Abstract
The aim of this study is to assess the toxicity of some heavy metals and parasitic infection in fishes as well as the immunological status of these fishes infected with different parasites. Between January 2018 and January 2019, 360 Nile tilapia (Oreochromis niloticus) near four farms and in the Nile River in Giza were examined for external and internal parasites. In addition, samples were expressed for two genes using quantitative real-time polymerase chain reaction. Samples were also taken from muscles and water for toxicological analysis of different heavy metals. In the skin, the mean TNF-α levels in the monogenea and mixed protozoan parasites (Trichodina and Myxobolus spp.) were 18.00 ± 0.67 and 13.65 ± 0.54, respectively. In the gills, the mean TNF-α of monogenea, Centrocestus formosanus, and encysted metacercaria (EMC) with monogenea group means was 20.45 ± 0.74, 23.00 ± 0.74, and 25.78 ± 0.74, respectively. In muscles with EMC, the mean TNF-α level was 14.67 ± 0.70. In livers with EMC, the mean TNF-α level was 26.78 ± 0.70. In the skin, the mean IL-1β level in monogenea and protozoan parasites (Trichodina and Myxobolus spp.) was significantly different (25.00 ± 0.69 and 15.00 ± 0.43, respectively). In the gills, the mean IL-1β level of the monogenean group was 21.00 ± 0.79, whereas C. formosanus showed the significantly highest value (27.00 ± 0.74). The mean IL-1β level of EMC with the monogenea group was 24.00 ± 1.54. Results showed that the level of Zn, Pb, Cu, and Cd was the highest in tissues of fish than permissible limits (PLs). Histopathological examination of different examined tissues exhibited serious pathological alterations. Monogenetic trematodes were detected in the examined fish, and their presence was accompanied by hyperplasia and fusion of the secondary gill lamellae. EMC of digenetic trematodes was frequently observed in the gills and in the cartilage rod. In conclusion, the parasite infection can upregulate the immunological cytokines with different levels with different infections.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main species of freshwater fish found in the Nile River of Egypt is Oreochromis niloticus (Nile tilapia). It is one of the most prevalent and low-cost fish consumed by the Egyptian population. This fish can tolerate adverse environmental conditions because of its somatic resistance to disease and can also survive low oxygen and high ammonia levels because of their low respiratory demands (Zhou et al. 1998).
Oreochromis niloticus was considered as the most prevalent and cheapest source of animal protein in Egypt as it enhances several human health conditions as recorded by Mahmoud et al. (2019a).
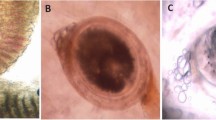
Different parasites were recorded in Nile tilapia (Oreochromis niloticus (Linnaeus, 1758)) such as Trichodina spp., Ichthyophthirius multifiliis, Chilodonella spp., Myxobolus spp., Cichlidogyrus spp., Gyrodactylus spp., Contracaecum sp., Argulus spp., Ergasilus spp., and Lernea spp. (Arguedas et al. 2017).
The contamination of freshwater by different heavy metals has been a great concern because of these substances’ threat to both human and aquatic life (Canli et al. 1998). Heavy metals present in the human population are due to either natural or manmade causes (wind-blown dust, ore-bearing rocks, forest fires, and vegetation) or anthropogenic activities (industrial effluents, sewage sludge disposal, pesticides, inorganic fertilizers, and atmospheric stones (Haiyan and Stuanes 2003; Ali and Abdel-satar 2005).
Heavy metal pollution of the aquatic environment results in the bioaccumulation of these high-density metals in fish tissues. The presence of heavy metals inside animals and human tissues can cause the disruption of essential molecules, including proteins and enzymes that are important for metabolism and DNA damage repair and the destruction of viable organs in living tissues such as cell membrane, mitochondria, and different organelles, including even nuclei (Suziki et al. 1988; Hodgson 2011; Tchounwou et al. 2012).
There are many indicators of heavy metal pollution. The first indicator includes aquatic insects, plants, protozoans, crustaceans, and fish (Burger et al. 2002). Because fishes are known to be susceptible to bioaccumulation of metals, they can be used for biomonitoring. In addition, they can be used to compare metal concentrations among sites where water samples are near or below the detection limits, the atomic absorption technique (Ramelow et al. 1989). The second indicator of pollution is water, as heavy metal concentrations in water mirror the pollution status of these areas (Ali and Abdel-satar 2005). The third indicator is sediment. Heavy metal pollution of aquatic ecosystems is often most obvious in sediment, macrophytes, and aquatic animals, followed by water (Linnik and Zubenko 2000).
Different immunological cytokines are evaluated against several pathogens which indicates the immune status of the fish against the infection; from these cytokines, IL-1β and TNFα that are considered as the pro-inflammatory cytokine and produced in early stages of infection.
Currently, there is a lack of studies on the relationship between different parasitic infections and the immunological health condition of the O. niloticus with different gene expression patterns. Thus, the aim of this study was to determine the relationship between the immunological status of fish infected with different parasites using gene expression analysis by quantitative real-time polymerase chain reaction (qRT-PCR) as well as assessment of the toxicity of some heavy metals.
Materials and methods
Collection of fish specimens
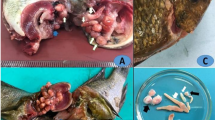
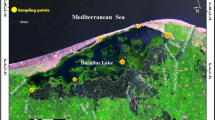
Between January 2018 and January 2019, a total of 360 Nile tilapia (O. niloticus; 1 year old, 10 cm in length) were collected near four farms (two in Kafr Elshikh and two in El Sharkia) and from the Nile River. These fishes were evaluated for common helminths and protozoan parasites. Thirty fishes were collected every month for 1 year for the examination studies. The fishes were brought in aquaria and transported to the laboratory for parasitological, toxicological, and histopathological examinations as well as molecular analysis. All of the collected fishes were kept in aerated 30-L aquaria (Mahmoud et al. 2019b). Tissues samples from fish were collected for heavy metal analysis. So, edible muscles, gills, and liver were removed and kept frozen at − 20 °C until toxicological analysis.
