Abstract
To investigate the effect of hypothermal (22 → 16 °C) and hyperthermal (22 → 28 °C) stress on the energy regulation of Litopenaeus vannamei cultured in long-term freshwater, the activities of hexokinase (HK), pyruvate kinase (PK), lactic dehydrogenase (LDH), and succinate dehydrogenase (SDH) were determined and compared with those kept in seawater. Results showed that at the early stage of thermal stress, HK, PK, and LDH activities increased and then decreased. At the end of the trial (48 h), all enzyme activities except PK and LDH activities in shrimps cultured in freshwater returned to the pre-experiment level. Following temperature stress, SDH activity of shrimps cultured in freshwater and seawater all decreased first and then increased, and finally reached the pre-experiment level, while SDH activity in gills of shrimps cultured in seawater was significantly lower than that before. At a constant temperature of 22 °C, compared with shrimps cultured in seawater, those cultured in freshwater had a lower level in HK, PK, and SDH activities, but a higher level in LDH activity. In summary, shrimps cultured in freshwater might rely more on anaerobic metabolism, while the opposite was true for glycolysis and aerobic metabolism. Because shrimps cultured in freshwater had a higher sensitivity to thermal stress, noticeable temperature variation especially when temperature decrease should be avoided in the freshwater intensive culture of L. vannamei.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal survival and growth are directly related with external environments which have considerable influence on internally biochemical and physiological processes (Magnuson et al. 1979; Reynolds and Casterlin 1979). In nature, aquatic organisms can adapt to change in their habitat environments by behavioral and metabolic regulation. For poikilotherm, this regulatory ability is of vital importance, which makes them physiologically flexible against thermal change in a certain range (Buckley et al. 2001).
As the basic physiological activity of organisms, energy metabolism can help to maintain the normal life activity. It also reflects internal metabolic condition, physiological mechanism, and organism adaptability to environment changes. Zhang et al. (1998) reported that in crustaceans, respiration consumes at least 50% of total assimilated energy. For metabolism, specific enzymes can accelerate the conversion of metabolites to meet the energy demand for organisms. In fact, enzyme activity and metabolite content are two key factors influencing metabolism and they are co-regulated by endogenous factors such as ages (Segner and Verreth 1995; Marsh et al. 1999) and exogenous factors such as temperature and light (Torres and Somero 1988; Vetter and Buchholz 1997). Enzyme activity is reported to be a direct indicator of metabolism in various organisms (Ding et al. 2009, 2014; Zeng et al. 2018). The biological characteristics of the Pacific white shrimp, Litopenaeus vannamei, make it easy to adapt to ambient environments and thus the shrimp have become the most widely cultured shrimp species. Due to its strong adaptability to low salinity, freshwater culture of this species become popular in many countries (Hu et al. 2004). Because the culture of L. vannamei yields higher profits than the culture of other freshwater aquaculture species, and because there is more land available for aquaculture in inland areas than in coastal areas, it is expected that fresh water culture of L. vannamei will continue to increase in many inland regions (Liao and Chien 2011). Compared to seawater aquaculture, freshwater aquaculture is higher in diurnal temperature variation and poor in water stability. Studying the effect of thermal stress on the metabolic enzymes of L. vannamei is significant for successful culture of freshwater shrimp. However, little detailed research has been conducted on this topic. We therefore designed this experiment to compare the effects of hypo- and hyperthermal stress in shrimp that are cultivated in either freshwater or seawater. The results of the study could provide an understanding of the physiological characteristics of L. vannamei cultured under two different water conditions and provide a scientific reference for improving freshwater culture techniques and environmental management of L. vannamei.
Materials and methods
Shrimps and acclimation
One thousand healthy shrimps (8.69 ± 0.27 cm, length) were obtained from a shrimp farm in Jiaozhou Qingdao, where the water salinity was 18–20 practical salinity units (psu). After transportation to the laboratory, shrimps were placed in two round fiberglass tanks (7000 L) filled with water (18 psu, 22.0 ± 0.5 °C), each containing 500 shrimps. After 7 days, water salinity in one tank was elevated at a rate of 2–3 psu/day by adding normal seawater (30 psu). When the salinity was near 30 psu, the normal seawater would be supplied. In another tank, the salinity was reduced to 5 psu at the same rate in the other tank by adding freshwater and then further reduced to < 0.5 psu at a rate of 0.5–1 psu/day. After target salinity was reached, shrimps were allowed to acclimate for another 30 days before experiments.
