Abstract
Amphiprion clarkii, a popular marine ornamental fish, despite having an already established reproduction technology, still presents divergences regarding the feeding protocol used for its larviculture in what concerns the ideal day of transition from rotifers to Artemia. This study aimed to determine the optimum time to start supplying Artemia to larvae. Survival, growth, weight gain and metamorphosis of treatments: (T2) start of Artemia supply at the 2nd day after hatching (DAH); (T4) start at the 4th DAH; and (T6) start at the 6th DAH were evaluated. Survival rates ranged from 60 to 66 %. Fish in T2 had a number of metamorphosed fish statistically higher than T6 and began to metamorphose 1 day sooner than other treatments. The positive results obtained for T2 can be related to the premature ability of this species to capture large live food, which can provide many benefits to the larvae. It is concluded that it is possible to offer Artemia nauplii to yellowtail clownfish at 2 DAH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphiprion clarkii, known as Clark’s anemone fish or yellowtail clownfish, is very popular in the marine ornamental trade. Clownfishes have long been bred in captivity (Alava and Gomes 1989); nevertheless, optimal hatchery feeding protocol has not been fully established.
One of the biggest obstacles in the larval rearing of marine fish is the transition from endogenous to exogenous food, when the larvae completely consumed its yolk sac and starts feeding on the food available in the environment (Olivotto et al. 2011). The problem lies mainly in the fact that many larvae are unable to digest inert diets, requiring the use of live food of adequate size and composition (Bengtson 2003).
Rotifers are widely produced as first food for marine fish larvae due to their small size (90–350 microns, depending on the species, strain and stage of development), their ability to be raised in mass cultures and the ease to improve their nutritional composition with enrichment diets (Lubzens and Zamora 2003). However, when larvae already have the capability to capture larger food, rotifers are replaced by brine shrimp, Artemia sp., nauplii (400–500 microns in size when newly hatched), whose cysts are commercially sold and easily hatched in laboratory (Dhont and Van Stappen 2003).
The timing of transitioning from rotifers to brine shrimp nauplii is of utmost importance for rearing of marine fish larvae because there is a time when the energy spent by larvae to capture rotifers is not compensated by the energy they contain. Therefore, if there is a delay in the supply of brine shrimp nauplii, there may be a delay in the development of the larvae (Côrtes and Tsuzuki 2011).
During the production of yellowtail clownfish, there are some discrepancies regarding the best time to start offering brine shrimp. Knowing this, the present study aimed to determine the optimum age to start offering Artemia to A. clarkii, in order to maximize survival, growth and larval metamorphosis.
Materials and methods
Animals and maintenance conditions
The experiments were carried out at the Fish and Marine Ornamentals Laboratory (LAPOM), Federal University of Santa Catarina (UFSC), Florianópolis, Santa Catarina, Brazil.
A couple of A. clarkii was kept in a 100-L net cage, submersed in a 8000-L concrete tank, with water maintained at 27 °C and 35 ‰ salinity. They were fed twice daily to apparent satiation with a varied diet that included commercial feed for marine ornamental fish (Tetra, Germany), and a paste made of fresh shrimp, fish and shellfish mixed in a blender with astaxanthin, cod liver oil, commercial premix and unflavored gelatin.
Inside the net cage, a clay pot was placed as a substrate for spawning. This substrate was observed daily for the occurrence of spawns. Seven days after spawning at 27 °C, the clay pot with the eggs was transferred to a 100-L cylindrical tank with black walls, with the same water parameters of the breeding tank, for hatching.
Experimental design
On the day of hatching, ten larvae were randomly collected, weighed with an analytical balance (0.0001 g) and measured with a stereoscope (Olympus SZ-CTV, Japan) with micrometer to determine their total length. The remaining larvae were then transferred to twelve 15-L aquariums at a density of 5 larvae per liter (75 fish per aquarium). Water quality parameters were measured daily (unionized ammonia, nitrate and pH measured with commercial kits, Labcon, Brazil; temperature measured with mercury thermometer and salinity with a refractometer) and were kept equal for all aquariums.
In order to evaluate the influence of the starting day of brine shrimp nauplii supply on survival, growth and beginning of metamorphosis of the larvae, three feeding transition treatments were tested in triplicate: brine shrimp nauplii offered from the 2nd (T2), 4th (T4) and 6th (T6) Day After Hatching (DAH) (Fig. 1).
In the days before transition to brine shrimp, rotifers Brachionus sp. cultivated with S.Parkle diet (INVE Co., Belgium) were offered at a density of 15 rotifers/mL together with microalgae Nannochloropsis oculata at 5 × 105 cells/mL. Brine shrimp was initially offered at a density of 1 nauplius/mL, and after 3 days, two enriched metanauplii/mL were provided. The brine shrimp metanauplii were enriched with lipid emulsion Super Selco DHA (INVE Co., Belgium). A sample of 20 brine shrimp nauplii was measured with a stereoscope (Olympus SZ-CTV, Japan) with micrometer to determine their total length.
