Abstract
Winter seasonal concentrations of dissolved rare earth elements (REE) of two major river systems (the Wujiang River system and the Yuanjiang River system) in karst-dominated regions in winter were measured by using a method involving solvent extraction and back-extraction and subsequent ICP-MS measurements. The dissolved REE concentrations in the rivers and their tributaries are lower than those in most of the large rivers in the world. High pH and high cation (i.e., Na+ + Ca2+) concentrations of the rivers are the most important factors controlling the concentrations of dissolved REE in the river water.
The dissolved load (<0.22 μm) REE distribution patterns of high-pH river waters are very different from those of low-pH river waters. The shale (PAAS)-normalized REE patterns for the dissolved loads are characterized by light REE-enrichment and heavy REE-enrichment. Water in the upper reaches of the Wujiang River generally shows light REE-enriched patterns, while that in the middle and lower reaches generally shows heavy REE-enriched patterns. The Yuanjiang River is heavy REE enriched with respect to the light REE in the same samples. Water of the Wuyanghe River draining dolomite-dominated terrains has the highest heavy REE-enrichment. Most river water samples show the shale-normalized REE patterns with negative Ce and Eu anomalies, especially water from Wuyanghe River. Y/Ho ratios show that the water/particle interaction might have played an important role in fractionation between HREE and LREE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rare earth elements have been used to study weathering of rocks and soil (Piper 1974; Nesbitt 1979), as well as the fundamental reactions and processes operating in rivers, estuaries and oceans (Elderfield and Greaves 1982; Goldstein and Jacobsen 1988; Sholkovitz 1989, 1993; Dia et al. 2000; Tang and Johannesson 2003). The fractionation of REE also occurs during geochemical reactions at low temperatures in river waters (Sholkovitz 1993). In the process of chemical weathering, the rare earth elements (REE) are relatively inert with respect to their behavior and tend to be retained in the weathering crust: only a little amount of REE enters river waters in the form of dissolved loads. Therefore, the dissolved REE in river waters are informative for a better understanding of geochemical processes such as complexation, water/rock and /particle interactions.
We measured the abundances of dissolved REE in waters from 3 rivers running through karst terrains. From the data we have obtained the typical REE patterns for the dissolved loads of karst river waters. The implication of these data is for the behavior of REE during weathering and river transport. This work could help us understand the background levels of REE in karst river waters so as to constrain the origin and behavior of REE in river waters from carbonate catchment areas.
2 Geological setting, sampling and analytical procedure
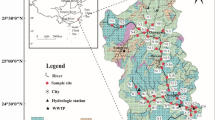
Guizhou Province is located in the center of the Southeast Asian Karst (an area where limestones are irregularly distributed, and due to their erosion fissures, sinkholes, underground stream and caverns were developed). It is a region where karstification is most developed and karst types are most diverse, with a largest karst area in the world. The Wujiang River is the largest river in Guizhou Province with a total length of 874 km. The Yuanjiang River is the second largest river in Guizhou Province, consisting of two tributaries, which are the Qingshuijiang and Wuyanghe Rivers. The Qingshuijiang and Wuyanghe rivers are as long as 459 and 258 km, respectively. The drainage systems of the Wujiang, Qingshuijiang and Wuyanghe Rivers are all located in the karst areas of Guizhou Province. The strata exposed in the Wujiang River catchments are mainly Pre-Jurassic in age (Fig. 1a). Along the upper reaches of the Wujiang River catchment are developed Permian and Triassic carbonate rocks and coal-bearing formations, with minor basaltic igneous rocks. In the middle reaches of the Wujiang River are widely distributed Permian and Triassic limestones, dolomitic limestones and dolomites, while in the lower reaches are mainly distributed carbonate rocks, shales, sands shales and siltstones. In the middle and lower reaches of the Qingshuijiang River are mostly developed clastic sedimentary rocks, while exposed in the upper and middle reaches of the Wuyanghe River are dolomitic limestones.