Parasitological examination
Each fish was carefully examined under light microscope, including the mucous membranes surrounding the skin, gills, and fins. A smear was prepared from each part. These smears were prepared for fixation using absolute methanol and then stained with Giemsa stain for protozoal inspection (Saha and Bandyopadhyay 2017). The smears were examined under a light microscope (Olympus CX41 microscope, Japan). To identify the encysted metacercaria (EMC) species present in the fish, one cm of the fish muscles (preferred around the gills and the tail) and in kidney was compressed between two slides and examined under dissecting and light microscopy for the presence of any EMC according to Paperna (1996). All the collected parasites were freshly examined under dissecting and light microscope and then identified using the key of Paperna (1996).
qRT-PCR
Sampling
Infected skin samples and muscles (0.5 cm2) as well as gills (with the parasites, protozoan parasites, monogenean spp., EMC, Centrocetus formosanus) were aseptically dissected. Samples from four uninfected fishes were collected in a similar manner and used as negative controls. The samples were classified as skin with monogenean spp., skin with protozoan parasites, gills with monogenean spp., gills with C. formosanus, gills with mixed infection of monogenean spp. and EMC, muscles with EMC, and liver with EMC.
RNA isolation
Fishes infected with the parasites under investigation were selected for gene expression analysis. One hundred milligrams of skin, gills, muscles, and liver was used for isolation of RNA using an RNA isolation kit (Ambion, Applied Biosystems). Firstly, the tissues were homogenized using lysing matrix D tubes (MP Biomedicals) in a FastPrep-24 homogenizer (MP Biomedicals, two cycles of 30 s at 6 m/s). The purity and quantity were measured using the RNA Nanodrop (Picard-Sanchez et al. 2019).
qRT-PCR protocol
The two gene primers used were tumor necrosis factor-α1 (TNF-α1) and interleukin-1β (IL-1β) specific for O. niloticus as follows: TNF-α1, F (GGTTAGTTGAGAAGAAATCACCTGCA), TNF-α1. R (GTCGTCGCTATTCCCGCAGATCA), IL-1β-F (TGCACTGTCACTGACAGCCAA), IL-1β-R (ATGTTCAGGTGCACTATGCGG). β-actin was used as a reference gene: β-actin. F (CAGCAAGCAGGAGTACGATGAG), β-actin. R (TGTGTGGTGTGTGGTTGTTTTG) (Praveen et al. 2006; Heinecke and Buchmann 2013; Akbari et al. 2017).
The cDNA synthesis, RNA extraction as well as the cycling condition were carried out in accordance with the methods of Abu-Elala et al. 2018.
The quantitative PCR assays were performed following the steps by Akbari et al. (2017). Ten microliters of mixture of SYBR® Premix Ex Taq™ (Tli RNase H Plus) and ROX plus, 1 μl of cDNA, and 0.5 μl of forward and reverse primers (100 nM) were mixed in a reaction and filled with pure water to a final volume of 20 μl. The cycling conditions were as reported in Abu-Elala et al. (2018). The ∆CT value was calculated by subtraction of the β-actin CT as an internal control, in which CT is the cycle number at which detectable signals are achieved.
Water samples
Water sample collection
Water samples were collected from two sites. The first site was the Nile River at Giza, and the second site was an aquaculture earthen pond. Samples were collected using clean glass bottles placed 50 cm below the water surface and then transported to the Faculty of Veterinary Medicine laboratory in an ice box, with minimum delay before examination (APHA, 2005).
Examination of water samples
Collected samples were examined physically for pH and chemically for total hardness, chloride, organic matter, phosphate, sulfate, ammonia nitrogen, nitrite nitrogen, nitrate, and some heavy metals.
Physical and chemical examination
Samples were immediately transported to the laboratory to measure hydrogen ion concentration (pH) using a pH meter (pH ep-tester®, Hanna instruments). Chemical oxygen demand was determined using the potassium permanganate method according to Golterman (1971). Colorimetric methods were used to determine ammonia and nitrite levels following the methods described by APHA (1995). Phosphate and nitrate levels were determined according to SMWW (1998). Chloride and sulfate were measured by using the method described in APHA (1985). Total hardness was determined using the EDTA titrimetric method mentioned in APHA et al. (2005).
Heavy metal analysis
The concentrations of some heavy metals, including Cd, Cr, Cu, iron (Fe+2), Pb, zinc (Zn), arsenic (As), Se, and silicon (Si), in water samples were determined using the inductivity coupled plasma technique (Horiba TY model ultima 2) at the Soils, Water & Environment Research Institute, Agricultural Research Center, Ministry of Agriculture and Reclamation, Egypt. The collected 50-ml unfiltered water sample from aquaria and the Nile River was added to 0.5 ml of concentrated sulfuric acid. Then, boiling occurred until white fumes were formed; this foaming was set to cooling and added to 1 ml of 60% HCLO3 and 5 ml of concentrated HNO3 until a clear digest was noticed. This digested samples were cooled and filtered with a Whatman paper No.1. Then, the digested water samples were subjected to heavy metal analysis using an atomic absorption spectrophotometer at the Soils, Water & Environment Research Institute, Agricultural Research Center, Ministry of Agriculture and Reclamation, Egypt (El-Sayed et al. 2011).
