Abstract
Twenty-three species of dry Traditional Chinese herbs (TCHs) were examined for the experiment. Bacteriostasis experiments in vitro were carried out for screening 23 TCHs on inhibiting Vibrio harveyi and four screened species (Galla chinensis, Terminalia chebula, Scutellaria baicalensis, Rheum officinale) which have antibacterial activity were decoct, concentrated, and mixed with diets at 2%, respectively. After feeding for 28 days, shrimps were challenged by V. harveyi, and then mortality was recorded. With regard to hemocyte phagocytic activity, activities of lysozyme (LZM) were measured on day 0, 7, 14, 21, and 28. Relative percentage survival (RPS) was also investigated in each TCH group. Phagocytic activity (PA) phagocytic index (PI), LZM, and RPS (with the exception of T. chebula and R. officinale) of Pacific white shrimp can be significantly increased when fed 2% TCH extracts. RPS of S. baicalensis was the highest, reaching 51.86% on day 28, then the G. chinensis group, whereas R. officinale group was the lowest, when the Pacific white shrimp challenged with V. harveyi. It can be concluded that S. baicalensis and G. chinensis could be used to enhance shrimp’s immunity and disease resistance in cultured Pacific white shrimp.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacific white shrimp (Litopenaeus vannamei) is cultured commonly in the Western Hemisphere and has been grown in inland saline waters. In China, Pacific white shrimp culture trials begun with the importation of larvae in the late 1980s. The species is now being economically important shrimp in China. In the recent years, with the higher density in the culture of Pacific white shrimp, Pacific white shrimp became susceptible to many disease outbreaks (Ganjoor 2015).
In order to reduce financial losses caused by shrimp diseases, effective preventive measures should be taken. Whereas, chemotherapeutics and antibiotics will pollute environment and accumulate residues in shrimp. Vaccines are very expensive to fish farmers and specific to diseases (Sakai 1999).
Immunostimulants can enhance immune mechanism and have been considered as an effective method in preventing fish diseases (Anderson 1992). In our research, TCHs were used to activate nonspecific defense mechanisms, and Pacific white shrimp’s specific immune response was elevated.
TCH can improve nonspecific immunity and have been used in aquaculture as immune stimulating factors for many years (Ardó et al. 2008; Yin et al. 2008; Yin et al. 2009; Pan et al. 2013; Adel et al. 2017). For instance, when different TCHs were fed to crucian carp, NBT positive cells, phagocytosis, and lysozyme activity increased (Jeney and Jeney 2002). The lysozyme activity significantly increased, and the cumulative mortality significantly decreased when oliver flounder Paralichthys olivaceus were fed with 0.1% and 1.0% monkey head mushroom, Hericium erinaceum-enriched diet once infected with Philasterides dicentrarchi (Harikrishnan et al. 2011). When Red drum were fed 2% of TCH, phagocytic percentage, phagocytic index, and lysozyme activity significantly increased in major groups, and Astragalus membranaceus et al. were effective in preventing the disease caused by Vibrio splendidus (Pan et al. 2013). In this study, TCH extracts from Artemisia argyi, Atractylodis macrocephalae, Rhizoma coptidis, Cortex moutan, Radix glycyrrhizae, Terminalia chebula, Fructus mume, Galla chinensis, Houttuynia cordata, Cortex phellodendri, Cistanche deserticola, Wolfiporia extensa, Fructus forsythia, Rheum officinale, Flos lonicerae, Polyporus umbellatus, Isatidis radix, Astragalus membranaceus, Eucommia ulmoides, Rhizoma cyrtomii, Scutellaria baicalensis, Radix bupleuri, and Radix sophorae were chosen for the bacteriostasis experiment in vitro (Jian and Wu 2003; Ardó et al. 2008; Yin et al. 2008, 2009; Yan et al. 2010; Pan et al. 2013).
In the study reported here, G. chinensis, T. chebula, S. baicalensis, and R. officinale had significant effects on bacterial inhibiting in vitro among the 23 species TCH.
Pacific white shrimp was fed with feed containing one of the four TCH, respectively. Key parameters of nonspecific immune response were measured for 4 weeks, and RPS of Pacific white shrimp was also evaluated when challenged with V. harveyi.
