Abstract
The present work shows the possibility of determining variations in the lipid composition in Tetraselmis suecica under different conditions of culture by means of flow cytometry in cells marked with Nile Red (NR). A significant correlation was observed between the cellular contents in polar and neutral lipids and the cytometric signal of the marked cells. Likewise, there was a significant correlation between the ratio of polar and neutral lipids, estimated by cytometry, and the relative composition of polyunsaturated fatty acids (PUFAs) in Tetraselmis, which corresponded to the greater content of PUFAs detected in the polar lipid fraction of this microalgae. This relationship between the polar/neutral ratio and the relative contents of PUFAs, together with the flow cytometry and the marking by means of NR, would make it possible to have an effective indicator of the abundance of PUFAs in Tetraselmis, as well as the development of techniques of massive screening of strains which are hyperproductive of PUFAs and of rapid checking of the variations in lipid composition in response to cultivation conditions, which are much simpler and more rapid than traditional techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae are considered to be one of the potentially most important sources of long-chain polyunsaturated fatty acids (PUFAs). On one hand, their use in aquaculture, which is already extensive, will very possibly be increased over the next few years (Muller-Feuga 2000; Patil et al. 2007). On the other hand, these organisms are already the basis of an incipient industry oriented toward the production of fatty acids for human consumption, and have displaced fish oil in the preparation of functional foodstuffs and special preparations in the food industry such as maternised milk (Ward and Singh 2005). However, and despite its importance, the production of microalgae on a large scale for obtaining oils is limited to a small number of species, among which the dinoflagellate Crypthecodinium cohnii (Pulz and Cross 2004) is of particular importance, which together with other marine protists, such as Schizochytrium, may be produced on a large scale in heterotrophic cultures (Yokochi et al. 1998). In reality, microalgae and their potential applications is a field which still needs to be explored. Of the ~40,000 species known, potential uses of no more than 1% have been studied (Radner and Parker 1994) and no more than some six species are exploited on a large scale. It is for this reason that screening work on new potentially culturable strains among this group of organisms is becoming an extraordinarily active field. This is especially true in the case of production of oils. Great interspecies variability has been detected both in the content and in the fatty-acid composition of microalgae (Petkov and García 2007; Patil et al. 2007). This variability is also clear as an effect of culture conditions (Roessler 1990), and it has been discovered that the production of fatty acids from intensive culture of microalgae is an enormously plastic process in which it is possible to operate by altering both the quantity and the quality of the lipid composition of the biomass by means of variation in culture conditions (Swaaf 2003). This diversity, which is both genotypic and associated with the processes of culturing, makes the work of screening for new species and strains, as well as prospecting for new systems of cultivation, key factors in the development and final viability of this activity, and it is for this reason that the availability of rapid and effective techniques for quantitative and qualitative estimation of lipid composition is fundamental, which will make it possible to deal with programmes of mass screening of new strains and of evaluation of the response to the great variety of parameters of cultivation. In this sense, flow cytometry is presented as one of the most valid and most interesting alternatives to traditional systems of analysis of composition in fatty acids in unicellular organisms (de la Jara et al. 2003). However, its use is still limited in microalgae, in large part as a result of the still insufficient definition of the cytometric procedures and parameters applicable in the estimation of the lipid composition and contents of PUFAs (de la Jara et al. 2003).
Tetraselmis suecica is one of the species of microalgae that is most extensively used in aquaculture and is considered to be an optimal source of long-chain PUFAs, and especially of eicosapentaenoic acid (EPA) (Fábregas et al. 2001). Its lipid composition is strongly determined by conditions of cultivation (Otero and Fábregas 1997; D’Souza and Kelly 2000), and the maximisation of production, especially of PUFAs, has been the object of numerous studies. In the present work, we show how the use of Nile Red (NR), a lipophilic fluorescent marker, and of flow cytometry permits an effective determination of the variations in cellular lipid composition induced by conditions of cultivation of Tetraselmis suecica.