During acclimation, water was aerated continuously, and temperature was maintained at 22.0 ± 0.5 °C. Half of the water was exchanged for aerated freshwater or sand filtrated normal seawater every day. Shrimps were provided with enough formulated feed three times a day (7:00, 14:00, and 21:00), and uneaten food and feces were siphoned out after 3 h. The photoperiod was 14L:10D.
Experiment process
According to Drach (1939) and Cesar et al. (2006), 600 size-similar shrimps (300 shrimps from freshwater group and 300 shrimps from seawater group) at intermolt stage were chosen and used for this experiment. After starvation for 24 h, 300 shrimps from freshwater or seawater respectively were randomly divided into three groups: control group (22 °C), hyperthermal (28 °C), and hypothermal (16 °C). Each group consisted of 20 glass aquaria (90 × 50 × 30 cm), with five shrimps in each aquarium filled with 60-L water. During the experiment, shrimps in each group were subject to corresponding thermal stress by being transferred from normal temperature (22 °C) to target temperature (16 and 28 °C). With the synergistic work of temperature controllers and water chillers or submerged heaters, the water in two groups was maintained at 16 ± 0.5 and 28 ± 0.5 °C respectively. After thermal stresses, 10 individuals were sampled at 1, 3, 6, 12, 24, and 48 h respectively. During experiments, water was aerated continuously, and water salinity was monitored and adjusted to keep constant every 3 h.
Sample collection and preparation
Sampled shrimps were dried with absorbent paper. And then shells were removed and tissues (gills and muscles) were dissected into a 1.5-mL centrifuge tube. Tubes were flash frozen in liquid nitrogen and finally stored in − 80 °C refrigerator (Thermo Scientific Forma 702, Thermo Fisher Scientific Co., MA, USA) until analysis.
Tissues of 0.1–0.3 g were cut into pieces, homogenized in 9 volume saline (4 °C, 0.86%), and centrifuged for 10 min (3000 rpm, 4 °C) (Universal 320R, Andreas Hettich GmbH & Co., KG, Germany). The supernatants were collected and stored at − 80 °C until measurement of hexokinase (HK), pyruvate kinase (PK), lactate dehydrogenase (LDH), and succinate dehydrogenase (SDH) activity.
Sample analysis
The activities of HK, PK, LDH, and SDH were measured via colorimetric methods with UV-VIS spectrophotometer (Thermo Scientific Evolution 300, Thermo Fisher Scientific Co., MA, USA) using Hexokinase Assay Kit, Pyruvate Kinase Assay Kit, Lactate Dehydrogenase Assay Kit and Succinate Dehydrogenase Assay Kit (Jiancheng Biological Engineering Institute, Nanjing, China) according to the manufacturer instructions.
HK activity was determined using 6-phosphogluconate dehydrogenase coupling colorimetric method (Tanaka et al. 1962). In the presence of HK, glucose was phosphorylated into glucose-6-phosphate (G-6-P). The reaction product was then dehydrogenized by 6-phosphaogluconate dehydrogenase. In this reaction, NADP was reduced to NADPH. Therefore, absorbance change at 340 nm was used to calculate the HK activity (Tanaka et al. 1962). One enzyme activity unit was defined as 1 mmol/L NADPH formed by per gram protein in the reaction system at 37 °C and pH 7.6.
PK activity was determined according to the method of Ireland et al. (Valentin et al. 1967). In the reaction system, PK could catalyze phosphoenolpyruvate (PEP) into pyruvate, which was converted into phenylhydrazone in the presence of 2,4-dinitrophenylhydrazine. The color of final solution was positively related with pyruvate concentration. Accordingly, activity of PK could be evaluated. One enzyme activity unit was defined as the conversion of 1 μmol PEP into pyruvate by per gram protein in the reaction system at 37 °C and pH 7.6.
LDH activity was determined with the method of Thébault (1984). In the reaction system, LDH catalyzed lactate into pyruvate, which was converted into phenylhydrazone in the presence of 2,4-dinitrophenylhydrazine. The absorbance of final solution at 340 nm was used to calculate PK activity. One enzyme activity unit was defined as the formation of 1 μmol pyruvate by per gram protein in the reaction of 15 min at 37 °C.
SDH activity was determined using commercial kits (Nanjing Jiancheng Bioengineering Institute, China). In the reaction system, flavin adenine dinucleotide (FAD) was reduced to FADH. The reaction was coupled with the reduction of 2,6-dichlorophenol indophenol (2,6-DCPIP). Absorbance changes of reaction system at 600 nm was used to calculate SDH activity. One enzyme activity unit was defined as the decrease of 0.01 absorbance by per gram protein in the reaction system at 37 °C.
