Abstract
Chitin is the most important natural polysaccharide after cellulose; however, it is not widely used for industrial application because of its insolubility in many solvents. The present study utilizes Indian major carps (Labeo rohita, Catla catla, and Cirrhinus mrigala) scale to extract chitin and prepare chitosan by a sequence of chemical processes involving demineralization, deproteinization, and deacetylation. The extracted chitosan is characterized by different analytical techniques viz. UV-Vis spectroscopy, FT-IR, TGA, and XRD, and SEM analysis reveals that fish scale waste are good source of chitosan. Compositional analysis of fish scale waste was done by standard methods which reveal that the extracted chitosan was having 0.691% moisture content, 0.53% ash, 109% fat-binding assay (FBC), 104% water-binding assay (WBC) indicating the value depend upon the source of origin and optimization of chemical process of extraction. The antibacterial activity of chitosan against Aeromonas hydrophila, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa was evaluated by agar well diffusion method. The chitosan also exhibited DPPH scavenging along with anti-inflammatory and anticoagulant activity in a dose-dependent manner. The extracted chitosan also demonstrated preservation and seed germination activities. The result of the present study strongly supports the use of chitosan in pharmaceutical, food industry, and agro-based industries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish processing industries generate solid wastes that include scales, bones, shells, skin, and head that can be as high as 50–80% of the original raw material. This waste is very resistant to biodegradation and also their disposal is problematic. If not properly treated, it may spoil quickly and contaminate the environment and cause pollution (Kumari et al. 2016; Islam et al. 2004). Therefore, proper management of fish waste is an essential requirement in the present scenario across the globe. In this context, the Indian Major Carps (IMC) that includes Labeo rohita, Catla catla, and Cirrhinus mrigala which is one of the most cultivable and consumable freshwater fishes in India and particularly in the state of Odisha produces a bulk amount of fish waste including scale. However, these scales are almost got unused and further add up environmental pollution. On the other hand, this scale can be converted into excellent, efficient products which can be utilized for various industrial uses including pharmaceutical, food industry, and agro-based industries. This includes bioactive peptides, collagen, chitosan, gelatin, which can be commercially marketed. Among this chitosan, a poly-β-(1-4)-2-amino-2-deoxy-D-glucopyranose which is derived from chitin is found fish scale. The wide range of applications in which chitosan is involved are chelating agents, drug carriers, water treatment (membrane filters), like wound-healing agents, biodegradable film in food coating, and biomaterials for nerve repair. It is also used as antibacterial agent as well as potential food preservative. Apart from these industries, chitosan is also used in making cosmetics, dietary supplements, pesticides, biopolymer, and paper (Walke et al. 2014).
In this context, the present study aimed production of chitosan by a sequence of chemical processes involving demineralization, deproteinization, and deacetylation utilizing the fish scale derived from Indian major carps. Furthermore, the prepared chitosan was evaluated for their antibacterial, radical scavenging, anti-inflammatory, anticoagulant activities along with preservation and seed germination potential.
Materials and methods
Collection of samples
The material investigated was the Indian major carps (IMC), i.e., (Labeo rohita, Catla catla, and Cirrhinus mrigala) scales obtained from various local fish markets of Bhubaneswar, Odisha. The scales were separated from other impurities like dust, leaf, fins, and gills by thorough washing with tap water followed by distilled water. The clean scales were then dried in a hot air oven at 60 °C for 48 h and stored in an air tight container at room temperature until further processing.
Extraction of chitosan
Chitosan extraction from fish scale was carried out by demineralization, deproteinization, and deacetylation processes (No and Meyers 1992).
Demineralization
Initially, the scales were demineralized using 0.1N HCl at a solid to solvent ratio of (1:4.5) (w/v) at 90 °C for 15 min on a hot plate. Then the solution is decanted leaving the sample behind. Then the sample was washed with deionized water for 15 min to attain neutrality. This process resulted chitin as the product (Kumari et al. 2016).
Deproteinization
The demineralized sample was deproteinated using 1% NaOH solution in the ratio of (1:4) (w/v) heated at 100 °C for 15 min with intermittent mixing. The synthesized sample was filtered and washed using distilled water for further neutralization.
Deacetylation
Deacetylation was achieved by refluxing the deproteinized sample with aqueous 15% NaOH at a ratio of 1:2 (w/v) for 15 min at 60 °C (Kumari et al. 2016). The chitosan extracted was washed with deionized water and dried at 60 °C for 48h and stored in airtight container.