(a) Sketch map showing the lithlogy of Guizhou Province (from Wang et al. 2004). Zone I has an average altitude of more than 1500 m, zone II, less than 700 m; and zone III, 700–1500 m. Legend A stands for clastic sedimentary rocks. B, E and G are consecutive carbonate rock assemblages (carbonate rocks account for more than 90%), respectively for limestone, dolomite and mixed assemblage of limestone and dolomite. C and F are the assemblages of carbonate rocks interbedded with clastic rocks (carbonate rocks account for 70–90%), respectively for linestones and dolomites interbedded with clastic rocks. D stands for clastic rocks interbedded with limestone (carbonate rocks account for 30–70%). (b) Map showing sampling locations and sample numbers
Sixty-five river water samples had been collected during the period from Jan. 7 to Feb. 11, 1999. The sampling locations are shown in Fig. 1b. The samplers were 2l polyethylene bottles, which were cleaned with acid (2–3N HCl) and finally rinsed with Milli-Q water and dried. The water samples were filtered on site through 0.22μm membrane filtered (Millipore) immediately after collection. The solution that passed through this filter refers to as the dissolved load, which consists of dissolved ions and colloids smaller than 0.22 μm in size. The filtered samples were acidified with ultra-purified hydrochloric acid until pH < 2 to prevent adsorption during storage. All of the samples were stored at 4° for analysis within 30 days.
The REE were determined by inductively coupled plasma mass spectrometry (ICP-MS, ELEMENT). The REE concentrations of our samples are below the detection limit of the ICP-MS. We hence used the method of Shabani et al. (1990) to concentrate the REE by a factor of at least 100. The analysis of dissolved REE concentrations includes the following steps: REE were extracted from water samples with 65% HDEHP (2-ethylhexyl hydrogen phosphate) and 35% H2MEHP (2-ethylhexyl dihydrogen phosphate) in heptane, then back-extracted with 6N ultra-purified hydrochloric acid and transformed into nitrates of REE for ICP-MS analysis. Indium was used as the internal standard to monitor the chemical procedure on the quantitative basis, and rhodium was used to monitor the stability of ICP-MS determination. Reagents and procedural blanks were determined in parallel to the sample treatment using the identical procedures. The entire analytical procedure was accomplished in the Class-100 laboratory and Millipore-Q water (18.2 MΩ cm) and high-purity reagents were used. The following masses were used for REE measurement: 89Y, 139La, 140Ce, 141Pr, 146Nd, 147Sm, 151Eu, 157Gd, 159Tb, 163Dy, 165Ho, 167Er, 169Tm, 173Yb and 175Lu. These isotopes are free of isobaric interferences and REEO+ and BaO+ were low. The detection limits are presented as below: 89Y 0.022 μg/l, 139La 0.018 μg/l,140Ce 0.028 μg/l, 141Pr 0.005 μg/l, 146Nd 0.076 μg/l, 147Sm 0.009 μg/l, 151Eu 0.002 μg/l, 157Gd 0.021 μg/l, 159Tb 0.002 μg/l, 163Dy 0.009 μg/l, 165Ho 0.003 μg/l, 167Er 0.005 μg/l, 169Tm 0.002 μg/l, 173Yb 0.003 μg/l and 175Lu 0.0005 μg/l. The blanks were 5% for La, 10% for Ce, and 2–3% for other REE and Y. Each calibration curve was evaluated by analysis of these quality control (QC) standards before, during and after the analysis of a set of samples. The accuracy and precision of heavy REE and Y measurements were better than ±3%, and that of light REE were better than ±5%.
3 Results and discussion
3.1 The variation of REE concentration
The concentrations of dissolved REE in karst river waters of Guizhou Province are listed in Table 1 (the data for the concentrations of major ions are cited from Han and Liu, 2004) and REE PAAS normalized patterns are shown in Fig. 2.
Listed in Table 2 are the concentrations of dissolved REE in different rivers. From Table 2, we can see that the dissolved REE contents of these three rivers are very low, mostly lower than 100 ng/l. From the upper to the lower reaches of the Wujiang River, the abundances of REE and light REE tend to decrease, while those of heavy REE show almost no change. The Qingshuijiang River has the highest REE concentrations in these three rivers. The Wuyanghe River has almost the lowest REE contents in these three rivers. Because the sample collection was carried out in winter season, under low-flow discharge, water/rock interaction would dominate over the solute in the river water. During the weathering and transport processes, REE are present mainly in the form of particulates, so the contents of the soluble load of REE in the river waters are very low.