Examination of fish tissues for heavy metals
Concentration analysis of some heavy metals, including Cd, Cu, Pb, and Zn, in fish samples from the aquaria and Nile River was determined as follows: 1 g of the fish tissue sample was digested using 10 ml of freshly prepared 1:1 concentrated HNO3-HCLO3 for 1 h with gentle heating at 160 °C. The digested materials were allowed to cool. Then, the digested fish tissues were subjected to heavy metal analysis using an atomic absorption spectrophotometer at the Soils, Water & Environment Research Institute, Agricultural Research Center, Ministry of Agriculture and Reclamation, Egypt (El-Sayed et al. 2011).
Histopathology
On fish dissection, tissue samples from the gills, eyes, skin, muscles, liver, spleen, and intestines were collected and kept in 10% neutral buffered formalin. After fixation, tissues were routinely processed in different grades of alcohol and then xylene, and finally embedded in melted paraffin wax. Sections of 5 μm were cut and stained by using hematoxylin and eosin according to Bancroft and Gamble (2008) and Mahmoud et al. 2019b. Slides were examined using an Olympus light microscope (Bx43), and images were captured by an Olympus digital camera (DP 27).
Statistical analysis
Group means were compared using one-way analysis of variance. Values of P < 0.05 were considered statistically significant. All statistical inference was performed using the PASW Statistics, version 18.0, software (SPSS Inc., Chicago, IL, USA). All measurements of the parasitic protozoa were expressed as means and standard errors.
Results
Identification of the collected parasites
All fishes were investigated for different external and internal parasites (Fig. 1). Trichodina spp. were collected from the gills, skin, and fins. One species of Myxobolus was collected (M. tilapiae). One monogenean parasite was found to be the most common in examined fishes (Cichlidogyrus tilapiae) and C. formosanus with different types of EMC (Prohmostomidae). All of the examined fishes were positive for one or two mixed parasitic infections: 300 fishes were infected with Trichodina spp. (83.33%), Myxobolus spp. (27.8%), and EMC (72.2%). In fish with monogenean infection,16.66% and 3.8% were infected with C. formosanus.
TNF-α and IL-1β gene expressions of infected skin, gills, muscles, and liver with different parasitic groups
In the skin, the mean TNF-α levels in the monogenea and mixed protozoan parasites (Trichodina and Myxobolus spp.) were 18.00 ± 0.67 and 13.65 ± 0.54, respectively. In the gills, the mean TNF-α of monogenea, C. formosanus, and EMC with monogenea group means were 20.45 ± 0.74, 23.00 ± 0.74, and 25.78 ± 0.74, respectively, which were significantly higher than those in the control group (3.00 ± 0.00; P = 0.0001).
In muscles with EMC, the mean TNF-α level was 14.67 ± 0.70, which was significantly higher than that in the control group (3.00 ± 0.00; P = 0.0001).
In livers with EMC, the mean TNF-α level was 26.78 ± 0.70, which was significantly higher than that in the control group (3.00 ± 0.00; P = 0.0001; Table 1).
In the skin, the mean IL-1β level in monogenea and protozoan parasites (Trichodina and Myxobolus spp.) was significantly different (25.00 ± 0.69 and 15.00 ± 0.43, respectively) and significantly higher than that in the control group (3.00 ± 0.00; P = 0.0001).
In the gills, mean IL-1β level of the monogenean group was 21.00 ± 0.79, whereas C. formosanus showed the significantly highest value (27.00 ± 0.74). The mean IL-1β level of EMC with the monogenea group was 24.00 ± 1.54, which was significantly higher than that of the control group (3.00 ± 0.00; P = 0.0001).
In muscles with EMC, the mean IL-1β level was 12.00 ± 1.4, which was significantly higher than that in the control group (3.00 ± 0.00; P = 0.0001).
In livers with EMC, the mean IL-1β was 26.00 ± 1.45, which was significantly higher than that in the control group (3.00 ± 0.00; P = 0.0001; Table 1).
Water quality
We found that the pH exceeded the permissible limit (PL) for freshwater fish based on the Environmental Protection Agency’s (EPA’s) 2001 guidelines (WHO 1993; Table 2). The ammonia, nitrite, and phosphate levels in the samples were also higher than PLs, whereas sulfate and hardness were within the PL. Chloride levels were found to be lower than the PL. The nitrate level of sample 2 exceeded the recommended limits of the EPA.
Heavy metals in tissues
All of the examined heavy metals were within the PL for surface water (Table 3). The arrangement of measure was Si, Fe, Zn, Cu, and Se. The levels of the other metals were lower than the detection limit.
The level of Zn was the highest in muscles, followed by the gills. Zn levels were the lowest in the liver and greatly exceeded the PL of the Food and Agriculture Organization/World Health Organization (FAO/WHO 1999) and the Egyptian Organization for Standardization and Quality Control (EOSQC 2005).
The levels of Pb were equivalent in the muscles and gills and higher in these two tissues than in the liver. All levels greatly exceeded the PLs of the FAO/WHO (1999) and EOSQC (2005).
Cu levels were highest in the muscles followed by the gills and then the liver, exceeding the PLs of the FAO/WHO (1999) and EOSQC (2005).
Cd levels were highest in the gills and exceeded the PLs of the FAO/WHO (1999) and EOSQC (2005).