Total protein content was determined with Coomassie Brilliant Blue G250 dye binding method (Bradford 1976).
Statistical analysis
Resulting data in this experiment were expressed as mean ± standard error (S.E.) and analyzed with SPSS 16.0 software. Three-way ANOVA test and SNK multiple comparison analysis were employed to determine the difference among time intervals. Independent sample T test was conducted to analyze differences of global means in both freshwater and seawater groups and differences between freshwater and seawater at the same time. P values< 0.05 were considered statistically significant for all variance tests. Data from analysis were made into pictures by Sigmaplot 12.0 software.
Results
The energy metabolic enzyme activity of L. vannamei cultured in freshwater and seawater at 22 °C
The levels of energy metabolic enzyme activities of L. vannamei at 22 °C were shown in Table 1. Shrimps cultured in freshwater at 22 °C had a lower value than shrimps cultured in seawater in activities of HK, PK, and SDH, while the former was higher than the latter in LDH activity.
Effects of thermal stresses on HK activity
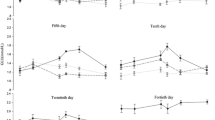
Figure 1a, d showed that compared to the control group, HK activities of gill and muscle, shrimps in freshwater and seawater all changed slightly during the experiment (P > 0.05). During the experiment, HK activity of gill in freshwater shrimp was significantly lower than that in seawater shrimp (P < 0.05), and it was similar to HK activity of muscle (P > 0.05). Figure 1b, c showed the response of HK activities of gill in shrimps cultured in freshwater and seawater conditions to thermal stress. Following hypothermal stress, HK activity in gill was elevated within 1 h (freshwater) and 6 h (seawater). After that, HK activity declined dramatically and maintained at a stable level after 6 and 24 h respectively. HK activity in freshwater shrimp increased within 1 h after hyperthermal stress and then returned to the pre-experiment level. For shrimps cultured in seawater, HK activity kept a relatively high level within 3–12 h, after which HK activity gradually returned to normal. During the thermal stress, gill HK activity of shrimps cultured in freshwater was significantly lower than that of shrimps cultured in seawater at every time point, except at 1 h (P < 0.05).
Variation of HK activities in L. vannamei after thermal stresses. Values are mean ± S.E. The “*” means the significant difference between seawater and freshwater at the same time. Different lowercase letters mean significant differences among time intervals in seawater group, whereas different capital letters mean significant differences among different exposure times in freshwater group (the same as below)
Figure 1e, f showed that HK activity of muscle varied with time elapsed under thermal stress. Under hypothermal stress, the HK activity of shrimps cultured in freshwater increased to a significant higher level at 3 h (P < 0.05) and then decreased, until 12 h the activity recovered to normal. For shrimps cultured in seawater, HK activity was reduced at 1 h and then dramatically increased at 3 h, after that HK activity remained stable but no significant difference was observed between time intervals (P > 0.05). Under hyperthermal stress, the HK activity of shrimps cultured in freshwater increased markedly within 3–6 h, rapidly decreased, and then maintain stable. The HK activity of seawater shrimp responded to hyperthermal stress in a similar manner with that under hypothermal stress. For muscle HK activity of shrimps cultured in freshwater, it was slightly lower than that cultured in seawater before thermal stress but higher after thermal stress, especially at 1, 6, and 24 h (P < 0.05).
Three-way ANOVA analysis results showed in Table 2. Culture salinity, thermal stress, and stress time all had significant effects on HK activity of gill (P < 0.05), but not their interaction (P > 0.05). For HK activity of muscle, it was significantly influenced by culture salinity and stress time (P < 0.05) but not by thermal stress and the interaction of these three factors (P > 0.05).
Effect of thermal stresses on PK activity
As showed in Fig. 2a, d, PK activities remained stable and there was no significant change in control group (P < 0.05). Figure 2b, c illustrated the response of PK activities of gill in shrimps cultured in freshwater and seawater conditions. Following hypothermal stress, PK activities of gill in both shrimps cultured in freshwater and seawater conditions showed a varying trend of increasing first and then decreasing. For shrimps cultured in freshwater, gill PK activity increased within 1 h and then returned to normal level after 6 h. While PK activity in shrimps cultured in seawater increased within 3 h and then decreased gradually. T test results showed that gill PK activity was lower in shrimps cultured in freshwater than that in shrimps cultured in seawater during experiment (P < 0.05). Under hyperthermal stress, PK activity of gill reached the maximum at 3 and 6 h for shrimps cultured in freshwater and seawater conditions respectively (P < 0.05). After that, PK activity decreased gradually. T test results showed that at 0, 1, and 6 h of treatments, gill PK activity was significantly lower in shrimps cultured in freshwater than that in shrimps cultured in seawater (P < 0.05).