Materials and methods
Biological material and experimental conditions
The work was carried out with Tetraselmis suecica from the collection of microalgae of ICMAN-CSIC (Spain) (culture code: 03/0203) deposited in the official collection of algae BNA (culture code: BNA-10037). This strain of Tetraselmis was cultivated in the medium f/2 (Guillard 1975). Cultures were bubbled with 3% CO2 in air (v/v) with continuous light (200 μmol photon m−2 s−1) and grown in 500 ml borosilicate flasks containing 500 ml media at 25 ± 2°C in a chamber with programmable temperature. The stock was maintained by subculturing every week. In all cases, the stock culture was used as inoculum.
The experimental culture conditions were established, maintaining three replicates in each: control condition, which corresponds to the maintenance condition of the stock culture; high-irradiation condition (high light), in which cultures were subjected to irradiation of 1,000 μmol m−2 s−1 achieved by means of low-consumption 20-W lamps (Mazda Eureka), with other conditions the same as for the stock culture; and low-temperature condition, in which the cultures were maintained at a suboptimal temperature for growth: 15°C.
Lipid assessment and chromatography analysis
For analysis of lipid composition, samples were taken in both the exponential phase of growth and in the stationary phase of the cultures. The growth phases were determined from the variations of cellular density, estimated by direct counting in the Thoma chamber every 24 h. Harvesting of samples was carried out by means of centrifugation (5 min, 5,000g, 5°C).
Gravimetric extraction and quantification of lipid composition was carried out according to the method described by de la Jara et al. (2003). Total lipid content was obtained from crude extract of each sample. First, frozen cells (50–100 μl aliquots) were extracted by adding methanol/chloroform (1:2, v/v). After 5 min centrifugation (5,000g), the supernatant was collected in a new tube and a solution of sodium chloride (0.9%, w/v) was added in a proportion of 1:5 v/v of lipid extract. This mix was roughly vortexed and left for 5 min until two phases were observed; supernatant was discarded as this phase is rich in nonlipid components. The oily phase was recovered as crude lipid extract. Total lipid content was obtained by evaporating the crude lipid extract, drying in the oven at 85°C for 15 min and accurately weighing. The polar and neutral lipidic fractions were separated by absorption chromatography on silica cartridges as described by Juaneda and Rocquelin (1985), according to the modification effected by Yongmanitchai and Ward (1992) for the separation of lipids in microalgae.
Fatty-acid composition was estimated according to the method described by Mendoza et al. (1999). Fatty-acid composition was obtained from fresh samples. Aliquots of biomass were transmethylated with MeOH–acetyl chloride. Gas chromatography analysis was performed in a Varian cp-38 with a flame ionisation detector. Fatty-acid methyl esters were identified comparing the retention times with those of authentic standards (Supelco FAME Mix C4-C24).
Apart from the composition in fatty acids of each sample, the composition in fatty acids of the polar and neutral lipid fraction in the cultures maintained in the control condition was analysed specifically. In this analysis the different lipid fractions were concentrated after separation by nitrogen evaporation, so as to avoid degradation of the fatty acids.
The relative content in PUFAs is expressed as the ratio between the percentages of saturated fatty acids (SATs), monounsaturated fatty acids (MUFAs) and PUFAs according to the following formula: PUFAs/(SATs + MUFAs) (Guimarães et al. 1991).
Flow cytometric analysis
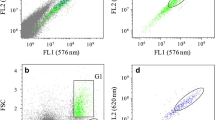
Cells from different growth conditions were stained with 15 mM NR (Sigma), as described by de la Jara et al. (2003). Yellow and red fluorescence of NR stained cells as well as deep red autofluorescence due to chlorophyll, size and complexity were determined by flow cytometry using EPICS XL flow cytometer (Beckman Coulter Instruments) equipped with an air-cooled 488-nm argon-ion laser. The optical system used in the EPICS XL flow cytometer collects yellow light (575 nm bandpass filter) in the FL2 channel and red light (620 bandpass filter) in the FL3 channel. These lengths are similar to the fluorescence of optimal emission described for the NR in a neutral (580 nm) and in a polar lipids fractions (610 nm), although the excitation length is substantially lower (neutral 530 nm, polar 560 nm) (Alonzo and Mayzaud 1999). Chlorophyll fluorescence was collected in the FL4 channel (675 nm bandpass filter). Cells were gated according to their characteristics of chlorophyll fluorescence in order to eliminate nonalgal particles. Approximately 3,000 cells were analysed using log amplification of the fluorescent signal. Calibration of the equipment was done every day using Flow-Check™ fluorospheres (Beckman Coulter). Nonstained cells were used as autofluorescence control. Data were expressed as fluorescence arbitrary units.