Histopathology
Histopathological examination of the gills from the collected fish exhibited serious pathological alterations. Severe acute telangiectasia of the blood capillaries in the secondary gill lamellae (Fig. 2a) was the most frequently encountered lesion, which was usually accompanied by severe congestion of the primary lamellar blood vessels (Fig. 2b). Moreover, fusion of the secondary gill lamellae was observed in many cases as a result of hyperplasia and infiltration of mononuclear inflammatory cells (Fig. 2c). Along with the lesions, hyperplasia and hyperactivity of mucous cells were noticed (Fig. 2d). The affected gills were heavily infiltrated by eosinophilic granule cells (EGCs; Fig. 2e) with marked degranulation (Fig. 2f) in the tissue. Bent and distorted secondary gill lamellae were also frequently observed. Some severely affected gills exhibited shortening or complete erosion of the secondary gill lamellae (Fig. 2g). Complete necrosis and destruction of the secondary gill lamellae (Fig. 2h) were detected in some sections, resulting in bare primary gill filaments with the presence of desquamated and sloughed epithelial lining (Fig. 2i). Monogenetic trematodes were detected in an examined case, and their presence was accompanied by hyperplasia and fusion of secondary gill lamellae as well as heavy infiltration of mononuclear inflammatory cells (Fig. 2 j and k). EMC of digenetic trematodes were frequently observed in the gills of infected fish, and some EMC were found in the cartilage rod supporting the primary gill lamellae (Fig. 2 l and m), causing hyperplasia in the surrounding chondrocytes. At the base of the gill filaments near the gill arch, severe areas of diffuse inflammatory edema were detected (Fig. 2n). Heavily infected fish had increased numbers of EMC at the gill arch, which appeared diffusely infiltrated by inflammatory cells (Fig. 2o). EGCs were the predominant inflammatory cells in cases of EMC infestation (Fig. 2p). Mononuclear inflammatory cells were also associated with other cases of EMC infestation (Fig. 2q), especially those detected in the connective tissue of the gills (Fig. 2r). Melano-macrophages (Fig. 2s) were also noticed as a reaction against the infesting parasites. Generally, in almost all cases, the presence of EMC typically resulted in mild mononuclear and EGC infiltration (Fig. 2t).
Photomicrograph of gills, H&E-stained, showing a lamellar telangiectasia (arrows); note the presence of necrotic gill filaments (N) and areas of focal lamellar fusion (F). b Congestion of the primary gill lamellae (C) with lamellar telangiectasis in some secondary gill lamellae (T); note the presence of necrotic gill filament. c Lamellar hyperplasia with fusion of the secondary gill lamellae. d Fusion of secondary gill lamellae with hyperactivity of mucous cells (arrow). e Lamellar hyperplasia and fusion with heavy eosinophilic granule cell infiltration (EGC). f Higher magnification showing degranulation of EGC. g Shortening and necrosis of the secondary gills lamellae. h Massive necrosis and destruction of the secondary gill lamellae. i Higher magnification showing necrosis and desquamation of the lining epithelium of the gills; note the congestion in the primary gill filaments. j Section in monogenetic trematode with hyperplasia and fusion of gill lamellae. k Higher magnification showing mononuclear inflammatory cells infiltrating the secondary gill filaments along with sloughing of the lining epithelium. l EMC in the cartilage rod supporting the primary filament with EGC infiltration. m Higher magnification showing hyperplasia in the chondrocytes surrounding the parasite. n Marked inflammatory edema causing expansion of the connective tissue at the gill arch. o Presence of multiple EMC at the gill arch that appear surrounded by intense inflammatory reaction. p Heavy EGC infiltration at the base of the gill filaments. q EMC at the gill arch surrounded by heavy mononuclear cell infiltration. r EMC embedded in the connective fibrous tissue with mononuclear inflammatory cell infiltration. s EMC with mononuclear inflammatory cell infiltration with the presence of melano-macrophages. t Mild mononuclear and EGC infiltrations around the EMC
Histology of the eye sections revealed EMC in the surrounding connective and adipose tissues (Fig. 3 a and b), which caused edema and mild infiltration of mononuclear inflammatory cells. Examination of the muscles revealed the presence of EMC either beneath the skin surface (Fig. 3c) or deeply embedded in between the muscle fibers (Fig. 3d). The presence of EMC in the muscles was usually accompanied by very mild or even no inflammatory reaction (Fig. 3e), but in some examined sections, the embedded cysts appeared to be surrounded by an intense mononuclear inflammatory reaction (Fig. 3 f and g). The other commonly detected alterations in muscle fibers included myodegeneration that was characterized by marked vacuolation of the myocytes (Fig. 3h) and myositis that was manifested by infiltration of mononuclear inflammatory cells along with hyalinization of muscle fibers (Fig. 3i). Livers of fish showed the presence of EMC (Fig. 3j). The hepatocytes suffered degenerative changes including vacuolar degeneration and fatty change (Fig. 3k). Some severely affected sections exhibited massive diffuse hepatocellular necrosis (Fig. 3l). With regard to the histopathological alterations in the spleen, diffuse lymphocytic depletion was a frequently encountered lesion (Fig. 3m) as well as hyperactivity of the melano-macrophage centers (Fig. 3n). Intestines of fish showed diffuse infiltrations of the mononuclear inflammatory cells with a necrotic epithelial lining as well as the presence of parasites in their lumen (Fig. 3o).