The change trend of PK activity in muscle under thermal stress was observed in Fig. 2e, f. In muscle, PK activity of freshwater shrimp in hypothermal group ascended within 12 h and dropped considerably at 24 h, after that kept stable. At the end of the experiment, PK activity was significantly higher than that before hypothermal stress (P < 0.05). For shrimps cultured in seawater, PK activity decreased within 1 h and then increased to the maximum at 3 h, after which it decreased to pre-experiment level until 48 h. T test results showed muscle PK activity was significantly lower in shrimps cultured in freshwater than that in seawater at 0, 3, and 24 h (P < 0.05). Following hyperthermal stress, muscle PK activity in freshwater shrimp increased slightly within 6 h and then decreased. No significant difference was observed between time intervals (P > 0.05). For shrimps cultured in seawater, muscle PK activity fluctuated and maintained at a higher level within 3–12 h of treatments. At 48 h, PK activity returned to the pre-experiment level. PK activity of muscle was significantly lower in shrimps cultured in freshwater than that in shrimps cultured in seawater during experiment (P < 0.05).
Three-way ANOVA analysis is demonstrated in Table 3. Culture salinity, thermal stress, and stress time significantly affected the PK activity in both gill and muscle of L. vannamei (P < 0.05). With respect to the interaction of these three factors, it had a significant effect on muscle PK activity (P < 0.05) but not on gill PK activity (P > 0.05).
Effect of thermal stresses on LDH activity
In the control group, LDH activities of gill and muscle in shrimps cultured in freshwater and seawater did not change significantly during the trial (P > 0.05) (Fig. 3a, d). Figure 3b, c showed the changing trend of LDH activities in gill after hypothermal stress. Under hypothermal stress, LDH activity of freshwater shrimp slightly descended within 1 h and then ascended acutely at 3 h, after that LDH activity declined and maintained a stable level which was near to the pre-experiment level. LDH activity of seawater shrimp elevated significantly within 12 h and decreased acutely at 24 and 48 h (P < 0.05). In comparison with shrimps cultured in seawater, shrimps cultured in freshwater were higher in LDH activity, especially at 0, 1, and 48 h (P < 0.05). During hyperthermal stress, LDH activity in shrimps cultured in freshwater changed slightly and there were no significant variations (P > 0.05). Whereas in shrimps cultured in seawater, LDH activity increased within 3 h and then decreased and returned to pre-experiment at 48 h. LDH activity of gill was significantly higher in shrimps cultured in freshwater than that in shrimps cultured in seawater at 0, 6, and 48 h (P < 0.05).
Under thermal stress, muscle LDH activity in both freshwater and shrimps cultured in seawater changed similarly, while all increased first and then declined. After hypothermal stress, LDH activity of shrimps cultured in freshwater ascended within 12 h and remaining at a higher level (P < 0.05). LDH activity in shrimps cultured in seawater increased within 6 h and then fell back to the pre-experiment level. Muscle LDH activity was significantly higher in shrimps cultured in freshwater than that in shrimps cultured in seawater during the experiment (P < 0.05). Following hyperthermal stress, the responses of LDH activity were similar between shrimps cultured in freshwater and seawater conditions. LDH activity in shrimps cultured in both freshwater and seawater increased within 3 h and then decreased. At the end of stress, LDH activity returned to normal level (P > 0.05). During thermal stress, LDH activity of muscle was higher in shrimps cultured in freshwater than that in shrimps cultured in seawater especially at 0 and 24 h (Fig. 3e, f).
It was shown by three-way ANOVA test in Table 4 that culture salinity, thermal stress, and stress time significantly affected the LDH activity in both gill and muscle of L. vannamei (P < 0.05), but only muscle LDH activity was significantly affected by the interaction of these three factors (P < 0.05).
Effect of thermal stresses on SDH activity
In the control group, SDH activities of gill and muscle in shrimps cultured in freshwater and seawater did not change significantly during the experiment (P > 0.05) (Fig. 4a, d). Figure 4b, c shows the variation tendency of gill SDH activity under thermal stress. Under hypothermal stress, SDH activity in seawater shrimp declined markedly within 6 h and then increased slightly but at the end of trial, the SDH activity was still lower than pre-experiment level. After hypothermal stress, the SDH activity in freshwater shrimp decreased first, reached the minimum value at 6 h, and subsequently ascended to the normal level. Following hyperthermal stress, gill SDH activity of shrimps cultured in freshwater decreased first and then increased, which was similar to that of shrimps cultured in seawater. The SDH activity of shrimps cultured in freshwater reached the maximum at 6 h, while for shrimps cultured in seawater, it kept a lower level within 3–24 h. After hyperthermal stress, there was a significant difference in gill SDH activity between shrimps cultured in seawater and freshwater at each stress interval, while the opposite was true for hypothermal group except at 24 h (P > 0.05).