Data analysis
In all cases data are mean of three replicates and were analysed for statistical significance using linear regression analysis. Statistically significant differences for the different cultured conditions were established by analysis of variance and t-test. In all cases the alpha level used was 0.05 and 0.01 in the correlation analysis.
Results
A significant correlation (P < 0.01) was observed between the cellular contents in polar and neutral lipids estimated by gravimetry and the signal of the cells marked with NR (FL2 signal-neutral lipids, r 2 = 0.87; FL3 signal-polar lipids, r 2 = 0.61) as depicted in Fig. 1.
On analysing the lipid composition in the different experimental conditions of culture, a significant increase in the total contents of lipids in the stationary phase of growth was observed in all of them. This increase was similar in all experimental conditions, with mean content in the stationary phase being 1.6 times higher than in the exponential one (Fig. 2).
The lowest lipids content corresponded with the cultures in high light, both in the cultures in the exponential phase and in the stationary phase (Fig. 2). The cultures at low temperature exhibited similar lipid contents to the control (Fig. 2). In contrast, the cultures exposed to high irradiation showed a PUFAs/(SATs + MUFAs) ratio significantly higher than the control in both exponential and stationary phases (Table 1). The PUFAs/(SATs + MUFAs) ratio was always significantly higher in the exponential phase than in the stationary phase (Table 1). The variations in this ratio arose fundamentally at the expense of the variations in the contents of MUFAs (always higher in the stationary phase) and that of the PUFAs, which were reduced in the stationary phase under all experimental conditions (Table 1). The polar/neutral lipid ratio presents a similar variation to the relative composition of fatty acids and was significantly higher in the exponential phase and in the high-light cultures (Table 1).
Analysis of the fatty-acid composition of the polar and neutral lipid fractions of the standard cultures (control) is reported in Table 2. The former, the polar fraction, showed lower relative content of SATs and MUFAs, with a PUFAs/(SATs + MUFAs) ratio of 0.34, while in the neutral fraction it was 0.21, this difference being significant (P < 0.05). In general, both lipid fractions presented a very similar profile of fatty acids, while in the polar fraction no 14:0 was detected, 16:4 was found, which did not appear in the neutral fraction. The variations detected in the contents of 18:1ω−9c and 20:5ω−3 were especially significant (P < 0.05), the former being higher in the neutral fraction, while the relative content of 20:5ω−3 was significantly higher in the polar fraction. In general, significant differences were detected in the contents of MUFAs, with higher content in the neutral fraction, and the PUFAs, which were greater in the polar lipids.
A significant correlation was appreciated, with an r 2 of 0.83 (P < 0.01), between the polar/neutral lipid ratio, estimated by flow cytometry in cells marked with NR, and the PUFAs/(SATs + MUFAs) ratio achieved in each phase of culture and replicate (Fig. 3). The largest content in PUFAs was achieved in cultures with the highest content in polar lipids, in the case of the experimental conditions studied, which matched the cultures subjected to high irradiation. In correspondence with these results and the fatty-acid profile of the lipids fraction, we observed a high correlation between the polar/neutral lipids ratio (FL3/FL2) and the relative content of 16:1, 18:1ω−9c, 25:5ω−3 and 18:3ω−3 in all experimental conditions (Fig. 4).
Correlation between the relative content of fatty acids (expressed as percentage of total fatty acids) and the polar/neutral lipids ratio (FL3/FL2 estimated by NR and flow cytometry) at different experimental conditions. (a) Positive correlation with 20:5ω−3 (r 2 = 0.79) and 18:3ω−3 (r 2 = 0.66); (b) negative correlation with 18:1ω−9c (r 2 = 0.76) and 16:1 (r 2 = 0.76)
Discussion
The possibility of estimating the lipid content in unicellular microalgae, fungus and yeasts marked with NR had already been shown (Cooksey et al. 1987; Kimura et al. 2004). In protozoa it is possible to identify and quantify, by fluorimetry and NR, contents in polar and neutral lipids (Alonzo and Mayzaud 1999), and even in some species of microalgae, such as Crypthecodinium cohnii, the neutral/polar ratio, estimated by flow cytometry, has been related to the relative content in docosahexaenoic acid (de la Jara et al. 2003). The present work takes a further step in this direction, demonstrating the possibility of estimating, by flow cytometry and marking with NR, the relative abundance of PUFAs in microalgae, and this is therefore a valid technique for rapid estimation of variations in contents of fatty acids in response to culture conditions.