Photomicrograph, H&E-stained. a, b Eye of a fish showing EMC (arrow) embedded in the connective tissue with edema and mild mononuclear inflammatory cell infiltration. c Muscle of a fish. EMC was detected under the skin and appeared surrounded by mononuclear (M) and eosinophilic granule (EG) cell infiltrations. d Muscle of a fish. EMC embedded deeply in between the muscle bundles. e Muscle of a fish, higher magnification. EMC surrounded by fine connective tissue layer and eliciting minimal tissue reaction. f Muscle of a fish. EMC embedded in between muscle bundles which appeared surrounded by intense mononuclear inflammatory cell infiltration; note hyalinization of muscle fibers. g Muscle of a fish, higher magnification, showing intense mononuclear inflammatory cell infiltration around the EMC. h Muscle of a fish showing severe diffuse myodegeneration and necrosis. i Muscle of a fish showing intense mononuclear and EG cell infiltration in between muscle bundles. j Liver of a fish showing the presence of EMC in between hepatocytes. k Liver of a fish showing vacuolar degeneration (V) and fatty change (F) in hepatocytes. l Liver of fish showing an area of severe diffuse hepatocellular necrosis. m Spleen of a fish showing diffuse lymphocytic depletion. n Spleen of a fish showing activation of melano-macrophage center. o Intestines of a fish showing mononuclear inflammatory cell infiltration in lamina propria and sloughing of the lining epithelium; note the presence of parasite in the intestinal lumen
Discussion
In this study, we found a strong relationship between the presence of parasites, toxicity, and immunogenicity, all of which impeded the effects on the histopathological condition of the affected tissues.
IL-1β and TNF-α are important pro-inflammatory cytokines which mediate immune regulation in both innate and acquired immunity and in turn regulate the inflammatory response. IL-1β was the first reported cytokine which secreted by different immunological cells stimulated by different pathogens these cells as endothelial cells, macrophages, T lymphocytes, and many other immunological cells. IL-1 has a chemoattractant effect on different leucocytes which was coordinated by different chemokines. This stimulation leads to rapid release of intracellular Ca+ ions, with consequence upregulation of the chemokine receptors on the target cells (Fujiki et al. 2000; Jiang et al. 2008).
The pro-inflammatory cytokine, TNF-α, is one of the early secreted gene in the immunological process, which has a good role in regulating inflammation and is expressed during the early stage of infection in fish (Attia et al. 2020), fish TNF-α with IL-1β had overlapping functions in different fishes which had a role in activation of macrophages/phagocytes and in turn promote the microbial killing activity (Zou and Secombes 2016).
Only fish infected with Ichthyophthirius multifilis which evaluated the immunological genes as TNF-α by Akbari et al. (2017) and Gonzalez et al. (2007) evaluated different genes as IL-1β, CXCa, and CXCb in common carp. Toll-like receptor and immunoglobulin were evaluated by Wang et al. (2019).
Cultured O. niloticus can respond to high upregulation of different genes stimulated by the different pathogens. The skin and gills of fishes are common sites of parasitic infection, which provide a 1st barrier against different parasitic diseases by secreting mucus which in turn limits the pathogen load (Simon Jones 2001).
We found that TNF-α was upregulated in the skin, gills, muscles, and liver which were infected with different parasites in comparison with the control group, which due to IL-1β expression had greater upregulation in the skin and gills as compared with the skin, also with greater upregulation in the liver than in the muscles due to the presence of the immunological cells, which secrete different cytokines.
These results indicate that the skin, gills, muscles, and liver, had high genes up-regulation which was due to the presence of mucous that supported with excessive macrophage secretion Zhu et al. (2013)
Physicochemical parameters were evaluated due to the solubility of toxic heavy metal increase with the decrease of pH, and when pH values increased, Se and Zn levels decreased. Environmental factors including pH and hardness are among factors affecting heavy metal concentration in water and consequently in the tissues (El-Sayed et al. 2011).
Many reviews have included data about parasites and their possible use as bioindicators of environmental impact. Acanthocephalans can act as indicators of bioaccumulation because of their capacity to accumulate heavy metals (Sures 2008a). However, there is conflicting evidence regarding the impact on aquatic parasite abundance (Sures 2008b).
A level of heavy metals in the human diet that exceeds the PLs can lead to several chronic illnesses, including cancer, headache, and liver and kidney failure (El-Sayed et al. 2011).
The Cu values detected in O. niloticus in this study disagreed with those of Aly (2016), as we detected the Cu concentrations to be in the following increasing order: muscles < liver < gills. However, Aly’s study showed the highest concentrations of Cd in the liver and gills.
The Zn concentrations of this study also disagree with the findings of Aly (2016), who found increasing Zn concentrations in O. niloticus tissues in the order of muscles < gills < liver.
Bioindicators are species that reflect the environmental impact as a response to habitat alterations. They can reveal changes in physiology or chemical composition of the environment, and they can be either accumulation or effect indicators. Accumulation indicators are those that accumulate substances from their environment without showing adverse effects. Parasites can uptake heavy metals higher than their hosts. On the other hand, effect indicators demonstrate functional molecular, physiological, population size, or status changes. These indicators can measure the effect of exposure to pollutants at different levels. Potential effect indicators include parasites (helminths, crustaceans, and protozoans) of both terrestrial and aquatic environments.
Several studies have reported that different parasites accumulate heavy metals in their bodies. Thus, nematodes, cestodes, or even Acanthocephalan spp. could be used as indicators of heavy metal toxicity of water and tissues (Sures et al. 1994; Tenora et al. 1999; Tenora et al. 2000; Palíková and Baruš 2003; Baruš et al. 2007). It could be concluded that marine fish can be used as biological indicators of heavy metal pollution while also minimizing the bioaccumulation of heavy metals in fish tissues (Table 4). Until now, understanding of the relationship between parasites and toxicity has not been satisfactory (Nachev et al. 2013).