Figure 4e, f shows that muscle SDH activity changed in a similar manner in both shrimps cultured in freshwater and seawater under thermal stress. Under hypothermal and hyperthermal stress, the SDH activity rapidly declined within 6 h and then gradually increased to pre-experiment level. At every time interval, SDH activity in seawater was higher than that in freshwater shrimp.
According to the three-way ANOVA test results in Table 5, culture salinity, thermal stress, and stress time all had significant impacts on muscle and gill SDH activity (P < 0.05), while these three factors did not interact significantly (P > 0.05).
Discussion
Comparison of metabolic enzyme activity of L. vannamei between cultured in freshwater and seawater
It is known that salinity could significantly influenced crustacean because more energy will be used by organisms to maintain osmotic balance. When ambient salinity changed, L. vannamei could adapt by depending on osmoregulation. In this process, stored energy would be consumed and therefore increasing metabolic rate (Rosas et al. 2001). Zhang and Dong (2002) reported that under iso-osmotic condition, Fenneropenaeus chinensis was the lowest in the ratio of respiratory energy to ingestion energy. Bindu and Diwan (2002) thought that the osmotic pressure was minimum for crustacean in the iso-osmotic environment and meanwhile less extra metabolic energy was needed. It was reported that crustacean could utilize fatty acid and glucose as energy substance to regulate osmotic pressure (Deering et al. 1997; Palacios et al. 2004a, b). In the low salinity condition, crustacean was inclined to utilize amino acids as effector for maintaining a stable osmotic pressure (Claybrook 1983). All these substances would be involved in energy metabolism with the help of such enzymes as HK, PK, SDH, and LDH. According to the report of Nelson et al. (1977), as osmoregulation is not the major reason of the changes in energy metabolism and also, metabolic rate was not always lowest under iso-osmotic condition. Lignot et al. (2000) reported that effects of salinity on crustacean metabolic rate were closely related with acclimation time, body size, and health condition. Long-term salinity acclimation could weaken the divergence between effects of different salinity level for L. vannamei, Fenneropenaeus indicus, Litopenaeus setiferus, and Cherax quadricarinatus (Villarreal and Rivera 1993; Kutty et al. 1974; Meade et al. 2002; Rosas et al. 1999). In the present study, HK and PK activity was lower in shrimps cultured in freshwater than seawater, indicating that freshwater condition reduced the glycolytic capability of gills and muscles in L. vannamei. In comparison with shrimps cultured in seawater, shrimps cultured in freshwater were higher in LDH activity and lower in SDH activity. It can be seen that more pyruvate was reduced to lactate rather than involved in TCA cycle, indicating that the freshwater condition depressed aerobic respiratory and increased the anaerobic respiratory. On the utilization of energy substance for shrimps cultured in low salinity or freshwater condition, it needs further study on the function of protein and lipid for regulating osmotic pressure and providing energy under different ambient salinity.
Effects of thermal stresses on HK and PK activity
For organisms, energy metabolism could reflect not only internally physiological condition but also the effect of external factors. Temperature is reported to be one of the most important environmental factors. In general, body temperature of aquatic animals can fluctuate with ambient environment, leading to a concomitant variation in metabolic rate (Tian et al. 2006). Wang et al. (2005a, b) reported that when organisms were faced with such a situation where body demand for oxygen increases or there is an internally hypoxia, the energy for consumption would be augmented and therefore there is a need for more produced energy. Glucose is the major substrate for energy production (Sancho et al. 1997). HK and PK are two key enzymes in glycolysis process and their activities have an important role in maintaining hemolymph or blood glucose level. For HK, it could cooperate with glucose transporter in the membrane to improve the utilization of exogenous glucose (Allert et al. 1991; Susana et al. 2006), while for PK, it could catalyze pyruvate and release ATP in the reaction. PK is a rate-limiting enzyme of the glycolysis (Lemos et al. 2003). Guo et al. (2010) found that when ambient temperature increased or decreased by 10 °C, hepatopancreatic HK and PK activities in L. vannamei showed a varying trend of increasing first and then decreasing. In this study, thermal stress affected HK activity in gill and the PK activity in gill and muscle significantly (P < 0.05). both hypothermal and hyperthermal stress resulted in a varying elevation in HK and PK activity in gills and muscles of both shrimps cultured in freshwater and seawater. However, with treatment time being prolonged, enzyme activity decreased gradually and returned to the normal level at 48 h. This implied that when faced with thermal stress, the normal energy-producing capability cannot meet the increased energy demand and glycolysis is enhanced to supply more energy. In fact, organisms could adapt to the thermal stress at a certain degree and therefore reduced the demand for energy. As a result, energy production pathways such as glycolysis would return to the normal level while HK and PK activities also relate with gluconeogenesis. Metón et al. (2003) reported that the glucose level is balanced by two pathways: glycolysis and its reversal pathway-gluconeogenesis. Following temperature change, more glucose is utilized for energy production. Therefore, glycolysis was enhanced, meanwhile gluconeogenesis was depressed. However, with the thermal stress being prolonged, the much consumed glucose needed to be replenished by re-activated gluconeogenesis. Due to the re-balance of glycolysis and glyconeogenesis, the energetic homeostasis was maintained, aiding in the self-regulation of internal metabolism in organisms (Hochachka and Lutz 2001).