Physiochemical properties
Solubility
The solubility % of chitosan sample was checked by dissolving 100 mg of chitosan sample in 10 ml of 1% acetic acid for 30 min by continuous stirring in a magnetic stirrer at 240 rpm.
Moisture content
Moisture content was checked by drying the extracted chitosan samples in a hot-air oven at 60 °C for 48 h. The moisture content was calculated as follows.
Ash content
The ash content was determined by muffle furnace (Helrich 1990). For this, 2 g of sample was taken in a pre weighed crucible with lid. The sample was charred. Both the sample and crucible were weighed. Then it is placed inside a preheated muffle furnace at 600 °C for 6 h. After cooling, the crucibles are taken out of the furnace using metal tongs and placed in desiccators. Then the ash content is found by weighing the ash.
Water-binding capacity and fat-binding capacity
Water-binding capacity (WBC) and fat-binding capacities (FBC) of chitosan were measured (Wang and Kinsella 1976). Water and fat absorption were initially carried out by weighing a centrifuge tube containing 0.5 g of sample, adding 10 ml of water or soybean oil and mixing on a vortex mixer for 1 min to disperse the sample. The contents were left at ambient temperature for 30 min with shaking for 5 s every 10 min and centrifuged at 3500 rpm for 25 min. After the supernatant was decanted, the tube was weighed again. WBC and FBC were determined as follows:
Characterization
Ultraviolet–visible spectroscopy
The prepared sample was studied using ultraviolet–visible spectrophotometer (Perkin Elmer Lambda 365) to verify the formation of chitosan. The absorbance of the sample was scanned in the range of 200 to 800 nm and the wavelength corresponding to maximum absorption (λmax) was recorded (Gokulalakshmi et al. 2017).
FT-IR
The extracted chitosan was characterized by Fourier-transform infrared spectroscopy (FT-IR) assay. A homogeneous sample disc was made by mixing KBr (200 mg) and chitosan (3 mg) thoroughly. The samples were analyzed in the range of 400 to 4000 cm−1 using FT-IR spectrophotometer (Shimadzu) (Gokulalakshmi et al. 2017).
Thermogravimetric analysis (TGA)
Thermogravimetric analysis (TGA) of the extracted chitosan sample was carried out using Mettle-Toledo Thermal analyzer.
Five milligrams of chitosan sample was taken for analyzing devolatilization characteristic in a temperature range of 30–600 °C at a rate of 10 °C per min under N2 atmosphere (Walke et al. 2014).
X-ray powder diffraction (XRD)
X-ray powder diffraction (XRD) is primarily used for phase identification of a crystalline material and to determine the polymorphic forms of a compound with different crystalline structures (Paul et al. 2014). The XRD measurement of the chitosan sample was performed on a XRD diffractometer (Rigaku Ultima IV) with CuKα radiation. The tube voltage was set at 40 kV, and the current was set at 40 mA with a wavelength of 1.54060 nm. The XRD diffraction patterns were taken in the range of 10 to 80° 2θ range at 1.0°/min scan rate.
Scanning electron microscopy (SEM)
The surface morphology of extracted chitosan sample was observed using scanning electron microscope (Hitachi S3400N). The chitosan sample was mounted on the SEM stubs with double-side adhesive tapes. However, the chitosan samples were not coated with platinum or gold (Zaku et al. 2011). Then stub containing the sample was placed in the SEM chamber under vacuum. Chitosan samples were studied under SEM with different magnifications. All the microscopic structures were carefully studied and analyzed for the identification of chitosan.
Biological activity
Antibacterial activity
Antibacterial activity of chitosan solution at different concentrations was tested against Gram-positive bacteria Staphylococcus aureus, and Gram-negative bacteria Aeromonas hydrophila, Escherichia coli, and Pseudomonas fluorescens by disc diffusion method (Paul et al. 2014). The antibacterial activity was assessed by measuring the diameter of zone of inhibition.
Anticoagulant activity
The anticoagulant activity of Chitosan was carried out following the method of Tarafdar and Biswas (2013) with slight modification. 0.1% of chitosan solution (3ml, 5ml, 7ml) were taken in sterile test tubes to which fresh chicken blood was poured and the volume was made up to 10 ml. Then the test tubes were plugged carefully and kept undisturbed at room temperature for observation of any clotting sign after 7, 30, 60, 90, and 120 min. The heparin was used as a standard anticoagulant against which the anticoagulant activity of the chitosan was compared.