Materials and methods
Shrimp
Apparently, healthy Pacific white shrimp (weight 9.80 ± 0.21 g) was maintained in 20,000-L concrete tanks with recirculation system in Zhejiang Mariculture Research Institute, Wenzhou, Zhejiang province, China. After 2 weeks, the shrimps were transferred into 2000-L tanks. During the experiment, water temperature 23–25 °C, salinity 26 g/L, pH 8.2, and dissolved oxygen 5.0 mg/L–6.0 mg/L were maintained. Shrimp were fed with a formulated feed.
Preparation of TCH extracts and supplementation diet
TCHs including A. argyi, A. macrocephalae, R. coptidis, C. moutan, R. glycyrrhizae, T. chebula, F. mume, G. chinensis, H. cordata, C. phellodendri, C. deserticola, W. extensa, F. forsythia, R. officinale, F. lonicerae, P. umbellatus, I. radix, A. membranaceus, E. ulmoides, R. cyrtomii, S. baicalensis, R. bupleuri, and R. sophorae were purchased in Wenzhou people’s large pharmacy, Zhejiang Province. A hot-water extract of the 23 TCHs was extracted as Wu et al. (2010) reported. Twenty-five grams of each TCH were added to 250 mL of deionized water, respectively, and boiled for 0.5 h and then filtered and boiled twice as the first time. The 750-mL filtrate of each TCH was concentrated to 25 mL (each TCH concentration was about 1 g/mL) and kept at 4 °C for bacteriostasis experiment in vitro. With the result of bacteriostasis experiment in vitro, four TCH including G. chinensis, T. chebula, S. baicalensis, and R. officinale were used to prepare TCH extracts as Pan et al. (2013). Ten grams of each TCH were added to 200 mL of deionized water, respectively, and boiled for 0.5 h and then filtered and boiled twice as before. The 600-mL filtrate of each TCH was concentrated to 50 mL, then added to 490 g crushed formula feed (Fuzhou Haima Feed Co., Ltd), respectively. The control group added 50 mL of 0.65% sodium chloride. Normal balanced feed composed of 46.4% protein, 22% carbohydrate, 3.6% lipid, and 10.8% ash. Diameter of the pellet is 1.2 mm. All pellets are stored at 4 °C in refrigerator.
Bacteriostasis experiment in vitro
Vibrioharveyi was isolated from diseased Pacific white shrimp as Parichat and Pongsak (2010) reported and identified using 16S rDNA sequencing (Frank et al. 2008). The bacteria were cultured in Luria-Bertani (LB) containing 2% NaCl, incubated at 30 °C for 24 h, centrifuged at 2500 g for 10 min, and washed in phosphate-buffered saline (PBS). V. harveyi was used to determine the lowest concentration of the assayed antimicrobial agent (minimal inhibitory concentration, MIC, minimum bactericidal concentration, MBC) at the concentration of 1 × 106 CFU/mL for the 23 TCHs (Irith et al. 2008).
Experiment design
Shrimps were allocated into 4 treated groups and one control group (300 shrimps per group) in triplicate and fed diets twice a day for 4 weeks. Then, remaining shrimps of all groups were fed with the same formula feed (Fuzhou Haima Feed Co., Ltd) for 1 week.
Preparation of hemolymph and hemocytes
Hemolymph samples (6 shrimps per group) were collected from the heart without anesthesia on day 0, 7, 14, 21, and 28 after start of feeding. Some sampled anesthesia were put into a tube to prepare hemolymph for assaying lysozyme; others were put into another tube containing sodium heparinate for measuring phagocytic activity.
Phagocytic activity
Phagocytic activity of hemocytes was performed as Enright and Jeffers (1984). To perform the essay, 100 μL of hemolymph was mixed with 100 μL Staphylococcus aureus (1.0 × 107 CFU / mL). The mixtures were incubated at 28 °C for 60 min and shaken once every 10 min during the water bath. After the culture, 3 ml of cold physiological saline was added and centrifuged at 2000 rpm for 3 min, and then supernatant was discarded. A 1-ml AO dye solution was added to the above phagocytic precipitate, mixed well, and stained for 1 min. After centrifugation at 2000 rpm for 3 min, the supernatant discarded and the hemocytes were suspended in physiological saline and placed in an ice bath for observation by fluorescence microscope. Phagocytic activity (PA) and phagocytic index (PI) were calculated by the following formula:
Lysozyme activity
Lysozyme activity was measured spectrophotometrically as Adel et al. (2017) reported. In brief, a series of dilutions was prepared by diluting a standard lysozyme sample (Amresco, Switzerland) mixed with a Micrococcus lysodeikticus (ATCC NO. 1698) suspension for establishing a calibration curve. For this, 500 ml of diluted hemolymph was added to 1 ml of a suspension of M. lysodeikticus (0.2 mg/ml) in a 0.05 M sodium phosphate buffer (pH 6.2). Absorbance was measured at 500 nm after 30 and 270 s by spectrophotometer (Biophotometer Eppendorf). The lysozyme content was determined using the calibration curve and the absorbance measured. One unit (U) of lysozyme activity was defined as the amount of enzyme that decreases the absorbance by 0.001 min−1 mL−1 hemolymph.