The lesser correlation observed between the gravimetric estimation of the polar lipids and the cytometric signal of the cells marked with NR might be explained by the difference that there is between the optimum excitation and emission lengths for the NR in polar lipids assessed by spectrofluoremetry and by flow cytometry, 560/610 nm and 480/620 nm respectively (Alonzo and Mayzaud 1999). This difference is less in the case of neutral lipids, which corresponds to the greater correlation observed between the gravimetric and cytometric estimations. Nevertheless, the degree of correlation achieved in both polar and neutral lipids is appropriate for the development of protocols for mass screening in which the processing of numerous samples is a priority, as well as in rapid control work on cellular response to culture conditions. In comparison with traditional techniques of analysis of lipid composition, flow cytometry makes it possible to process a large quantity of samples rapidly and cleanly (Davey and Kell 1996), which, together with the possibility of linking cytometry with automatic separation or sorting techniques, makes it an ideal way to develop work on mass screening and rapid characterisation of cellular response to culture conditions.
In microalgae, PUFAs are associated with polar lipids, fundamentally with membrane structures (Roessler 1990; Ward and Singh 2005). In Tetraselmis suecica the percentage of EPA is greater in the polar lipid fraction, with the result that the neutral/polar ratio may be an effective indicator of content of this fatty acid. To date, there have been no direct fluorescent markers of cellular contents of fatty acids in microalgae. Any fluorimetric technique for estimating fatty acids content must resort to indirect measures. In the present work, it is demonstrated how NR, given its different fluorescence in a matrix of polar or neutral lipids, can constitute an effective marker for this purpose and therefore for the selection of strains that are hyperproductive of PUFAs or the rapid optimisation of culture conditions. The effectiveness of NR is such that it becomes possible to make an almost quantitative estimate of cellular variation in lipid contents, at least of neutral lipids, and a qualitative estimate of potential PUFAs content. On the other hand, in the development of techniques of nutritional supplement by green water in aquaculture, in which the nutritional profiles and cellular integrity of microalgae are of great importance (Lavens and Sorgeloos 1996), it is important that techniques should be available that, like flow cytometry, enable precise and rapid determination of cell lipids contents and possible variability under different conditions of culture and use, as well as other cellular variables such as size, viability or cellular integrity. At present, joint use of NR and relatively low-cost equipment such as the fluorescent spectrophotometer would be possible for systematic and quantitative control of variation in lipid content of microalgae cultures (Liu et al. 2008).
In general, microalgae present a great inter- and intraspecific variability in fatty-acid composition, and their fatty-acid profile is strongly affected by culture conditions (Roessler 1990; Hu et al. 2008). These are the most common explanations for the differences found in the lipidic composition of studied species by different authors; Tetraselmis suecica is no exception. However, most of the previous works agree on the predominance in Tetraselmis suecica of the fatty acids 16:0 and of 18:1 in SATs and MUFAs, respectively, whereas the most abundant fatty acid in the PUFAs is 18:3ω−3 (Otero and Fábregas 1997; El-Dakar et al. 2001; D′Souza and Kelly 2000; Rosa et al. 2005; Bonaldo et al. 2005; Pratoomyot et al. 2005; Patil et al. 2007). The present study agrees with these results, although we observed increased contents of 18:0 compared with in previous works.
The fatty-acids composition of microalgae is significantly affected by irradiation and phase of cellular growth (Tzovenis et al. 2003; de la Pena and Villegas 2005). Increases in photon flux density have been related to increase in content in PUFAs in various species of microalgae (Thomson et al. 1990). In the present work, a similar reaction was observed in Tetraselmis suecica, with an increase in content of PUFAs and a higher polar/neutral lipids ratio. These variations have been associated in microalgae with adaptive response of photosynthetic membranes to increase in irradiation (Floreto et al. 1994; Mendoza et al. 1999). The increase in total lipids content and reduction in PUFAs content under conditions of stationary growth, already described in Tetraselmis (De la Pena and Villegas 2005) and characteristic of the majority of species of microalgae (Roessler 1990), would correspond to the general mechanism of adaptation of cells to conditions of limited growth due to low availability of nutrients (Sukenik and Carmeli 1990).