DPPH radical scavenging activity
DPPH (1,1-diphenyl-2picrylhydrazyl) radical scavenging activity of chitosan was measured by following the standard procedure (Das et al. 2018). Different concentrations of Chitosan sample (0.75, 1.0, 1.5 mg/ml) were mixed with methanolic DPPH solution and incubated for 30 min in a dark condition. The absorbance was measured at 517 nm using a spectrophotometer. The DPPH scavenging potential of the chitosan was calculated as follows.
Anti-inflammatory activity by protein denaturation study
The in vitro anti-inflammatory activity of chitosan was determined by protein denaturation method by following the method of Murugan and Parimelazhagan (2014) with slight modification. The reaction mixture (0.5 ml, pH 6.3) consisted of 0.45 ml of Bovine serum albumin (5% aqueous solution) and 0.05 ml of distilled water and pH was adjusted at 6.3 using a small amount of 0.1N HCl. Chitosan samples at various concentrations (0.75, 1.0, 1.5 mg/ml) were added to the reaction mixture and incubated at 37°C for 20 min. Then the reaction mixture was heated at 72 °C for 15 min. After cooling the samples, 2.5 ml of phosphate buffer solution was added and absorbance was taken at 600 nm against the control containing BSA and phosphate buffer. The protein denaturation activity was calculated as follows.
Effect of chitosan on seed germination
The effect of chitosan on seed germination was assessed by following the method of Jeyaraman et al. (2017). Briefly, 20 mung bean seeds were taken and 10 seeds were placed in water and another 10 seeds were placed in a solution containing 0.1% of chitosan. Seeds were incubated for 6 h following which seeds were tied in double-layered muslin cloth and placed in a damp place. The growth promotions of seeds were studied after 4 days of incubation.
Food preservative activity of chitosan
Food preservative activity of chitosan was studied by the method of Tarafdar and Biswas (2013). Five different types of vegetable and fruits namely capsicums, tomatoes, eggplant, apples, and cucumbers were taken in this study. Five sets of each experiment were prepared viz. Set-I using capsicum, Set-II using tomato, Set-III using eggplant, Set-IV using apple and Set-V using cucumber were coated with 0.1% IMC chitosan by dipping the vegetable in chitosan solution. One set from each fruit or vegetable was kept as control. Both chitosan coated and control samples were kept for observation for 2 weeks at room-temperature. The experiments were performed in triplicate.
Results and discussion
The physicochemical properties of the chitosan prepared from IMC scale were given in Table 1.The prepared chitosan is further characterized by employing different techniques (Table 2).
Physicochemical properties
In the present study, the prepared chitosan from IMC scale was insoluble in water but found to be soluble in 1% acetic acid. Chitosan is a semi-crystalline biopolymer, and it is not soluble in most of the solvents like water, alkali, or aqueous solution (pH ≈ 7) and common organic solvents. At certain pH values under continued stirring, chitosan is soluble in few acids such as hydrochloric, lactic, propionic, phosphoric, tartaric, citric, succinic, acetic, and formic acids (Chung et al. 2005; Krajewska 2004; Qin et al. 2006). The moisture content was found to be 0.676 %.
The ash content is an important parameter that affects chitosan solubility, viscosity, and also other important characteristics. The ash content of the extracted chitosan was reported to be 0.37% that indicated the efficiency of the demineralization step in chitosan preparation process in removing minerals. However, earlier studies have shown that the ash content of chitosan may differ on the basis of methodology employed (Mohanasrinivasan et al. 2013).
FBC assay signifies fat binding or absorption capacity of chitosan. The FBC of prepared chitosan is 109%. It has been reported that the FBC of chitosan depends upon molecular weight and density (Panith et al. 2016). WBC is stated with respect to the molecular weight and degree of crystalline of chitosan. The present study demonstrated the WBC of prepared chitosan as 104%. The WBC of chitosan depends upon crystallinity of the products, differences in the number of salt-forming groups, and differences in the protein content of the materials (Knorr 1982).
Characterization of chitosan
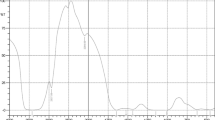
UV-Vis spectral study indicated a peak in the range of 250–270 nm that showed the formation of chitosan (Fig. 1). The present study is in accordance with the earlier study by Gokulalakshmi et al. (2017).