Several toxic substances can be present in higher concentrations in farmed as compared with wild fish (Cole et al. 2009). Thus, it is necessary to have a sufficient supply of good-quality water for the construction of ponds to maintain high growth rates and good product quality (Agoz et al. 2005).
With regard to our histopathological findings, much of the histological alterations in the gills were associated with the nonspecific defense mechanism of the host fish against infestation (Buchmann et al. 2001; Paruruckumani et al. 2015). Pooling of blood in the lamellar capillaries (lamellar telangiectasis or aneurysm) could be regarded as a rupture of the retaining pillar cells (Roberts and Rodger 2012). Lamellar hyperplasia observed in the gills is considered a long-term response of the malpighian cells to lower levels of irritation, which may result in secondary lamellar fusion.
Our findings of edema, hyperplasia, and fusion of the secondary gill lamellae related to the presence of parasites in the gills were similar to those observed by Vinobaba (1994), Shinn et al. (2004), Hossain et al. (2007), and Fujimoto et al. (2014). Such lesions were attributed to the attachment mode of the parasite on the gill epithelium. Excessive mucus secretion due to parasitism was also reported by Santos et al. (2017), who considered this to be a cause of respiratory failure and fish death. Infiltration by EGCs observed in the gills could be attributed to parasitism, as previously mentioned by Kantham and Richards (1995).
Similar to our findings, Mood et al. (2010) reported the presence of metacercariae lodged next to the cartilage filaments, resulting in an intensive inflammatory response and hyperplasia of the cartilage of the primary lamellae. The extensive proliferation of gill cartilage as a response to metacercariae infestation was confirmed by Blazer and Gratzek (1985), which was similar to our results. Unlike our findings, Santos et al. (2017) reported that there were no histological alterations related to metacercaria infestation and attributed this observation to the decreased load of infection.
The histological findings in the present study are in agreement with previous reports indicating that parasites can cause considerable damage to the viscera, including the liver and musculature of many fish species (Adeyemo and Agbede 2008; Shareef and Abidi 2012).
Conclusion
In this study, an explanation was provided for the toxicity of O. niloticus, including reduced water quality and a high parasitic load, based on the histopathological profile of the fishes. These parasites as well as the heavy metals present in the fish tissues can have destructive effects on human health. Thus, the water in aquaria must be changed and cleaned regularly. So, different rules for cleaning and health conditions for ponds must be taken into consideration.
References
Abu-elala NM, Younis NA, Abubakr HO et al (2018) Efficacy of dietary yeast cell wall supplementation on the nutrition and immune response of Nile tilapia. Egypt J Aquatic Res 44(4):333–341
Adeyemo AO, Agbede SA (2008) Histopathology of tilapia tissues harbouring Clinostomum tilapiae parasites. Afr J Biomed Res 11:115–118
Agoz HM, Abbas HH, Mahmoud HM (2005) Rice-fish-azolla integrated culture systems. J Egypt Acad Soc Environ Dev 6(2):65–85
Akbari M, Taghizadeh V, Heidarieh M, Hajimoradloo A (2017) The key role of tumor necrosis factor alpha (TNF-α) in vaccinated rainbow trout via irradiated Ichthyophthirius multifiliis trophont. Vet Arhiv 87(2):229–237
Ali MHH, Abdel-satar AM (2005) Studies of some heavy metals in water, sediment, fish and fish diets in some fish farms in El-Fayoum province, Egypt. Egypt J Aquatic Res, 1687–4285 31(2):261–273
Aly (2016) Comparison of heavy metals levels in muscles, liver, and gills of three fish species collected from agricultural drainage water AT El- Abbassa fish farm, Sharkia, Egypt. Egypt J Aquat Biol & Fish 20(3):103–112
APHA (American Public Health Association) (1985) Standard methods for the examination of water and wastewater. 16th Edition. American Public Health Association, Washington, D.C., New York
APHA (American Public Health Association) (1995) Standard methods for the analysis of water and wastewater
APHA (American Public Health Association) (2005): Standard Methods for the Examination of Water and Wastewater, 21st ed. American public health association, Washington, D.C
APHA, AWWA, WEF, Rice EW, Eaton AD, Clesceri LS (2005) American Public Health Association, American Water Work Association, Water Environment Federation. Standard methods for the examination of water and wastewater, 21st edn 800, I St. NW., Washington DC 20001–3710
Arguedas, C. Cesare Ortega S, Simón Imon Martínez C. &ÁngelAstroza C. (2017) Parasites of Nile tilapia larvae Oreochromis niloticus (Pisces: Cichlidae) in concrete ponds in Guanacaste, northern Costa Rica Donald. Cuadernos de Investigación UNED (ISSN: 1659–4266) Vol. 9(2): 313–319
Attia MM, El-Gameel SM, Ismael E (2020) Evaluation of tumor necrosis factor-alpha (TNF-α); gamma interferon (IFN-γ) genes and oxidative stress in sheep: immunological responses induced by Oestrus ovis (Diptera: Oestridae) infestation. J Parasit Dis 44:332–337. https://doi.org/10.1007/s12639-020-01220-w