These variations in lipid composition may arise in a relatively rapid manner and may be the basis of the design of systems of cultivation in which accumulation of lipids is favoured before harvesting of microalgae, such as the techniques of biphasic culture (Mendoza et al. 2008) in which a biomass production phase, in optimal cultivation conditions, is combined with a phase of induction of accumulation of lipids by means of suppression of a nutrient, generally nitrogen. In this type of culture system rapid control of lipids contents and of cellular response is fundamental, which again makes clear the potential usefulness of flow cytometry and marking with NR in estimating lipid composition of cultures and its advantages over traditional techniques as a rapid means of control.
Abbreviations
- PUFAs:
-
Polyunsaturated fatty acids
- NR:
-
Nile Red
- SATs:
-
Saturated fatty acids
- MUFAs:
-
Monounsaturated fatty acids
References
Alonzo F, Mayzaud P (1999) Spectrofluorometric quantification of neutral and polar lipids in zooplankton using Nile Red. Mar Chem 67:289–301. doi:10.1016/S0304-4203(99)00075-4
Bonaldo A, Badiani A, Testi S, Corso G, Mordenti AL, Gatta PP (2005) Use of centrifuged and preserved microalgae for feeding juvenile Manila clam (Tapes philippinarum): effects on growth and fatty acid composition. Ital J Anim Sci 4:375–384
Cooksey KE, Guckert JB, Williams GA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6:333–346. doi:10.1016/0167-7012(87)90019-4
D’Souza FML, Kelly GJ (2000) Effects of a diet of a nitrogen-limited alga (Tetraselmis suecica) on growth, survival and biochemical composition of tiger prawn (Penaeus semisulcatus) larvae. Aquaculture 181:311–329. doi:10.1016/S0044-8486(99)00231-8
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations—the importance of single-cell analyses. Microbiol Rev 60:641–696
De la Jara A, Mendoza H, Martel A, Molina C, Nordströn C, de la Rosa V, Díaz R (2003) Flow cytometric determination of lipid content in marine dinoflagellate Crypthecodinium cohnii. J Appl Phycol 15:433–438. doi:10.1023/A:1026007902078
De la Pena MR, Villegas CT (2005) Cell growth, effect of filtrate and nutritive value of the tropical Prasinophyte Tetraselmis tetrathele (Butcher) at different phases of culture. Aquacult Res 36:1500–1508. doi:10.1111/j.1365-2109.2005.01371.x
El-Dakar AY, Shalaby SM, Hassanein GD, Ghoneim SI (2001) Use of rotifers cultured on different microalgal species in larval feeding of sea bass Dicentrarchus labrax. Asian Fish Sci 14:43–52
Fábregas J, Otero A, Domínguez A, Patino M (2001) Growth rate of the microalga Tetraselmis suecica changes the biochemical composition of Artemia species. Marin Biotechnol 3:256–263. doi:10.1007/s101260000074
Floreto EAT, Hirata H, Yamasaki S, Castro SC (1994) Influence of light intensity on the fatty acid composition of Ulva pertuosa kjellman (Chlorophyta). Bot Mar 37:143–149
Guillard RRL (1975) Culture of phytoplakton for feeding marine invertebrates. In: Smith WL, Chanle MH (eds) Culture invertebrate animals. Plenum, New York, pp 26–60
Guimarães ARP, Costa Rosa LFBP, Sitnik RH, Curi R (1991) Effect of polyunsaturaes (PUFA n-6) and saturated fatty acid-rich diets on macrophage metabolism and function. Biochem Int 23:1739–1751
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Darzins A (2008) Micoalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639. doi:10.1111/j.1365-313X.2008.03492.x
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids in rat heart using silica cartridges. Lipids 20:40–41. doi:10.1007/BF02534360
Kimura K, Yamaoka M, Kamisaka Y (2004) Rapid estimation of lipids in oleaginous fungi and yeast using Nile Red fluorescence. J Microbiol Methods 56:331–338. doi:10.1016/j.mimet.2003.10.018
Lavens P, Sorgeloos P (1996) Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper no. 361, Roma, 295 pp
