloticus and Clarias gariepinus. Aquat Living Resour 7:79–85. https://doi.org/10.1051/alr:1994010
FAO (Food and Agriculture Organization of the UN) (2016) The State of World Fisheries and Aquaculture. Contributing to food security and nutrition for all. Italy, Rome, pp 200
Fardiaz D, Markakis P (1981) Degradation of phytic acid in oncom (fermented peanut press cake). J Food Sci 46(2):523–525. https://doi.org/10.1111/j.1365-2621.1981.tb04901.x
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Francis G, Makkar HPS, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227. https://doi.org/10.1016/S0044-8486(01)00526-9
Furukawa A, Tsukahara H (1966) On the acid digestion method for determination of chromic oxide as an indicator substance in the study of digestibility in fish. Bull Japan Soc Sci Fish 32:502–506
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38(6):551–579. https://doi.org/10.1111/j.1365-2109.2007.01704.x
German DP, Horn MH, Gawlicka A (2004) Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Physiol Biochem Zool 77:789–804. https://doi.org/10.1086/422228
Ghosh K, Roy M, Kar N, Ringø E (2010) Gastrointestinal bacteria in Rohu, Labeo rohita (Actinopterygii: cypriniformes: Cyprinidae): scanning electron microscopy and bacteriological study. Acta Ichthyol Piscat 40(2):129–135. https://doi.org/10.3750/AIP2010.40.2.05
Gupta RK, Gangoliya SS, Singh NK (2015) Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol 52:676–684. https://doi.org/10.1007/s13197-013-0978-y
Haider MS, Ashraf M, Azmat H, Khalique A, Javid A, Atique U, Zia M, Iqbal KJ, Akram S (2016) Nutritive evaluation of fish acid silage in Labeo rohita fingerlings feed. J Appl Anim Res 44(1):158–164. https://doi.org/10.1080/09712119.2015.1021811
Hardy RW (2010) Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquac Res 41(5):770–776. https://doi.org/10.1111/j.1365-2109.2009.02349.x
Hidalgo MC, Urea E, Sanz A (1999) Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic Amylase Act Aquac 170:267–283
Hlophe SN, Moyo NAG (2013) The aquaculture potential of Tilapia rendalli in relation to its feeding habits and digestive capabilities. Phys Chem Earth 66:33–37. https://doi.org/10.1016/j.pce.2013.09.006
Jauncey K, Tacon AGJ, Jackson AJ (1983) The quantitative essential amino acid requirements of Oreochromis (Sarotherodon) mossambicus. In: Fishelson L, Yaron Z (eds) Proceedings of the international symposium on Tilapia in Aquaculture. Tel Aviv University, Tel Aviv, pp 328–337
Kaviraj A, Mondal K, Mukhopadhyay PK, Turchini GM (2013) Impact of fermented mulberry leaf and fish silage in diet formulation of Indian major carp (Labeo rohita). Proc Zool Soc 66(1):64–73. https://doi.org/10.1007/s12595-012-0052-1
Kirk JR (1979) Nutritional changes upon processing. In: Clydesdale F (ed) Food Science and Nutrition. Current Issues and Answers. Prentice Hall, Inc., Englewood Cliffs, NJ, pp 62
Lazzari R, Neto JR, Pedron FA, Loro VL, Pretto A, Gioda CR (2010) Protein sources and digestive enzyme activities in jundiá (Rhamdia quelen). Sci Agric 67:259–266. https://doi.org/10.1590/S0103-90162010000300002
Lee SM (2002) Apparent digestibility coefficients of various feed ingredients for juvenile and grower rockfish (Sebastes schlegeli). Aquaculture 207:79–95. https://doi.org/10.1016/S0044-8486(01)00751-7
Lee YJ, Kim BK, Lee BH, Jo KI, Lee NK, Chung CH, Lee YC, Lee JW (2008) Purification and characterization of cellulase produced by Bacillus amyoliquefaciens DL-3 utilizing rice hull. Bioresour Technol 99:378–386. https://doi.org/10.1016/j.biortech.2006.12.013
Makkar HPS, Francis G, Becker K (2007) Bioactivity of phytochemicals in some lesser-known plants and their effects and potential applications in livestock and aquaculture production systems. Animal 1:1371–1391. https://doi.org/10.1017/S1751731107000298
Manikandavelu D, Neethiselvan J, Jagatheeshan G, Sundarsanam K (1992) Use of fermented ensilage based diet in enhancing the growth of common carp (Cyprinus carpio var communis). Fish Technol 29(2):111–113
Markweg H, Lang MS, Wagner F (1995) Dodecanoic acid inhibition of lipase from Acinetobacter sp. OPA 55. Enzyme Microb Technol 17:512–516. https://doi.org/10.1016/0141-0229(94)00067-2
McFadzen IRB, Coombs SH, Halliday NC (1997) Histological indices of the nutritional condition of sardine, Sardina pilchardus (Walbaum) larvae of the north coast of Spain. J Exp Mar Biol Ecol 212:239–258
Mondal S, Roy T, Sen SK, Ray AK (2008) Distribution of enzyme-producing bacteria in the digestive tracts of some freshwater fish. Acta Ichthyol Piscat 38(1):1–8. https://doi.org/10.3750/AIP2008.38.1.01
Mukhopadhyay N, Ray AK (1999) Effect of fermentation on the nutritive value of sesame seed meal in the diets for rohu, Labeo rohita (Hamilton), fingerlings. Aquac Nutr 5:229–236. https://doi.org/10.1046/j.1365-2095.1999.00101.x
Nasser N, Babikian J, Hatem MG, Saoud IP, Abiad MG (2019) Evaluation of post-consumer food waste as partial replacement of commercial feed in marbled rabbitfish, Siganus rivulatus aquaculture. Int J Environ Sci Technol 16:4059–4068. https://doi.org/10.1007/s13762-018-2051-x