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques. Elsevier health sciences
Baruš V, Jrkovský J, Prokeš M (2007) Philometra ovata (Nematoda: Philometroidea): a potential sentinel species of heavy metal accumulation. Parasitol Res 100:929–933
Blazer VS, Gratzek JB (1985) Cartilage proliferation in response to metacercarial infections of fish gills. J Comp Pathol 95(2):273–280
Buchmann K, Sigh J, Nielsen CV, Dalgaard M (2001) Host responses against the fish parasitizing ciliate Ichthyophthirius multifiliis. Vet Parasitol 100:105–116
Burger J, Gaines KF, Boring S, Stephens L, Snodgrass J, Dixon C, McMahon M, Shukla S, Shukla T, Gochfeld M (2002) Metal levels in fish from the Savannah River: potential hazards to fish and other receptors. Environ Res 89:85–97
Canli M, Ay Ö, Kalay M (1998) Levels of heavy metals (Cd, Pb, Cu, Cr and Ni) in tissue of Cyprinus Carpio, Barbus Capito and Chondrostoma Regium from the Seyhan River, Turkey. Tr J of Zoology 22:149–157
Cole DW, Cole R, Gaydos SJ, Gray J, Hyland G, Cole ML, Powell-Dunford N, Sawhney C, Au WW (2009) Aquaculture: environmental, toxicological, and health issues. Int J Hyg Environ Health 212:369–377
E O S Q C (Egyptian Organization for Standardization and Quality Control) (2005) The permissible limits for fish., 1–889
El-Sayed AE-S, El-Ayyat MS, Nasr E-S, Khater ZZK (2011) Assessment of heavy metals in water, sediment and fish tissues, from Sharkia province, Egypt Khater, Egypt. J. Aquat. Biol. & Fish. 15(2):125–144 ISSN 1110–1131)
Environmental Protection Agency (EPA) (2001) Parameters of water quality; Interpretation and Standards, EPA, Ireland. ISBN 1-84096-015-3
FAO/WHO, Expert Committee on Food Additives. (1999) Summary and conclusion, 53rd meeting, Rome, 1–10 June
Fujiki K, Shin DH, Nakao M, Yano T (2000) Molecular cloning and expression analysis of carp (Cyprinus carpio) interleukin-1 beta, high affinity immunoglobulin E Fc receptor gamma subunit and serum amyloid A. Fish Shellfish Immunol 10:229–242
Fujimoto RY, Neves M d S, Santos RFB, Cruz C, Diniz DG, Eiras JC (2014) Histopathological evaluation of seven Amazon species of freshwater ornamental armored catfish. Acta Sci 36:349
Golterman HL (1971): Methods for chemical analysis of freshwaters IBP handbook 3:1-166
Gonzalez SF, Buchmann K, Nielsen ME (2007) Real-time gene expression analysis in carp (Cyprinus carpio L.) skin: inflammatory responses caused by the ectoparasite Ichthyophthirius multifiliis. Fish Shellfish Immunol 22:641–650
Haiyan W, Stuanes AO (2003) Heavy metal pollution in air-water-soil-plant system of Zhuzhou City, Hunan Province, China. Water Air Soil Pollut 147:79–107
Heinecke RD, Buchmann K (2013) Inflammatory response of rainbow trout Oncorhynchus mykiss (Walbaum, 1792) larvae against Ichthyophthirius multifiliis. Fish Shellfish Immunol 34:521–528
Hodgson E (2011) A textbook of modern toxicology. http://scihub.tw/https://books.google.com.eg/books?isbn=1118211294
Hossain MK, Hossain MD, Rahman MH (2007) Histopathology of some diseased fishes. J Life Earth Sci 2:47–50
Jiang S, Zhang D, Li J, Liu Z (2008) Molecular characterization, recombinant expression and bioactivity analysis of the interleukin-1 beta from the yellowfin sea bream, Acanthopagrus latus (Houttuyn). Fish Shellfish Immunol. 24:323–336
Kantham KPL, Richards RH (1995) Effect of buffers on the gill structure of common carp, Cyprinus carpio L., and rainbow trout, Oncorhynchus mykiss. J Fish Dis 18:411–423
Linnik PM, Zubenko IB (2000) Role of bottom sediments in the secondary pollution of aquatic environments by heavy-metal compounds. Lakes and Reservoirs: Res and Man 5:11–21
Mahmoud MA, Mansour HA, Abdelsalam M, AbuBakr HO, Aljuaydi SH, Afify M (2019a) Evaluation of electrofishing adopted by Egyptian fish farmers. Aquaculture 498:380–387
Mahmoud MA, Abd El-Rahim AH, Mahrous KF, Abdelsalam M, Abu-Aita NA, Afify M (2019b) The impact of several hydraulic fracking chemicals on Nile tilapia and evaluation of the protective effects of Spirulina platensis. Environ Sci Pollut Res Int 26(19):19453–19467. https://doi.org/10.1007/s11356-019-05246-3
Mood SM, Ebrahimzadeh Mousavi HA, Mokhayer B, Ahmadi M, Soltani M, Sharifpour I (2010) Centrocestus formosanus metacercarial infection of four ornamental fish species imported into Iran. Bull Eur Ass Fish Pathol 30(30):146–149
Nachev M, Schertzinger G, Sures B (2013) Comparison of the metal accumulation capacity between the acanthocephalan Pomphorhynchus laevis and larval nematodes of the genus Eustrongylides sp. infecting barbel (Barbus barbus)., Parasites & Vectors 2013, 6:21, http://www.parasitesandvectors.com/content/6/1/21
Palíková M, Baruš V (2003) Mercury content in Aguillicola crassus (Nematoda) and its host Anguilla anguilla. Acta Vet Brno 72:289–294
Paperna I (1996) Parasites, infections and diseases of fishes in Africa. An update. CIFA Tech. Pap. No. 31. 220 p. FAO, Rome
Paruruckumani PS, Maharajan A, Ganapiriya V, Narayanaswamy Y, Raja Jeyasekar R (2015) Surface ultrastructural changes in the gill and liver tissue of Asian sea bass Lates calcarifer (Bloch) exposed to copper. Biol Trace Elem Res 168:500–507