The FT-IR study showed different absorption bands in the range of 500 to 4500 cm−1 (Fig. 2). The major absorption band at 1036.78 and 1048.1 represent the CO stretching vibration of the structure. The peak noticed at 1566.27 corresponds to N–H bending of secondary amide II band of –CONH. Furthermore, the sample showed the absorption band at 1496.83 indicating the presence of –CH3 group. Peak at 3510.6 showed the presence of NH stretch. Similarly, absorption band at 872.83 corresponds to ring stretching of the structure (Table 1). This showed the confirmation of formation of chitosan (Paul et al. 2014; Di Martino et al. 2005).
It is observed from the thermogram that the chitosan has stage-wise weight loss in two different temperature ranges. The initial weight loss is in the range of 30 to 120 °C corresponds to the removal of moisture content, and the second loss in the range between 210 and 360 °C may be attributed to deacetylation, depolymerization, and decomposition of chitosan (Fig. 3). The present study is in accordance with earlier study of Kumari et al. (2016). TGA provides quantitative measurement of mass change in materials associated with transition and thermal degradation. The loss in weight over specific temperature ranges provides an indication of the composition of the sample as well as indications of thermal stability. The study indicates the lower thermal stability of chitosan which may be due to introduction of weak linkage into the polymer chain and arises due to process condition and impurities (Nising 2006; Andrade et al. 2012).
The XRD study is used to determine the polymorphic form of compounds having different crystalline structures. In the present study, chitosan extracted from IMC was studied for XRD pattern. The IMC-extracted chitosan exhibited peaks 2θ were 25.81°, 32.12°, 53.81°, and 63.94° which correspond to characteristic peaks of chitosan (Fig. 4). The intense peak at 32.12 indicates the presence of hydroxyapatite (Kaya et al. 2014). This is in accordance with earlier study made by Allison et al. (2014) and Zaku et al. (2011). The sharp peak at 25.81° corresponds to chitosan which was in accordance with the earlier findings of Kumari et al. (2016) and Zaku et al. (2011).
It is important to study the morphological characteristics of the sample to check the presence of chitosan. In the present study, chitosan was selected morphological examination by SEM (Fig. 5). The extracted chitosan was observed to have layer of flakes. The sample exhibited rough and thick surface morphology under electron microscopic examination at × 50 magnification which was in accordance to the previous studies (Kumari and Rath 2014).
Biological activity
Antibacterial activity
The antibacterial activity of extracted chitosan was carried out against the microbial strains S. aureus, P. fluorescens, A. hydrophila, and E. coli. The formation of clear zone around the paper disc impregnated with chitosan indicated the antibacterial activities. The extracted chitosan exhibited antibacterial activity against all the four bacterial strains studied with the inhibition zone of 22.5 mm (P. fluorescens), 23 mm (A. hydrophila), 19.5 mm (E. coli), and 21 mm (S. aureus). Earlier studies have indicated that chitosan exhibited better antibacterial properties against Gram-negative bacteria. However the antibacterial potential of inhibition may vary depending upon the source of chitosan and the types of microbial strains. The present study indicated that the extracted chitosan could inhibit the growth of both Gram-positive bacteria and Gram-negative bacteria (Table 3). The antibacterial potential of chitosan is due to the large surface area that provides better contact with microbes (Suman et al. 2013). The mechanism behind antibacterial activity of chitosan is due to the interaction between polycationic groups of chitosan with cell surface of bacteria leading to the formation of an impermeable layer around the cell and preventing transportation of essential nutrients. The results of the present study showed encouraging results against bacterial pathogens with maximum inhibitory activity against the A.hydrophila.
Anticoagulant activity
The anticoagulant activity of the extracted chitosan was evaluated by taking three different concentrations at different time points. The result indicated that chitosan exhibited anticoagulant activity at different concentrations at different time points. After 7 min and 30 min, there was no sign of clotting in all the experimental test tubes containing 3 ml, 5 ml, or 7 ml chitosan and heparin solutions. After 1 h, minor clotting has just appeared in test tube containing 3 ml chitosan, but there was no sign of clotting in the other test tubes. After 1 h 30 min and 2h of experiment, test tube containing 7 ml chitosan solution showed no signs of clot formation whereas test tube containing 3 and 5 ml of chitosan showed minor clotting (Table 4). It has been reported that, chitosan sulphates with lower molecular weight possess a regular increase of anti-coagulant activity like heparin.