Relative percent survival (RPS) (Amend 1981)
Shrimps were challenged by injecting LD50 dose (0.05 mL PBS containing 1 × 106 cells) of live V. harveyi into the ventral sinus of the cephalothorax on day 28 as He et al. (2017) reported. Mortality was recorded for 7 days. From the organs of the just dead shrimp, V. harveyi was re-isolated to confirm the mortality due to the bacterial infection. RPS was calculated by the following formula:
Statistical analysis
The data were expressed as arithmetic mean ± standard error. Statistical analysis involved one-way analysis of variance (ANOVA) followed by Duncan’s multiple pair comparison test (SPSS 13.0).
Results
Effect of bacteriostasis experiment in vitro
All the 23 TCHs have different effects on the bacteriostasis in vitro. Four of the 23 TCHs, i.e., G. chinensis, T. chebula, S. baicalensis, and R. officinale, have more significant effects on bacteriostasis for V. harveyi in vitro; MIC of them is 0.001 mg/mL, 0.03 mg/mL, 0.49 mg/mL, and 0.24 mg/mL; MBC of them is 0.008 mg/mL, 0.24 mg/mL, 0.98 mg/mL, and 0.98 mg/mL, respectively.
Phagocytic activity
The PA of leucocyte varied significantly (P < 0.05) on day 28 in control group. The PA activity increased significantly (P < 0.05) when fed with 2% G. chinensis- or S. baicalensis-enriched diets on day 7 or fed with 2% R. officinale- or T. chebula-enriched diets on day 14 compared to the control (Table 1).
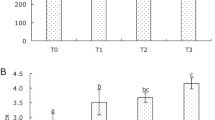
During the experiment period, PI of the control group is 1.67–1.84, and the S. baicalensis group is the highest among all the experiment groups, reaching 3.32 on day 21. PI of groups of S. baicalensis and G. chinensis has significant difference (P < 0.05) on day 7, when compared to the control group. Groups of R. officinale and T. chebula have significant difference on day 14 when compared to the control. PI of groups of S. baicalensis and G. chinensis has significant difference (P < 0.05) on day 14 compared to the group R. officinale and T. chebula (Table 2).
Lysozyme activity
The plasma lysozyme activity was enhanced significantly (P < 0.05) on day 7 on being fed with S. baicalensis-, G. chinensis-, R. officinale-, or T. chebula-enriched diets compared to the control group, and this high lysozyme activity can be maintained to day 28 (Table 3).
Disease resistance
The highest RPS were 51.9% and 48.1% in groups fed with S. baicalensis- or G. chinensis-enriched diets, and the lowest relative percent survival was only 14.8% in groups fed with R. officinale-enriched diets when challenged with V. harveyi.
The highest mortality is 90% in the control diet (Table 4). Shrimps began to die on the first day in group treated with R. officinale and on the second day in groups treated with S. baicalensis, G. chinensis, and T. chebula after challenged with V. harveyi (Table 4).
Discussion
Bacteriostasis in vitro of A. argyi, A. macrocephalae, R. coptidis, C. moutan, R. glycyrrhizae, T. chebula, F. mume, G. chinensis, H. cordata, C. phellodendri, C. deserticola, W. extensa, F. forsythia, R. officinale, F. lonicerae, P. umbellatus, I. radix, A. membranaceus, E. ulmoides, R. cyrtomii, S. baicalensis, R. bupleuri, and R. sophorae extracts on V. harveyi was analyzed in this study. Results showed that 4 TCHs (G. chinensis, T. chebula, S. baicalensis, R. officinale) extracts have the lowest MIC and MBC for V. harveyi at the concentration of 1 × 106 CFU/mL. The immunostimulating effect of the 4 TCH extracts in the Pacific white shrimp was also studied. The results showed that experiment shrimps fed with 2% dose of TCH extracts significantly enhanced PA, PI, lysozyme activities, and RPS (with the exception of R. officinale and T. chebula).