Effects of thermal stresses on LDH and SDH activity
LDH, a cytoplasmic enzyme, is a key link between glycolysis and tricarboxylic acid cycle (TCA). Under aerobic condition, pyruvate was oxidized to CO2, H2O, and plenty of ATP, while under anaerobic condition, LDH reduced pyruvate into L-lactate and release a small amount of ATP. Accordingly, LDH is a useful indicator used to evaluate the capability of anaerobic metabolism (Viruu 1994; Zietara et al. 1996). SDH is an important composition of mitochondrial inner membrane. This enzyme not only is a constituent of succinate-Q-reductase but also plays a key role in TCA cycle. SDH activity could influence the oxidative phosphorylation and consequently reflect the level of aerobic metabolism (Wang et al. 2005a, b). Many environment stress factors could affect the LDH and SDH activities in crustacean. For example, LDH activity of Palaemon serratus was affected significantly by ambient temperature (Thébault 1984), and hypoxia stress increased LDH activity and decreased SDH activity significantly in Macrobrachium nipponense (Li 2010). In this study, hypothermal and hyperthermal stress affected the LDH and SDH activity significantly (P < 0.05). After thermal stress, LDH activity in shrimps cultured in freshwater and seawater increased first and then decreased, which was opposite to SDH activity. In the early stage of thermal stress, the aerobic respiratory of shrimps was weaken, leading to an insufficient energy supply. To compensate for energy production, anaerobic respiratory was enhanced and as a result, the produced lactate accumulated in the body. At 24–48 h of thermal stress, LDH and SDH activity in shrimps returned to the normal level, indicating the recovery of aerobic respiration. At the same time, more pyruvate were introduced into TCA cycle for more energy production. Following temperature change, activities of metabolic enzymes were activated to a higher level, implying that shrimps could adjust metabolic processes to counteract the effect of thermal stress at a certain degree.
Conclusion
In this study, shrimps cultured in freshwater and seawater enhanced metabolic enzyme activity to increase metabolic level and therefore replenished energy for adaption to thermal variation. This indicated a possible metabolic compensation in L. vannamei. In comparison with shrimps cultured in seawater, those cultured in freshwater had a reduced level of aerobic metabolism and an elevated level of anaerobic metabolism. Additionally, shrimps cultured in freshwater had a higher sensitivity to thermal stress in particular the temperature decrease than to temperature increase. For freshwater intensive culture of L. vannamei, noticeable temperature variation especially temperature decrease should be avoided to guarantee less energy for consumption.