National Research Council (NRC) (2011) Nutrient requirements of fish and shrimp. National Academy Press, Washington DC
Peace RW, Gilani GS (2005) Chromatographic determination of amino acids in foods. J AOAC Int 88(3):877–887. https://doi.org/10.1093/jaoac/88.3.877
Rahimnejad S, Lu K, Wang L, Song K, Mai K, Davis DA, Zhang C (2019) Replacement of fishmeal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol 84:987–997. https://doi.org/10.1016/j.fsi.2018.11.009
Ramírez-Ramírez JC, Huerta S, Arias L, Prado A, Shirai K (2008) Utilization of fisheries by-catch and processing wastes for lactic acid fermented silage and evaluation of degree of protein hydrolysis and in vitro digestibility. Rev Mex Ing Quim 7(3):195–204
Rangacharyulu PV, Giri SS, Paul BN, Yashoda KP, Rao RJ, Mahendrakar NS, Mohanty SN, Mukhopadhyay PK (2003) Utilization of fermented silkworm pupae silage in feed for carps. Bioresour Technol 86:29–32. https://doi.org/10.1016/s0960-8524(02)00113-x
Rao RJ, Mahendrakar NS, Chakrabarthy NM, Raghavan SL (1996) Utilisation of fermented fish and poultry silages in feed for common carp (Cyprinus carpio). Seafood Export J 27(3):17–23
Saha S, Roy RN, Sen SK, Ray AK (2006) Characterization of cellulase-producing bacteria from the digestive tract of tilapia, Oreochromis mossambicus (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes). Aquac Res 37:380–388. https://doi.org/10.1111/j.1365-2109.2006.01442.x
Samaddara A, Kaviraja A, Saha A (2015) Utilization of fermented animal by-product blend as fishmeal replacer in the diet of Labeo rohita. Aquac Rep 1:28–36. https://doi.org/10.1016/j.aqrep.2015.03.004
Samtiya M, Aluko RE, Puniya AK, Dhewa T (2021) Enhancing micronutrients bioavailability through fermentation of plant-based foods: a concise review. Fermentation 7(2):63. https://doi.org/10.3390/fermentation7020063
Sinha AK, Kumar V, Makkar HP, De Boeck G, Becker K (2011) Non-starch polysaccharides and their role in fish nutrition–a review. Food Chem 127:1409–1426
Sudarmadji K, Markakis P (1977) The phytate and phytase of soyabean tempeh. J Sci Food Agric 28(4):381–383. https://doi.org/10.1002/jsfa.2740280410
Sutardi A, Buckle KA (1985) Reduction in phytic acid levels in soybean during tempeh production, storage and frying. Food Sci 50(1):260–263. https://doi.org/10.1111/j.1365-2621.1985.tb13325.x|
Teutino RA, Knorr D (1985) Impact of biotechnology on nutritional quality of food plants. Food Technol 39:127–134
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
Vidotti RM, Carneiro DJ, Viegas EMM (2002) Growth rate of Pacu, Piaractus mesopotamicus, fingerlings fed diets containing co-dried fish silage as replacement of fish meal. J Appl Aquac 12(4):77–88
Wilson RP (2002) Amino acids and proteins. In: Halver JE, Hardy RW (eds) Fish Nutrition, 3rd edn. Academic Press, San Diego, California, pp 143–179
Winberg GG (1956) Rate of metabolism and food requirements of fishes. J Fish Res Board Can 194:1–202
Zeineddine R, Ireland B, Monzer S, Saoud IP (2021) Preliminary assessment of restaurant food waste as a feed ingredient for small juvenile rainbow trout Oncorhynchus mykiss). Aquac Int 29:669–679. https://doi.org/10.1007/s10499-021-00650-4
Acknowledgements
The authors would like express their heartfelt gratitude to Mr. Nell Wiid, a fish farmer who helped to collect the chicken carcasses and process it on his farm. We are also grateful to honours students at ARU who assisted during fish sampling and dissections. We are equally thankful to the anonymous reviewers whose criticism and guidance led to an improved final product.
Funding
The Department of Forestry, Fisheries and the Environment provided funds for this study.
Author information
Authors and Affiliations
Contributions
Chris Henri Fouche and Esau M. Mbokane conceived the study and wrote the manuscript. Esau M. Mbokane and Lucia Matlale Mbokane carried out the experiments, collected data, analyzed and interpreted it.
Corresponding author
Ethics declarations
Ethics approval
This study is part of a multi-year project focusing on the use of locally-available plant and animal waste resources in an effort to develop cheaper and high-quality diets for freshwater fish species in South Africa. Ethical clearance was granted by the University of Limpopo Animal Research Ethics Committee (AREC) of the Faculty of Science and Agriculture. Handling and sacrificing of fish was executed in a humane manner, and international, national and/or institutional guidelines governing the use of animals were observed at all times.
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Gavin Burnell.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mbokane, E.M., Mbokane, L.M. & Fouche, C.H. The effect of fishmeal replacement with acid-fermented chicken silage on growth, digestive enzyme activity and histology of the intestine and liver of juvenile Mozambique tilapia (Oreochromis mossambicus). Aquacult Int 30, 2491–2512 (2022). https://doi.org/10.1007/s10499-022-00916-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-00916-5