Picard-Sanchez A, Estensoro I, del Pozo R, Piazzon MC, Palenzuela O, Sitja-Bobadilla A (2019) Acquired protective immune response in a fish-myxozoan model encompasses specific antibodies and inflammation resolution. Fish Shellfish Immunol 90:349–362
Praveen K, Evans DL, Jaso-Friedmann L (2006) Constitutive expression of tumor necrosis factor-alpha in cytotoxic cells of teleosts and its role in regulation of cell-mediated cytotoxicity. Mol Immunol 43(3):279–291
Ramelow GJ, Webre CL, Mueller CL, Beck JN, Young JC, Langley MP (1989) Variations of heavy metals and arsenic in fish and other organisms from the Calcasien River and Lake, Louisiana. Archive of Environ Contam and Toxicol 18:804–818
Roberts RJ, Rodger HD (2012) The pathophysiology and systematic pathology of teleosts. Fish Pathol:62–143
Saha M, Bandyopadhyay PK (2017) Parasitological and histological analysis of a new species of the genus Thalohanellus and description of a myxozoan parasite (Myxosporea: Bivalvulida) from cultured ornamental goldfish, Carassius auratus L. Aquacult Rep 8:8–15
Santos MA, Jerônimo GT, Cardoso L, Tancredo KR, Medeiros PB, Ferrarezi JV, Gonçalves ELT, da Costa Assis G, Martins ML (2017) Parasitic fauna and histopathology of farmed freshwater ornamental fish in Brazil. Aquaculture 470:103–109
Shareef PA, Abidi SMA (2012) Incidence and histopathology of encysted progenetic metacercaria of Clinostomum complanatum (Digenea: Clinostomidae) in Channa punctatus and its development in experimental host. Asian Pac J Trop Biomed 2(6):421–426
Shinn AP, Hansen H, Bachmann OK, Bakke L, T.A (2004) The use of morphometric characters to discriminate specimens of laboratory-reared and wild populations of Gyrodactylus salaris and G. thymalli (Monogenea). Folia Parasitol 51:239–252
Simon Jones RM (2001) The occurrence and mechanisms of innate immunity against parasites in fish. Dev Comp Immunol 25:841–852
SMWW (1998) Standard methods for the examination of water and wastewater. 20th edition
Sures B (2008a ) Environmental parasitology. Interactions between parasites and pollutants in the aquatic environment. Parasite 15, 434–438) numerical or physiological responses to pollutants can be positive, negative, or absent depending on the species, [ref:
Sures B (2008b) Host parasite interactions in polluted environments. J Fish Biol 73:1–10
Sures B, Taraschewski H, Jackwerth E (1994) Lead content of Paratenuisentis ambiguus (Acanthocephala), Anguillicola crassus (Nematodes) and their host Anguilla anguilla. Dis Aquat Organ 19:105–107
Suziki KT, Sunaga H, Aoki Y, Hatakeyama S, Sumi Y, Suziki T (1988) Binding of cadmium and copper in the mayfly Baetis thermicus larvae that inhabit in a river polluted with heavy metals. Comp Biochem Physiol 91(C):487–492
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. EXS. 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Tenora F, Baruš V, Kráčmar S, Dvořáček J, Srnková J (1999) Parallel analysis of some heavy metals concentrations in the Anguillicola crassus (Nematoda) and the European eel Anguilla anguilla (Osteichthyes). Helminthologia 36:79–81
Tenora F, Baruš V, Kráčmar S, Dvořáček J (2000) Concentration of some heavy metals in Ligula intestinalis plerocercoids (Cestoda) and Philometra ovate (Nematoda) compared to some their hosts (Osteichthyes). Helminthologia 37:15–18
Vinobaba P (1994) Some aspects of the biology of Dactylogyrus vastator Nybelin, 1924, (Monogenea) a gill parasite of Cyprinus carpio L. PhD Thesis. Institute of Aquaculture, University of Stirling, Stirling, U.K. pp. 1–46
Wang Q, Yu Y, Xiaoting, Zhen X (2019) Immune responses of fish to Ichthyophthirius multifiliis (Ich): a model for understanding immunity against protozoan parasites. Dev Comp Immunol 93:93–102
WHO (1993) Evaluation of certain food additives and contaminates (Forty-first report of joint FAO/WHO export committee on food Additives). WHO Technical Report Series NO. 837, WHO, Geneva
Zhou H, Cheung R, Chan K, Wong M (1998) Metal concentrations in sediments and tilapia collected from inland waters of Hong Kong. Water Res 32:3331–3340
Zhu L, Nie L, Zhu G, Xiang LX, Shao J-Z (2013) Advances in research of fish immune-relevant genes: a comparative overview of innate and adaptive immunity in teleosts. Dev Comp Immunol 39(2013):39–62
Zou J, Secombes CJ (2016) The function of fish cytokines. Biology 5:23. https://doi.org/10.3390/biology5020023
Author information
Authors and Affiliations
Contributions
All authors share in the aim of works. Nehal A. Younis: collect the samples and identify the clinical sign on the fish, Samah E. Laban: analyze the water quality and toxic substance in fish tissues, Asmaa K. Al-Mokaddem: examine the histopathological changes of the fish tissues, Marwa M. attia: examine the parasites inside the fishes and analyze the gene expression. All authors share in writing this manuscript and revise it.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Younis, N.A., Laban, S.E., Al-Mokaddem, A.K. et al. Immunological status and histopathological appraisal of farmed Oreochromis niloticus exposed to parasitic infections and heavy metal toxicity. Aquacult Int 28, 2247–2262 (2020). https://doi.org/10.1007/s10499-020-00589-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-020-00589-y