References
Allert S, Ernest I, Poliszczak A, Opperdoes FR, Michels PA (1991) Molecular cloning and analysis of two tandemly linked genes for pyruvate kinase of Trypanosoma brucei. Eur J Biochem 200(1):19–27
Bindu RR, Diwan AD (2002) Effects of acute salinity stress on oxygen consumption and ammonia excretion rates of the marine shrimp Metapenaeus monoceros. J Crustac Biol 22(1):45–52
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72(12):248–254
Buckley BA, Owen ME, Hofmann GE (2001) Adjusting the thermostat: the threshold induction temperature for the heat-shock response in intertidal mussels (genus Mytilus) changes as a function of thermal history. J Exp Biol 204(20):3571–3579
Cesar JRO, Zhao BP, Malecha S, Ako H, Yang JZ (2006) Morphological and biochemical changes in the muscle of the marine shrimp Litopenaeus vannamei during the molt cycle. Aquaculture 261:688–694
Claybrook DL (1983) Nitrogen metabolism. In: Mantel LH (ed) The biology of crustacea, volume 5. Internal Anatomy and Physiological Regulation, pp 163–213
Deering MJ, Fielder R, Hewitt DR (1997) Growth and fatty acid composition of juvenile leader prawns, Penaeus monodon, fed different lipids. Aquaculture 151:131–141
Ding S, Wang F, Dong SL, Gao QF (2009) Effects of salinity fluctuation amplitudes on growth, osmolarity, Na+-K+-ATPase activity and Hsp70 of juvenile Chinese shrimp Fenneropenaeus chinensis Osbeck. Chin J Oceanol Limnol 27(4):723–728
Ding S, Wang F, Dong SL, Li Y (2014) Comparison of the respiratory metabolism of juvenile Litopenaeus vannamei cultured in seawater and freshwater. J Ocean Univ China 13(2):331–337
Drach P (1939) Mue et cycle d’intermue chez les crustacés décapodes. Ann I Océan 14:103–391
Guo B, Wang F, Dong SL, Hou CQ (2010) The effects of rapid temperature changes on HK, PK and HSP70 of Litopenaeus vannamei in different seasons. J Ocean Univ China 9(3):303–308
Hochachka PW, Lutz PL (2001) Mechanism, origin, and evolution of anoxia tolerance in animals. Comp Biochem Physiol B 130(4):435–459
Hu CQ, Shen Q, Zhang LP, Ren CH, Du SB (2004) Present status and prospect of the Pacific white shrimp Litopenaeus vannamei culture in China. China/ FAO/ NACA Workshop on “Healthy, Safe and Environmentally Sound Shrimp Farming”. China Society of Fisheries, Beijing, pp 72–82
Kutty MN, Murugapoopathy G, Krishnan TS (1974) Influence of salinity and temperature on the oxygen consumption in young juveniles of the Indian prawn Penaeus indicus. Mar Biol 11:125–131
Lemos D, Salomon M, Gomes V, Phan VN, Buchholz F (2003) Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): effects of development and temperature. Comp Biochem Physiol B 135(4):707–719
Li L (2010) Effects of hypoxia on respiratory metabolism, energy metabolism and antioxidant capability of Macrobrachium nipponense. HeBei University, Shijiazhuang, pp 18–24
Liao IC, Chien YH (2011) The Pacific white shrimp, Litopenaeus vannamei, in Asia: the world’s most widely cultured alien crustacean. In the wrong place—alien marine crustaceans: distribution, biology and impacts, invading nature - Springer Series in invasion Ecology 6:489
Lignot JH, Spanings-Pierrot C, Charmantier G (2000) Osmoregulatory capacity as a tool in monitoring the physiological condition and the effect of stress in crustaceans. Aquaculture 191:209–245
Magnuson JJ, Crowder LB, Medvick R (1979) Temperature as an ecological resource. Integr Comp Biol 19(1):331–343
Marsh AG, Leong PK, Manahan DT (1999) Energy metabolism during embryonic development and larval growth of an Antarctic sea urchin. J Exp Biol 202(15):2041–2050
Meade ME, Doeller JE, Kraus DW, Watts SA (2002) Effects of temperature and salinity on weight gain, oxygen consumption rate, and growth efficiency in juvenile red-claw crayfish Cherax quadricarinatus. J World Aquacult Soc 33(2):188–198
Metón I, Fernández F, Baanante IV (2003) Short- and long-term effects of refeeding on key enzyme activities in glycolysis–gluconeogenesis in the liver of gilthead seabream (Sparus aurata). Aquaculture 225:99–107
Nelson SG, Knight AW, Li HW (1977) The metabolic cost of food utilization and ammonia production by juvenile Macrobrachium rosenbergii (Crustacea: Palaemonidae). Comp Biochem Physiol A 57(1):67–72
Palacios E, Bonilla A, Luna D, Racotta IS (2004a) Survival, Na+/K+-ATPase and lipid responses to salinity challenge in fed and starved white pacific shrimp (Litopenaeus vannamei) postlarvae. Aquaculture 234:497–511