Different TCHs may have different effects on bacteriostasis. Progression of bacteriostasis experiment in vitro can evaluate antibacterial activity in vitro. In this study, 23 TCH extracts were used to evaluate the effects of antibacteriostasis in vitro; the results showed that 23 TCH extracts have a large range of MIC (0.001 mg/mL −500 mg/mL)and MBC (0.008 mg/mL −500 mg/mL). Four TCHs (G. chinensis, T. chebula, S. baicalensis, R. officinale) extracts have the lowest MIC and MBC; the results indicate that these 4 TCHs have better antibacterial activity among the 23 TCHs. A previous study showed that G. chinensis extract significantly inhibited the growth of Vibrio parahaemolyticus and Listeria monocytogenes (Wu 2014; Wu et al. 2016). The ethanol extract of T. chebula had total phenolic and flavonoid content of 136 ± 1.5 mg of gallic acid equivalent/g d.w and 113 ± 1.6 mg of quercetin equivalent/g d.w, respectively, and some of the extracts such as alkaloids, tannins, and phenols have significant cytotoxic activity (Eshwarappa et al. 2016). The major bioactive components in roots of S. baicalensis were baicalein, baicalin, and wogonin (Li et al. 2009). Baicalin has anti-inflammatory, anti-allergic, antioxidant, and hepatoprotective activities (Chen et al. 2009). The hemolymph lysozyme activity of Macrobrachium rosenbergii in the group fed with 0.05% anthraquinone extract from Rheum officinale for 8 weeks were significantly higher than those of the control (Liu et al. 2010).
Phagocytic activity is important to primitive defense mechanism and nonspecific immune system (Neumann et al. 2001; Seeley et al. 1990). Sakai (1999) stated that fish phagocytosis can be increased when treated with immunostimulants. Phagocytosis can be enhanced by TCH extracts in many fish and shrimp species (Galina et al. 2009; Zhang et al. 2009; Huang et al. 2011; Ahmadi et al. 2012; Pan et al. 2013; Adel et al. 2017; He et al. 2017). Previous studies showed that S. baicalensis can enhance the nonspecific immune response and have a high RPS in Sciaenops ocellatus (Pan et al. 2013). Calocybe indica (milky mushroom) extract was used as a feed additive to improve Babylonia spirata antioxidant levels (Chelladuraia and Maran 2019). When pacific white shrimps were treated with polysaccharides from mycelia of Cordyceps sinensis, hemolymph immunity indicators, including phenoloxidase, alkaline phosphatase, acid phosphatase, and lysozyme were remarkably greater in the treated group (P < 0.05) (Deng et al. 2014). In our experiments, phagocytic activity of leukocytes can be enhanced significantly by G. chinensis, T. chebula, S. baicalensis, or R. officinale extracts during the 28 days feeding, even 7 days after start of feeding. This elevated activity can be maintained to the end, when compared to the control experiment. All of the four TCH extracts have phagocytic activity and nonspecific immunity to shrimp, suggesting that all TCH extracts may have immunostimulatory effects when they have bacteriostatic effects in vitro.
Lysozyme level is an important measurable element for the nonspecific defense system. Lysozyme can hydrolyze β-1-4-glucosidic linkages in the mucopolysaccharide cell wall of bacterial pathogens. In this study, lysozyme activity of the Pacific white shrimp was enhanced 7 days after start of feeding extract of the 4 TCH. Many authors have also found that values of fish and shrimp lysozyme can be increased after feed with TCH (Yin et al. 2008; Zhang et al. 2009; Ahmadi et al. 2012; Pan et al. 2013; Deng et al. 2014).
In our research, after challenged with V. harveyi, mortality of all treated shrimps reduced when compared to the control. RPS of S. baicalensis and G. chinensis are significantly higher than that of R. officinale, T. chebula and the control group. The group that was treated with S. baicalensis had the highest RPS. Among the remaining three groups, shrimps treated with G. chinensis had a higher RPS than that of R. officinale or T. chebula. Immunostimulants, vaccines, or probiotics could increase infected fish’s survival rates (Sakai 1999; Bakopoulos et al. 2003; Brunt et al. 2007). After challenging with V. harveyi, the survival rate of large yellow croaker increased in the group that was treated with glucan (Ai et al. 2007). Adel et al. (2017) reported that Pediococcus pentosaceus improved the growth performance, digestive enzyme activity, immunity, and tolerance against Vibrio anguillarum of white shrimp. Dietary administration of yeast can protect shrimp against a decline in resistance to bacterial disease (Burgents et al. 2004). Adding 0.3 g/kg of organic acids and essential oils that blend to the control diet significantly enhances disease resistance of Pacific white shrimp to Vibrio parahaemolyticus (He et al. 2017).