Furthermore, the pH of these three rivers vary from 7.6 to 8.4, higher than 8.0 for most samples. The high pH values are the result of dissolution of limestones and dolomites in the watershed. In comparison to the rivers in other parts of the world, the dissolved REE concentrations are lower than those in the Amazon River (Table 2, Fig. 3). The Amazon River has lower Na+ and Ca2+ concentrations and lower pH and the concentrations of dissolved REE are one order of magnitude higher than those of other rivers. The possible reason may be that the higher pH and higher cation concentrations (Na+ + Ca2+) make the concentrations of colloids so low in river water, that the dissolved REE concentrations are very low as well. Similar phenomena were observed in the Ohio River and Mississippi River in the United States and the Indus River in Pakistan (Fig. 3), i.e., Na+ and Ca2+ concentrations and pH are high but the levels of dissolved REE are relatively low (Goldstein and Jacobsen 1988).
PAAS-normalized REE patterns of dissolved loads in the river waters. Data source: Goldstein and Jacobsen, 1988
3.2 The fraction of LREE/HREE
The PAAS-normalized REE patterns for the matter dissolved in river water are shown in Fig. 2, where the REE concentrations of Post Archean Australian Shales (PAAS) compiled by Taylor and McLennan (1985) were chosen for normalization. In this work, PAAS prefers over NASC because the average REE analyses of the individual samples are known whereas in NASC, a single sample has been analyzed and the chance for aberrant material in the composite always exists. Nevertheless, there are only slight differences between these values. From Fig. 2, it is clear that these waters do not show flat PAAS-normalized REE patterns. The river waters were rich in Ca2+, HCO −3 and Mg2+ with pH > 7, so soluble complexation is possible in the Wujiang River catchment. From Table 2 and Fig. 4, it can be seen that the concentrations of LREE decrease from the upper to the lower reaches of the Wujiang River. The upper reaches of the Wujiang River, running through the Permian and Triassic carbonate rocks, show little light REE flat patterns with (La/Yb)N = 0.68−1.05, mean value = 0.99, the HREE distribution patterns are characterized by slight HREE depletion. The LREE concentrations in the middle reaches of the Wujiang River are lower than those in the upper reaches, (La/Yb)N = 0.34−1.78, mean value = 0.73, the LREE distribution patterns in most river water samples are characterized by LREE depletion. River waters in the lower reaches of the Wujiang River have the lowest LREE concentrations, (La/Yb)N = 0.46−0.81, mean value = 0.60, the LREE distribution patterns are characterized by LREE depletion. From the upper to the lower reaches, the dissolved REE in river waters show HREE enrichment. Most river water samples from the Qingshuijiang River show HREE enrichment patterns with (La/Yb)N = 0.27−1.96 and mean value = 0.80. Waters of the Wuyanghe River also show HREE enrichment patterns with (La/Yb)N = 0.29−1.28 and mean value = 0.55. The HREE enrichments of high-pH and low REE concentration are most easily explained by the formation of carbonate and hydroxide complexes in solution.
Usually, REE fractionation occurs during weathering and transport processes. Moreover, relative to the LREE, the HREE are of preferential complexation with ligands : (1) HREE will be preferentially released to the solution during weathering of source rocks, (2) LREE will be preferentially adsorbed on particle surfaces in the absorption/equilibrium reaction in rivers (Elderfield et al. 1990). The extent of complexation of heavy versus light rare earth elements has been used to explain the relative enhancement of HREE in dissolved loads. The data presented above suggest that pH is a major factor controlling both the absolute abundances of the light REE in solution and the relative REE patterns of dissolved material. Carbonate complexation is dominant in neutral- to alkaline-pH, natural waters, whereas free metal ion species (REE3+) and sulfate complexes are most important in acidic waters.
The heavy REE can form stronger complexes than the light REE with such inorganic ions as CO 2−3 , OH− and F− (Turner et al. 1981), thus high-pH river waters with abundant CO 2−3 and OH− are expected to be enriched in heavy REE relative to the source rocks in their drainage basins. The abundances of various inorganic REE complexes can be calculated, as the stability constants for REE and OH−, F−, Cl−, SO 2−4 and CO 2−3 are available (Lee and Byrne 1993). HREE enrichments of karst river waters and low LREE concentrations are most easily explained by the formation of carbonate and hydroxide complexes in the solution. The HCO −3 activity is a magnitude greater than the CO 2−3 activity in the river waters flowing through karst areas. So the bicarbonate species are possibly one order of magnitude higher than carbonate species such as [REE(CO3)4]5−, the latter seems to be less unimportant. Cantrell and Byrne (1987) indicated that [REE(CO3)2]− and [REE(CO3)]+ are the potentially important carbonate species. Of these two species, [REE(CO3)2]− is the mostly related to the pH range under consideration (Wood 1990). We estimated the dissolved REE species in the river waters by an equilibrium model (Wood 1990; Lee and Byrne 1993). From Fig. 5, it can be seen that the sum of [REE(CO3)2]− and [REE(CO3)]+ is almost 100%, and [REE(CO3)2]− is the main species in the river water. From La to Lu, the ability to form carbonate complexes in the solution trends to increase, but the [REECO3]+ species trends to decrease with increasing atomic number. So the significant HREE enrichments in these river waters are primarily the result of preferential formation of stronger carbonate complexes with the HREE. So the concentrations of HCO −3 are another important factor controlling HREE/LREE fractionation.