Since the experiment started on the 2nd DAH, larvae that died due to manipulation from the hatching tank to the experimental units before this date (0–1 DAH) were replaced. Every day during the experimental period (2 DAH onwards), dead animals were counted to determine the survivorship rate. After removal of dead larvae and organic matter, about 50 % of the water was exchanged.
The number of metamorphosed fish in each experimental unit was counted daily. Larvae were considered metamorphosed when showing pigmentation similar to an adult (three white bands and darkened pigmentation of the body). With the survivorship rate and the number of metamorphosed fish, it was possible to determine the percentage of metamorphosed fish per treatment.
The experiment was stopped when all treatments had more than 70 % of metamorphosed larvae. This percentage was set as normally some larvae show slower development and delay in metamorphose than the average group of fish. At this time, in order to determine larvae total length and weight, ten larvae from each aquarium were randomly collected, anesthetized with benzocaine (Reagen Quimibrás, Brazil) at a concentration of 12.9 mg/L, weighed on an analytical scale (0.0001 g) and measured with calipers (1 mm).
Another unreplicated experiment (n = 10) was simultaneously conducted using one extra aquarium per treatment, following the same feeding protocols of the main experiment, in order to determine mouth size (width and height of the mouth opening) of larvae at 0 DAH, 2 DAH, 4 DAH and 6 DAH.
Statistical analysis
All data are presented as mean and standard error. Repeated-measures analysis of variance (rANOVA) was performed on survivorship and metamorphosis data with a 5 % significance level. For comparison of larvae weight and total length, one-way ANOVA was performed with a P ≤ 5 % significance level. ANOVA assumptions were previously tested using Kolmogorov–Smirnov’s, Bartlett’s and Mauchly’s tests for normality, homoscedasticity and sphericity, respectively. Data transformations were used when necessary, and Tukey’s test was used for means comparison. Mouth size data were submitted to descriptive statistics only.
Results
Water quality parameters did not vary throughout the experiment. Temperature remained at 26.0 ± 0.04 °C and salinity at 30.0 ± 0.1 ‰. Toxic ammonia and nitrite stayed close to zero throughout the experimental period.
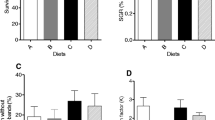
Survivorship data showed no statistical difference throughout experiment and corresponded at the end of the experiment to 60.0 ± 4.4, 66.0 ± 7.8 and 61.0 ± 6.3 % for T2, T4 and T6, respectively (Fig. 2).
Newly hatched larvae had 3.78 ± 0.0.3 mm of total length and 1.56 ± 0.07 mg of weight. At the end of the experiment, growth as total length (Fig. 3) was significantly higher (P = 0.003) for T2 (8.33 ± 0.38 mm) when compared to T6 (7.43 ± 0.25 mm), with no significant difference between T4 (7.98 ± 0.30) and other treatments. Larvae weight (Fig. 4) was significantly higher (P = 0.0003) for T2 (16.52 ± 1.48) than for T4 (14.04 ± 0.99) and T6 (12.53 ± 0.79), T2 being 18 and 32 % higher, respectively.
Length (mean ± SE) of yellowtail clownfish larvae at 13 day after hatching (DAH). (T2) brine shrimp provided from the 2nd DAH onwards; (T4) brine shrimp provided from the 4th DAH; and (T6) brine shrimp provided from the 6th DAH. Different letters indicate statistical difference between the treatments (P < 0.05)
Figure 5 shows the cumulative percentage of metamorphosed fish. T2 larvae started metamorphosis earlier (8th DAH) and showed overall higher mean of metamorphosed fish in comparison with other treatments; on the 10th DAH, T2 had a significantly higher percentage of juveniles than T6 (P = 0.007). From the 11th DAH onwards, percentage of metamorphosed larvae did not differ between treatments.
Cumulative percentage (mean ± SE) of metamorphosed yellowtail clownfish larvae throughout the experimental period. (T2) brine shrimp provided from the 2nd DAH onwards; (T4) brine shrimp provided from the 4th DAH; and (T6) brine shrimp provided from the 6th DAH. Different letters indicate statistical difference between the treatments (P < 0.05)
Newly hatched larvae had mouth width of 0.47 ± 0.0172 mm and height of 0.69 ± 0.018 mm. On 2nd DAH, larvae showed little increase on mouth size, with mean width and height of 0.49 ± 0.022 and 0.63 ± 0.023 mm, respectively. On the 4th and 6th DAH, larvae showed an increase in mouth width, 0.63 ± 0.018 and 0.67 ± 0.028 mm, respectively, and also in mouth height 0.76 ± 0.03 and 0.85 ± 0.033 mm, respectively. Newly hatched brine shrimp offered ranged from 0.3 to 0.6 mm (mean of 0.5 mm) in length.
Discussion
The present study showed high survivorship rates (60–66 %), increased weight gain (up to 2.5 times) and anticipation in metamorphosis when compared to other studies performed with the same species (Olivotto et al. 2008a, b, 2009; Le et al. 2011).