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722. doi:10.1016/j.biortech.2007.09.073
Mendoza H, Martel A, Jiménez del Río M, García-Reina G (1999) Oleic acid is the main fatty acid related with carotenogenesis in Dunaliella salina. J Appl Phycol 11:15–19. doi:10.1023/A:1008014332067
Mendoza H, Molina Cedrés C, de la Jara A, Nordström L, Freijanes K, Carmona L (2008) Quantitative and qualitative variation of the fatty acid composition in the dinoflagellate Cryothecodinium cohnii under nitrogen starvation conditions. Grasas Aceites 59:27–32. doi:10.3989/gya.2008.v59.i1.486
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12:527–534. doi:10.1023/A:1008106304417
Otero A, Fábregas J (1997) Changes in the nutrient composition of Tetraselmis suecica cultured semicontinuously with different nutrient concentrations and renewal rates. Aquaculture 159:111–123. doi:10.1016/S0044-8486(97)00214-7
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquacult Int 15:1–9. doi:10.1007/s10499-006-9060-3
Petkov G, García G (2007) Which are fatty acids of the green alga Chlorella? Biochem Syst Ecol 35:281–285. doi:10.1016/j.bse.2006.10.017
Pratoomyot J, Srivilas P, Noiraksar T (2005) Fatty acids composition of 10 microalgal species. Songklanakarin J Sci Technol 27:1179–1187
Pulz O, Cross W (2004) Valuable products from biotechnology of microalgae. J Appl Microbiol Biot 65:635–648. doi:10.1007/s00253-004-1647-x
Radner RJ, Parker BC (1994) Commercial applications of algae: opportunities and constraints. J Appl Phycol 6:93–98. doi:10.1007/BF02186062
Roessler PG (1990) Environmental control of glycerolipids metabolism in microalgae: commercial implications and future research directions. J Phycol 26:393–399. doi:10.1111/j.0022-3646.1990.00393.x
Rosa A, Deidda D, Serra A, Deiana M, Dessì MA, Pompei R (2005) Omega-3 fatty acid composition and biological activity of three microalgae species. J Food Agric Environ 3:120–124
Sukenik A, Carmeli Y (1990) Lipid synthesis and fatty acid composition in nannochloropsis sp (Eustigmatophyceae) grown in light-dark cycle. J Phycol 26:463–469. doi:10.1111/j.0022-3646.1990.00463.x
Swaaf ME (2003) Docohexaenoic acid production by the marine alga Crypthecodinium cohnii. University Press, Netherlands, p 125
Thomson PA, Harrison PJ, Whyte JNC (1990) Influence of irradiance on the fatty acid composition of phytoplankton. J Phycol 26:278–288. doi:10.1111/j.0022-3646.1990.00278.x
Tzovenis I, De Pauw N, Sorgeloos P (2003) Optimisation of T-ISO biomass production rich in essential fatty acids II. Effect of different light regimes on the production of fatty acids. Aquaculture 216:223–242. doi:10.1016/S0044-8486(02)00375-7
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of productions. Process Biochem 40:3267–3652. doi:10.1016/j.procbio.2005.02.020
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docohexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76. doi:10.1007/s002530051139
Yongmanitchai W, Ward OP (1992) Separation of lipid classes from Phaeodactylum tricornutum using silica cartridges. Phytochemistry 31:3405–3408. doi:10.1016/0031-9422(92)83694-T
Acknowledgment
This work was financed by the program “Canarias Objetivo de Progreso” (2007-2013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzmán, H.M., de la Jara Valido, A., Duarte, L.C. et al. Estimate by means of flow cytometry of variation in composition of fatty acids from Tetraselmis suecica in response to culture conditions. Aquacult Int 18, 189–199 (2010). https://doi.org/10.1007/s10499-008-9235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9235-1





 , low temperature ■). A significant difference was appreciated between the exponential and stationary phase under all experimental conditions
, low temperature ■). A significant difference was appreciated between the exponential and stationary phase under all experimental conditions