Through the experiment, a relationship between the phagocytic tests and survival in the challenge can be found. PA activity and PI had significant difference (P < 0.05) on day 7 in the TCH-enriched diets when compared to the control; RPS is also significantly different (P < 0.05) when challenged with V. harveyi.
In brief, our research showed that G. chinensis, T. chebula, S. baicalensis, and R. officinale extracts alone could significantly enhance phagocytic activity of leucocyte and hemocyte lysozyme activity. S. baicalensis or G. chinensis significantly enhanced the RPS.
In summary, G. chinensis or S. baicalensis were effective in preventing Pacific white shrimp from outbreaking diseases after challenged by V. harveyi.
References
Adel M, Yeganeh S, Dawood MAO et al (2017) Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquac Nutr 23(6):1401–1409
Ahmadi K, Banaee M, Vosoghei AR et al (2012) Evaluation of the immunomodulatory effects of silymarin extract (Silybum marianum) on some immune parameters of rainbow trout, Oncorhynchus mykiss (Actinopterygii: Salmoniformes: Salmonidae). Acta Ichthyol Piscat 42(2):113–120
Ai Q, Mai K, Zhang L et al (2007) Effects of dietary β-1, 3 glucan on innate immune response of large yellow croaker, Pseudosciaena crocea. Fish Shellfish Immun 22(4):394–402
Amend DF (1981) Potency testing of fish vaccines. Develop Biol Standard 49:447–454
Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu Rev Fish Dis 2:281–307
Ardó L, Yin G, Xu P et al (2008) Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture 275(1):26–33
Bakopoulos V, Volpatti D, Gusmani L, Galeotti M, Adams A, Dimitriadis GJ (2003) Vaccination trials of sea bass, Dicentrarchus labrax (L.), against Photobacterium damsela subsp. piscicida, using novel vaccine mixtures. J Fish Dis 26(2):77–90
Brunt J, Newaj-Fyzul A, Austin B (2007) The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 30(10):573–579
Burgents JE, Burnett KG, Burnett LE (2004) Disease resistance of Pacific white shrimp, Litopenaeus vannamei, following the dietary administration of a yeast culture food supplement. Aquaculture 231:1–8
Chelladuraia G, Maran BAV (2019) Dietary supplementation of mushroom extract enhances growth and antioxidant levels of Babylonia spirata (Mollusca: Gastropoda). Aquacult Rep 15:100218
Chen Y, Lu N, Ling Y et al (2009) Wogonoside inhibits lipopolysaccharide-induced angiogenesis in vitro and in vivo via toll-like receptor 4 signal transduction. Toxicology 259:10–17
Deng B, Wang ZP, Tao WJ et al (2014) Effects of polysaccharides from mycelia of Cordyceps sinensis on growth performance, immunity and antioxidant indicators of the white shrimp Litopenaeus vannamei. Aquac Nutr 21(2):173–179
Enright FM, Jeffers GW (1984) In: Barta O (ed) In laboratory techniques of veterinary clinical immunology. Springfield, Illinois, pp 58–65
Eshwarappa RSB, Ramachandra YL, Subaramaihha SR et al (2016) In vivo toxicity studies on gall extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae). Pharm Res 8(3):199–201
Frank JA, Reich CI, Sharma S et al (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microb 74(8):2461–2470
Galina J, Yin G, Ardó L et al (2009) The use of immunostimulating herbs in fish an overview of research. Fish Physiol Biochem 35(4):669–676
Ganjoor M (2015) A short review on infectious viruses in cultural shrimps (Penaeidae family). Fish Aquac J 9(3):1
Harikrishnan R, Kim JS, Kim MC et al (2011) Hericium erinaceum enriched diets enhance the immune response in Paralichthys olivaceus and protect from Philasterides dicentrarchi infection. Aquaculture 318(1):48–53
He WQ, Rahimnejad S, Wang L et al (2017) Effects of organic acids and essential oils blend on growth, gut microbiota, immune response and disease resistance of Pacific white shrimp (Litopenaeus vannamei) against Vibrio parahaemolyticus. Fish Shellfish Immun 70:164–173