DPPH scavenging activity
The present study was the first to examine the antioxidant properties of the chitosan obtained from IMC by using DPPH scavenging assay. The DPPH scavenging potential of the extracted chitosan at 0.75, 1.0, and 1.5 mg/ml concentrations were found to be 10.13%, 15.4%, and 21.47% respectively (Table 5). The study showed that the DPPH scavenging effect increased with increasing concentrations of the chitosan. In another study, chitosan prepared from white shrimp waste could scavenge DPPH radicals with 3.7 to 16.8% in the concentration range of 1 to 2 mg/ml (Trung and Bao 2015). Several earlier studies have also reported the DPPH scavenging potential of chitosan (Gokulalakshmi et al. 2017; Rajalakshmi et al. 2013).
Protein denaturation activity
The anti-inflammatory activity of chitosan prepared from scales of IMC was studied by protein denaturation method. The present study showed that chitosan showed good activities in a dose-dependent manner and there is a gradual increase in % of inhibition with the increase of concentrations of chitosan. At 0.75, 1.0, and 1.5 mg/ml concentrations, chitosan could inhibit the protein denaturation with 44.93%, 70.6%, and 93.63% respectively (Table 5).
Effect on seed germination
One of the major performance indexes of mung bean growth is germination percentage which was studied under chitosan-treated condition. In the present study, chitosan-treated seed showed 100% germination whereas under similar condition, the non-treated mung bean showed only 90% of germination. Similar results were also reported in earlier study carried out by Jeyaraman et al. (2017). Chitosan being cationic in nature can easily bind to the different binding sites in the plant root surface which leads to prolong the contact time between plant surface and agrochemicals (Li et al. 2019). It has been reported that germination of seed get reduced with reduction in water absorption by seeds (Al-Tawaha and Al-Ghzawi 2013). So treatment with chitosan might have helped in increasing the absorption of water into seed leading to increase in germination of seed.
Food preservative and shelf-life enhancing activities of chitosan
Chitosan is widely used in food industry due to its several distinctive biological activities and functional properties. The antimicrobial activity and film-forming property of chitosan prove it as a potential source of food preservative. It was observed that the various control samples had started to show signs of spoilage by fungal on various days. The experimental sets, which were coated by chitosan showed no signs of spoilage after 1 week of storage and looked fresh (Table 6). By the sixteenth day, the experimental samples had partially ripened but no signs of fungal or microbial attack were observed. The present study corroborated with the earlier study of Tarafdar and Biswas (2013).
Conclusion
The present findings prove that chitin obtained from the scales of the fish could be used in variety of applications especially when converted into chitosan. This study also demonstrates that the extracted chitosan has good anticoagulant, antimicrobial, antioxidant, and food preservation activity. Based on the results obtained, nutraceutical or pharmaceutical industries may develop and utilize chitosan from fish scales of Indian major carps which is being thrown as waste. However, further investigations are required to determine the factors responsible for changes in textural properties of the products during storage and processing also to compare the activity with chitosan derivatives in the fish products.
Data availability
Not applicable
References
Allison PG, Rodriguez RI, Moser RD, Williams BA, Poda AR, Seiter JM, Chandler MQ (2014) Characterization of multi-layered fish scales (Atractosteus spatula) using nanoindentation, X-ray CT, FTIR, and SEM. J Vis Exp 89:e51535
Al-Tawaha ARM, Al-Ghzawi ALA (2013) Effect of chitosan coating on seed germination and salt tolerance of lentil (Lens culinaris L.). Res Crops 14:489–491
Andrade S, Ladchumananandasivam R, Rocha B, Belarmino D, Galvao A (2012) The use of exoskeletons of shrimp (Litopenaeus vanammei) and crab (Ucides cordatus) for the extraction of chitosan and production of nanomembrane. Enzyme Microb Tech 3:495–508
Chung YC, Kuo CL, Chen CC (2005) Preparation and important functional properties of water-soluble chitosan produced through Maillard reaction. Bioresour Technol 96:1473–1482
Das SK, Samantaray D, Mahapatra A, Pal N, Munda R, Thatoi HN (2018) Pharmacological activities of leaf and bark extracts of a medicinal mangrove plant Avicennia officinalis L. Clin Phytosci 4:13. https://doi.org/10.1186/s40816-018-0072-0