Distribution of different complexing-forms of REE in the river waters. (a) The Wujiang River; (b) The Qingshuijiang River; (c) The Wuyanghe River. Stability constants come from Lee and Byrne (1993)
3.3 Ce, Eu anomalies
As a simple means, Ce anomaly is need to examine redox processes (e.g. Elderfield 1988; De Baar et al. 1985), which is defined as follows:
where Ce*, La*, Pr*, Eu*, Sm* and Gd* represent the concentrations of the respective REE in the average shale. The Ce anomaly represents the deviation of the Ce concentrations from those expected by linear interpolation between the concentrations of La and Pr in the shale-normalized REE pattern of the samples. The Eu anomaly represents the deviation of the Eu concentrations from those expected by linear interpolation between the concentrations of Sm and Gd in the shale-normalized REE pattern of the samples. It can be easily to distinguish the deviation (fractionation) of Ce and Eu from the expected (trivalent) REE.
The behavior of Ce is different from that of the other REE because of its redox chemistry. Although there are uncertainties pertaining to what controls aqueous Ce chemistry (Liu et al. 1989; Elderfield et al. 1990), we do consider that the oxidation of Ce3+ is much more sensitive to pH than to pe (Elderfield and Sholkovitz 1987):
Ce is controlled by a redox equilibrium between the dissolved Ce3+ and Ce4+ species. Ce depletion in high-pH river waters is probably due to the formation of the solid phase CeO2 that is preferential by removed from the solution. Ce anomalies vary widely from one river to another and some of the samples show no Ce anomaly at all (Table 1). Most samples from the Wujiang River show negative Ce anomalies with a few exceptions (samples 12, 13, 23, 36, 38) showing positive Ce anomalies. All of the samples from Qingshuijiang and Wuyanghe River show Ce negative anomalies. Our Ce anomaly data are presented in Table 1. Although the data are some what scattered, the general trend is similar to that reported by previous workers for surface waters (Liu et al. 1989; Goldstein and Jacobsen 1988; Tricca et al. 1999; Elderfield et al. 1990; Dia et al. 2000) that always show negative Ce anomalies (less than one). So the negative Ce anomaly is strongly pH-dependent in alkaline rivers.
Eu anomalies were found in the Wujiang River, Qingshuijiang River and Wuyanghe River. Negative Eu anomalies in the upper reaches of the Wujiang River range from 0.63 to 1.03, with a mean value of 0.83, those in the middle reaches from 0.39 to 1.13 with a mean value of 0.71, those in the lower reaches from 0.56 to 0.88 with a mean value of 0.75 and those in the Qingshuijiang River from 0.42 to 0.93 with a mean value of 0.79. The Wuyanghe River water samples show both highest La/Lu ratios and biggest negative Eu anomalies. The negative Eu anomalies in the Wuyanghe River range from 0.37 to 1.00 with a mean value of 0.66.
The Eu anomaly is strongly lithology-dependent (Tricca et al. 1999; Möller et al. 2004). The Eu anomalies found in river waters were originally derived from carbonate rocks. The source rocks show negative Eu anomalies (Ji et al. 2004) so water samples from the drainage system along P and T carbonate terrains show negative Eu anomalies.
3.4 Non-chondritic Y/Ho ratios
Y/Ho ratios in these three rivers (Fig. 6) are higher than those of continental rocks. These high Y/Ho ratios may be primarily attributed to their different chemical behaviors during water/particle reaction processes. From Fig. 6, it can be seen that Y/Ho ratios in these river waters lie between those of the continental rocks and those of seawater, indicating that these two elements, like the REE group, are probably fractionated during weathering and transport.