Palacios E, Bonilla A, Perez AS Racotta IS, Civera R (2004b) Influence of highly unsaturated fatty acids on the responses of white shrimp (Litopenaeus vannamei) postlarvae to low salinity. J Exp Mar Biol Ecol 299:201–215
Reynolds WW, Casterlin ME (1979) Behavioral thermoregulation and the final preferendum paradigm. Am Zool 19(1):211–224
Rosas C, Martinez E, Gaxiola G, Brito R, Sánchez A, Soto LA (1999) The effect of dissolved oxygen and salinity on oxygen consumption, ammonia excretion and osmotic pressure of Penaeus setiferus (Linnaeus) juveniles. J Exp Mar Biol Ecol 234(1):41–57
Rosas C, Lopez N, Mercado P, Martinez E (2001) Effect of salinity acclimation on oxygen consumption of juveniles of the white shrimp Litopenaeus vannamei. J Crustac Biol 21(4):912–922
Sancho E, Ferrando MD, Andreu E (1997) Sublethal effects of an organophosphate insecticide on the European Eel, Anguilla anguilla. Ecotoxicol Environ Safe 36:57–65
Segner H, Verreth J (1995) Metabolic enzyme activities in larvae of the African catfish, Clarias gariepinus: changes in relation to age and nutrition. Fish Physiol Biochem 14(5):385–398
Susana SA, Francisco JA, Jesús MM, María PMR, José LS, Juan MM (2006) Growth hormone and prolactin actions on osmoregulation and energy metabolism of gilthead sea bream (Sparus auratus). Comp Biochem Physiol A 144(4):491–500
Tanaka KR, Valentine WN, Miwa S (1962) Pyruvate kinase (PK) deficiency hereditary nonspherocytic hemolytic anemia. Blood 19(3):267–295
Thébault MT (1984) Lactate content and lactate dehydrogenase activity in Palaemon serratus abdominal muscle during temperature changes. J Comp Physiol B 154(1):85–89
Tian XL, Dong SL, Wang F, Wu LX (2006) The growth of juvenile Chinese shrimp, Fenneropenaeus chinensis Osbeck, at constant and diel fluctuating temperatures. J Shellfish Res 25:1007–1011
Torres JJ, Somero GN (1988) Metabolism, enzymic activities and cold adaptation in Antarctic mesopelagic fishes. Mar Biol 98(2):169–180
Valentin WN, Oski FA, Paglia DE, Baughan MA, Schneider AS, Naiman JL (1967) Hereditary hemolytic anemia with hexokinase deficiency. Role of hexokinase in erythrocyte aging. N Engl J Med 276(1):1–11
Vetter RAH, Buchholz F (1997) Catalytic properties of two pyruvate kinase isoforms in Nordic krill, Meganyctiphanes norvegica, with respect to seasonal temperature adaptation. Comp Biochem Physiol A 116(1):1–10
Villarreal H, Rivera JA (1993) Effect of temperature and salinity on the oxygen consumption of laboratory produced Penaeus californiensis postlarvae. Comp Biochem Phys A 106(1):103-107.
Viruu M (1994) Difference in effects of various training regimens on metabolism of skeletal muscles. J Sport Med Phys Fitness 34:217–227
Wang JY, Zhu SG, Xu CF (2005a) Biochemistry. Higher Education Press, Beijing (in Chinese)
Wang YS, Zou SX, Zhang YJ (2005b) Modem Animal Biochemistry. Higher Education Press, Beijing, pp 466–490 (in Chinese)
Zeng QH, Rahimnejad S, Wang L, Song K, Lu KL, Zhang CX (2018) Effects of guanidinoacetic acid supplementation in all-plant protein diets on growth, antioxidant capacity and muscle energy metabolism of bullfrog Rana (Lithobates catesbeiana). Aquac Res 49(2):748–756
Zhang S, Dong SL (2002) The effects of food and salinity on energy budget of juvenile shrimp of Penaeus Chinensis. J Dalian Fish Univ 17:227–233 (in Chinese)
Zhang S, Wang F, Dong S (1998) Studies on bioenergetics of Penaeus chinensis II. Effects of temperature and body weight on energy budget. J Ocean Univ Qingdao 28:228–232 (in Chinese)
Zietara MS, Gronczewska J, Stachowiak K, Skorkowski EF (1996) Lactate dehydrogenase in abdominal muscle of crayfish Orconectes limosus and shrimp crangon crangon (Decapoda: Crustacea): properties and evolutionary relationship. Comp Biochem Physiol B 114(4):395–401
Funding
This research work was supported by the Project of Shandong Province Natural Science Fund (ZR2018MC028) and Project of Ministry of Science and Technology of Agriculture Lee Technical Achievements Transformation Fund (2014GB2A100511).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jia, XY., Zhong, DS., Zhang, D. et al. Energy metabolic enzyme responses of Litopenaeus vannamei to thermal stress: a comparative study in freshwater and seawater conditions. Aquacult Int 26, 1067–1081 (2018). https://doi.org/10.1007/s10499-018-0268-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-018-0268-9