Di Martino A, Sittinger M, Risbud MV (2005) Chitosan: a versatile biopolymer for orthopaedic tissue engineering. Biomaterials 26:5983–5990
Gokulalakshmi E, Ramalingam K, Umasankari VMC (2017) Extraction and characterization of chitosan obtained from scales of Clarias gariepinus (catfish). Biotechnol J Int 18:1–8
Helrich K (1990) Official methods of analysis of the association of official analytical chemists. 15th ed. Virginia, USA
Islam MS, Khan S, Tanaka M (2004) Waste loading in shrimp and fish processing effluents: potential source of hazards to the coastal and near shore environments. Mar Pollut Bull 49:103–110
Jeyaraman M, Vignesh R, Sumathi N (2017) Extraction, characterization and applications of chitosan from fish scales. Int J Mod Trends Eng Res 4:137–141
Kaya M, Seyyar O, Baran T, Türkeş T (2014) Bat guano as new and attractive chitin and chitosan source. Front Zool 11:59. https://doi.org/10.1186/s12983-014-0059-8
Knorr D (1982) Functional properties of chitin and chitosan. J Food Sci 47:593–595
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139
Kumari S, Rath P (2014) Extraction and characterization of chitin and chitosan from (Labeo rohita) fish scales. Procedia Mater Sci 6:482–489
Kumari S, Rath P, Kumar ASH (2016) Chitosan from shrimp shell (Crangon crangon) and fish scales (Labeo rohita): extraction and characterization. Afr J Biotechnol 15:1258–1268
Li R, He J, Xie H, Wang W, Bose SK, Sun Y, Hu J, Yin H (2019) Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int J Biol Macromol 126:91–100
Mohanasrinivasan V, Mishra M, Paliwal JS, Singh K, Selvarajan E, Suganthi V, Devi CS (2013) Studies on heavy metal removal efficiency and antibacterial activity of chitosan prepared from shrimp shell waste. 3 Biotech 4:167–175
Murugan R, Parimelazhagan T (2014) Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckiaparvi folia Arn.–an in vitro approach. J King Saud Univ Sci 26:267–275
Nising P (2006) High-temperature radical polymerization of methyl methacrylate in a continuous pilot scale process [Ph.D. Thesis]. Ecole Polytechnique Federale De Lausanne Lausanne: EPFL
No HK, Meyers SP (1992) Utilization of crawfish processing waste as carotenoids, chitin and chitosan sources. J Korean Soc Food Nutr 21:319–326
Panith N, Wichaphon J, Lertsiri S, Niamsiri N (2016) Effect of physical and physicochemical characteristics of chitosan on fat-binding capacities under in vitro gastrointestinal conditions. LWT-Food Sci Technol 71:25–32
Paul S, Jayan A, Sasikumar CS, Cherian SM (2014) Extraction and purification of chitosan from chitin isolated from sea prawn Fenneropenaeus indicus. Asian J Pharm Clin Res 7:201–204
Qin C, Li H, Xiao Q, Liu Y, Zhu J, Du Y (2006) Water-solubility of chitosan and its antimicrobial activity. Carbohydr Polym 63:367–374
Rajalakshmi A, Narayanaswamy K, Jayachitra A (2013) Antioxidant activity of the chitosan extracted from shrimp exoskeleton. Middle East J Sci 16:1446–1451
Suman TY, Rajasree SR, Ramkumar R, Rajthilak C, Perumal P (2013) The green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim Acta A 118C:11–16
Tarafdar A, Biswas G (2013) Extraction of chitosan from prawn shell wastes and examination of its viable commercial applications. Int J Theor Appl Res Mech Engg 2:2319–3182
Trung TS, Bao HND (2015) Physicochemical Properties and antioxidant activity of chitin and chitosan prepared from pacific white shrimp waste. Int J Carbohydr Chem, pp 1–6. https://doi.org/10.1155/2015/706259
Walke S, Srivastava GK, Nikalje MD, Doshi JB, Kumar R, Ravetkar SD, Doshi PJ (2014) Physicochemical and functional characterization of chitosan prepared from shrimp shells and investigation of its antibacterial, antioxidant and tetanus toxoid entrapment efficiency. Int J Pharm Sci Rev Res 26:215–225
Wang JC, Kinsella JE (1976) Functional properties of novel proteins: alfalfa leaf protein. J Food Sci 41:286–292
Zaku SG, Emmanuel SA, Aguzue OC, Thomas SA (2011) Extraction and characterization of chitin; a functional biopolymer obtained from scales of common carp fish (Cyprinus carpio l.): a lesser known source. Afr J Food Sci 5(8):478–483
Consent for publication
The authors gave their consent for publication of the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest/Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable
Consent to participate
The authors gave their consent to participate in the research work.
Code availability
Not applicable
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Satpathy, A.A., Dash, S., Das, S.K. et al. Functional and bioactive properties of chitosan from Indian major carp scale. Aquacult Int 29, 417–430 (2021). https://doi.org/10.1007/s10499-020-00622-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00622-0