Plots showing the relationship between Y and Ho in the river waters with respect to their variations. Seawater: Y/Ho = 105; continental rock: Y/Ho = 52 from Nozaki et al. (1997)
Liu and Byrne (1995) showed that the carbonate complexation stability constant of Y resembles that of Tb and is, therefore, similar to that of Ho. Furthermore, Lee and Byrne (1993) showed the complexation behavior of Y on the soft organic surface of particulate matter is similar to that of light REE (e.g. Sm). Kawabe et al. (1991) pointed out that despite the similarity in their ionic radii, Y does not behave like Ho in the aquatic environment, due to the thermo chemical effect related to the absence of presence of 4f electrons in Y and trivalent REE. Nozaki et al. (1997) suggested that decoupling of Y and Ho probably occurs during scavenging by particulate matter, further indicating a possibility that Y distinctly deviates from Ho and other REEs due to competitive reactions during scavenging by particulate matter. The particulate-scavenging mechanism may be also valid to account for the fractionation of Y and Ho in river waters, and the extent of fractionation is possibly related to water/particle interaction.
4 Conclusions
-
1.
High-pH and high-carbonate Chinese rivers have low dissolved (<0.22 μm filtrate) REE concentrations relative to many of the world’s large rivers. The concentrations of dissolved REE in the Wujiang River water varied from 25.35 to 221.28 ng/l, those of in the Qingshuijiang River water varied from 20.65 to 410.45 ng/l, and those of the Wuyanghe River water from 22.50 to 62.69 ng/l. The high pH values and high ion concentrations of the rivers in the karst regions of Guizhou Province are the main factors leading to the lower concentrations of dissolved REE in river waters.
-
2.
The PAAS-normalized REE patterns of the three river systems, like those of many rivers in the world, are characterized by a slight LREE depletion. The concentrations of LREE in the dissolved loads are high relative to HREE in the upper reaches of the Wujiang River. But the concentrations of LREE in the dissolved loads are low relative to HREE in the middle and lower reaches of the Wujiang River, Qingshuijiang River and Wuyanghe River. The fractionation between heavy and light REE can be ascribed to high pH values. Ce, Eu anomalies were found in the Wujiang River, Qingshuijiang River and Wuyanghe River. Negative Ce anomalies are strongly dependent on redox chemistry, while negative Eu anomalies are dependent on the source rocks.
-
3.
Modeling of the data indicated that all of the REE are complexed with carbonate ions and that the sum of [REE(CO3)2]− + [REECO3]+ is almost 100%. [REE(CO3)2]− is a major species in the river water.
-
4.
Non-chondritic Y/Ho ratios in these river waters lie between those of the continental rocks and those of seawater, indicating that these two elements, like the REE group, are probably fractionated during weathering and transport. Y/Ho ratios observed in the REE patterns are the promising tools to understand water/rock interactions affecting the migration of REE.
References
Cantrell KJ, Byrne RH (1987) Rare earth element complexation by carbonate and oxalate ions Geochimica et Cosmochimica. Acta 51:597–605
DeBaar HJW, Bacon MP, Brewer PG, Bruland KW (1985) Rare earth elements in the Pacific and Atlantic Oceans. Geochimica et Cosmochimica Acta 49(9):1943–1959
Dia A, Gruau G, Olivie-Lauquet G, Riou C, Molenat J, Curmi P (2000) The distribution of rare earth elements in groundwaters: assessing the role of source-rock composition redox changes and colloidal particles. Geochimica et Cosmochimica Acta 64:4131–4251
Elderfied H, Greaves M (1982) The rare earth elements in seawater. Nature 296:214–219
Elderfield H (1988) The oceanic chemistry of the rare-earth elements. Phil Trans Roy Soc London, A325:105–126
Elderfield H, Sholkovitz ER (1987) Rare earth elements in the pore waters of reducing near shore sediments. Earth and Planetary Science Letters 82:280–288
Elderfield H, Upstill-Goddard R, Sholkovitz ER (1990) The rare earth elements in rivers estuaries and coastal seas and their significance to the composition of ocean waters. Geochimica et Cosmochimica Acta 54:971–991