Some studies with yellowtail clownfish larvae offered brine shrimp much later on in the feeding protocol, as in Ghosh et al. (2012), who started supplying brine shrimp nauplii on the 11th DAH and only obtained larvae with over 1 cm in total length at 45 DAH. In our study, larvae were able to reach that same length on the 13th DAH, a difference of more than a month to achieve the same size.
According to Shirota (1978), the apparent optimal size of the prey must measure up to 50 % of the height of the larval mouth. We observed that during the first 6 days larvae mouth height ranged from 0.6 to 1.07 mm, and although mean Artemia size corresponded to 79 % of mean mouth height for larvae in T2, the smallest Artemia nauplii measured only 45 % of mean mouth height. Therefore, yellowtail clownfish larvae are able to capture smaller brine shrimp nauplii on the 2nd DAH, which may have resulted in higher growth and superior weight gain in T2 when compared to the other treatments. Anto et al. (2009) found that, on the first day of life, some yellowtail clownfish larvae already had the ability to capture brine shrimp nauplii, although they preferred to capture smaller foods, especially wild zooplankton. This study corroborates with that, since newly hatched larvae mouth size did not differ from 2 DAH.
From the moment the larvae are able to capture brine shrimp nauplii, the supply of rotifers can be gradually reduced until ceasing it completely. The reduction or absence of rotifers supply in hatchery can greatly reduce handling and therefore production costs of this live food, as well as microalgae production, which is normally associated with rotifer culture.
Taking into account that the weaning diet may be provided at the time of metamorphosis and that T2 had a faster larval development, this treatment could receive inert feed earlier, also reducing production costs.
In conclusion, it can be stated that it is possible to anticipate the supply of brine shrimp in the hatchery of yellowtail clownfish to the 2nd DAH (T2), since the results of growth and weight gain at this treatment were better, especially when compared with T6. The anticipation of metamorphosis verified is also very important, since the sooner the fish metamorphose, the sooner they can be sold by the producer.
References
Alava VR, Gomes LAO (1989) Breeding marine aquarium animals: the anemonefish. Naga ICLARM Q 12(4):12–13
Anto J, Majoris J, Turingan RG (2009) Prey selection and functional morphology through ontogeny of Amphiprion clarkii with a congeneric comparison. J Fish Biol 75:575–590
Bengtson DA (2003) Status of marine aquaculture in relation to live prey: past, present, future. In: Stotrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell Science, Oxford, pp 1–16
Côrtes GF, Tsuzuki MY (2011) Effect of different live food on survival and growth of first feeding barber goby, Elacatinus figaro (Sazima, Moura and Rosa 1997) larvae. Aquac Res 43:831–834
Dhont J, Van Stappen G (2003) Biology, tank production and nutritional value of Artemia. In: Stotrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell Science, Oxford, pp 65–121
Ghosh S, Kumar TTA, Nanthinidevi K, Balasubrananian T (2012) Reef fish breeding and hatchery production using brackishwater, a sustainable technology with special reference to Clark’s clownfish, Amphiprion clarkii (Bennett, 1830). Int J Environ Sci Dev 3(1):56–60
Le Y, Sheng-Yun Y, Xiao-Ming Z, Min L, Jing-Yi L, Kai-Chang W (2011) Effects of temperature on survival, development, growth and feeding of larvae of yellowtail clownfish Amphiprion clarkii (Pisces: Perciformes). Acta Ecol Sin 31:241–245
Lubzens E, Zamora O (2003) Production and nutritional value of rotifers. In: Stotrup JG, McEvoy LA (eds) Live feeds in marine aquaculture. Blackwell Science, Oxford, pp 17–64
Olivotto I, Capriotti F, Buttino I, Avella AM, Vitiello V, Maradonna F, Carnevali O (2008a) The use of harpacticoid copepods as live prey for Amphiprion clarkii larviculture: effects on larval survival and growth. Aquaculture 274:347–352
Olivotto I, Buttino I, Borroni M, Piccinetti CC, Malzone MG, Carnevali O (2008b) The use of the Mediterranean calanoid copepod Centropages typicus in yellowtail clownfish (Amphiprion clarkii) larviculture. Aquaculture 284:211–216
Olivotto I, Avella MA, Buttino I, Cutignano A, Carnevali O (2009) Calanoid copepod administration improves yellowtail clownfish (Amphiprion clarkii) larviculture: biochemical and molecular implications. Aquac Aquar Conserv Legisl 2(3):355–367
Olivotto I, Planas M, Simões N, Holt GJ, Avella MA, Calado R (2011) Advances in breeding and rearing marine ornamentals. J World Aquac Soc 42(2):135–166
Shirota A (1978) Studies on the mouth size of fish larvae II: specific characteristics of the upper jaw length. Bull Jpn Soc Sci Fish 44(11):1171–1177
Acknowledgments
The authors thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for the Master’s scholarship to the first author and for Grant to M.Y. Tsuzuki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nass, D.H., Gonçalves, E.L.T. & Tsuzuki, M.Y. Effect of live food transition time on survival, growth and metamorphosis of yellowtail clownfish, Amphiprion clarkii, larvae. Aquacult Int 24, 1255–1261 (2016). https://doi.org/10.1007/s10499-016-9982-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-9982-3