Huang C, Lee T, Shih Y et al (2011) Effects of dietary supplementation of Chinese medicinal herbs on polymorphonuclear neutrophil immune activity and small intestinal morphology in weanling pigs. J Anim Physiol An N 96(2):285–294
Irith W, Kai H, Robert H (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3(2):163–175
Jeney G, Jeney Z (2002) Application of immunostimulants for modulation of the non-specific defense mechanisms in sturgeon hybrid: Acipenser ruthenus × A. baerii. J Appl Ichthyol 18(4–6):416–419
Jian J, Wu Z (2003) Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218(1):1–9
Li C, Zhou L, Lin G et al (2009) Contents of major bioactive flavones in proprietary traditional Chinese medicine products and reference herb of radix Scutellariae. J Pharmaceut and Biomed 50:298–306
Liu B, Ge XP, He YH et al (2010) Effects of anthraquinones extracted from Rheum officinale Bail on the growth, non-specific immune response of Macrobrachium rosenbergii. Aquaculture 310:13–19
Neumann NF, Stafford JL, Barreda D et al (2001) Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev Comp Immunol 25(8):807–825
Pan T, Yan M, Chen S et al (2013) Effects of ten traditional Chinese herbs on immune response and disease resistance of Sciaenops ocellatus (Actinopterygii: Perciformes: Sciaenidae). Acta Ichthyol Piscat 43(1):41–49
Parichat P, Pongsak R (2010) Isolation and partial characterization of a bacteriophage infecting the shrimp pathogen Vibrio harveyi. Afr J Microbiol Res 4(16):1794–1800
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172(1):63–92
Seeley KR, Gillespie PD, Weeks BA (1990) A simple technique for the rapid spectrophotometric determination of phagocytosis by fish macrophages. Mar Environ Res 30(1):37–41
Wu J (2014) Inhibiting Listeria monocytogenes, Vibrio parahaemolyticus and Morganella morganii with aqueous methanol extracts of Punica granatum and Galla chinensis. Ph.D. dissertation. Blacksburg, Virginia, USA: Virginia Tech
Wu CC, Liu CH, Chang YP et al (2010) Effects of hot-water extract of Toona sinensis on immune response and resistance to Aeromonas hydrophila in Oreochromis mossambicus. Fish Shellfish Immun 29(2):258–263
Wu J, Jahncke ML, Eifert JD et al (2016) Pomegranate peel (Punica granatum L) extract and Chinese gall (Galla chinensis) extract inhibit Vibrio parahaemolyticus and Listeria monocytogenes on cooked shrimp and raw tuna. Food Control 59:695–699
Yan M, Chen S, Shan L et al (2010) Selection of 22 kinds of Chinese herbal medicines on Bacteriostasis in vitro on Vibrio vulnificus and Vibrio splendidus. Journal of hydroecology 2:95–98
Yin GJ, Ardó L, Jeney Z et al (2008) Chinese herbs (Lonicera japonica and Ganoderma lucidum) enhance non-specific immune response of tilapia, Oreochromis niloticus, and protection against Aeromonas hydrophila. Diseases in Asian Aquaculture VI. Fish Health Section, Asian Fisheries Society, Manila, pp 269–282
Yin GJ, Ardó L, Thompson KD et al (2009) Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immun 26(1):140–145
Zhang G, Gong S, Yu D et al (2009) Propolis and Herba Epimedii extracts enhance the non-specific immune response and disease resistance of Chinese sucker, Myxocyprinus asiaticus. Fish Shellfish Immun 26(3):467–472
Acknowledgments
Science and Technology Project of Zhejiang Province (2016C02055-5, 2015C03005, 2016F10021), the China Agriculture Research System (CARS-47).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest to declare.
Compliance with ethical guidelines
The experimental procedure was approved by the Animal Ethics Committee at the Zhejiang Mariculture Research Institute. (No. 30051.0076/2013–46).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pan, T., Yan, M. The screening of traditional Chinese herbs on nonspecific immune response and protection of Pacific white shrimp (Litopenaeus vannamei) from Vibrio harveyi infection. Aquacult Int 28, 767–776 (2020). https://doi.org/10.1007/s10499-019-00493-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-019-00493-0