Goldstein SJ, Jacobsen SB (1988) Rare earth elements in river waters. Earth Planet Sci Lett 89:35–47
Han G, Liu C-Q (2004) Water geochemistry controlled by carbonate dissolution: a study of the river waters draining karst-dominated terrain, Guizhou Province China. Chem Geol 204(1–2):1–21
Ji H, Wang S, Ouyang Z, Zhang S, Sun X, Liu X, Zhou D (2004) Geochemistry of red residua underlying dolomites in karst terrains of Yunnan-Guizhou Plateau II The mobility of rare earth elements during weathering. Chem Geol 203:29–50
Kawabe I, Kitahara Y, Naito K (1991) Nonchondritic yttrium/holmium ratio and lanthanide tetrad effect observed in pre Cenozoic limestones. Geochem J 25:31–44
Lee JH, Byrne RH (1993) Complexation of trivalent rare earth elements (Ce, Eu, Gd, Tb,Yb) by carbonate ions. Geochimica et Cosmochimica Acta 57:295–302
Liu X, Byrne RH (1995) Comparative carbonate complexation of yttrium and gadolimium at 25° and 0.7 mol/cm−3 ionic strength. Mar Chem 51:213–221
Liu Y-G, Miah MRU, Schmitt RA (1989) Reliability of the reported stablility constant for CePO4 as related to Ce redox formulations in sea water. Geochimica et Cosmochimica Acta 53:1477–1479
Möller P, Dulski P, Sarascin Y, Conrad M (2004) Rare earth elements Yttrium and Pb isotope ratios in thermal spring and well waters of west Anatolia Turkey: a hydrochemical study of their origin. Chem Geol 206:97–118
Nesbitt HW (1979) Mobility and fractionation of rare earth elements during weathering of granodiorite. Nature 279:206–210
Nozaki Y, Zhang J, Amakawa H (1997) The fractionation between Y and Ho in the marine environment. Earth Planet Sci Lett 148:329–340
Piper DZ (1974) Rare earth elements in the sedimentary cycle: A Summary. Chem Geol 14(4):255–304
Shabani MB, Akagi T, Shimizu H, Masuda A (1990) Determination of trace lanthanides and yttrium in seawater by inductively coupled plasma mass spectrometry after preconcentration with solved extraction and back-extraction. Anal Chem 62:2709–2714
Sholkolitz ER (1993) The geochemistry of rare earth elements in the Amazon River estuary. Geochimica et Cosmochimica Acta 57:2181–2210
Sholkovitz ER (1989) Artifacts associated with the chemical leaching of sediments for rare-earth elements. Chem Geol 77:47–51
Tang J, Johannesson K (2003) Speciation of the rare earth elements in natural terrestrial waters: assessing the role of dissolved organic water from the modeling approach. Geochimica et Cosmochimica Acta 67:2321–2339
Taylor SR, McLennann SM (1985) The continental crust: its composition and evolution. Blackwell, London
Tricca A, Stille P, Steinmann M, Kiefel B, Samuel J, Eikenberg J (1999) Rare earth elements and Sr and Nd isotopic compositions of dissolved and suspended loads from small river systems in the Vosges mountains France the river Rhine and groundwater. Chem Geol 160:139–158
Turner DR, Whitfield M, Dickson AG (1981) The equilibrium speciation of dissolved components in fresh water and seawater at 25° and 1 atm pressure. Geochimica et Cosmochimica Acta 45:855–881
Wang S-J, Li R-L, Sun C-X, Zhang D-F, Li F-Q, Zhou D-Q, Xiong K-N, Zhou Z-F (2004) How types of carbonate rock assemblages constrain the distribution fof karst rocky desertified land in Guizhou Province PR China: Phenomena and mechanisms. Land Degrad Develop 15:123–131
Wood SA (1990) The aqueous geochemistry of the rare-earth elements and yttrium: 1 Review of available low-temperature data for inorganic complexes of the inorganic REE speciation of natural waters. Chem Geol 82:159–186
Acknowledgements
This work was supported jointly by National Basic Research Program of China (973 Program) (no. 2006CB403206), the Chinese National Natural Science Foundation (no. 40372108, 40673010).The authors gratefully acknowledge Prof. E.R. Sholkovitz and Johan Schijf for significant improvements on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, G., Liu, CQ. Dissolved rare earth elements in river waters draining karst terrains in Guizhou Province, China. Aquat Geochem 13, 95–107 (2007). https://doi.org/10.1007/s10498-006-9009-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-006-9009-1