Abstract
Myrtucommulone (MC) is a unique, nonprenylated acylphloroglucinol contained in the leaves of myrtle (Myrtus communis). Here, we addressed the potential of MC to induce apoptosis of cancer cells. MC potently induced cell death of different cancer cell lines (EC50 3–8 μM) with characteristics of apoptosis, visualized by the activation of caspase-3, -8 and -9, cleavage of poly(ADP-ribose)polymerase (PARP), release of nucleosomes into the cytosol, and DNA fragmentation. MC was much less cytotoxic for non-transformed human peripheral blood mononuclear cells (PBMC) or foreskin fibroblasts (EC50 cell death = 20–50 μM), and MC up to 30 μM hardly caused processing of PARP, caspase-3, -8 and -9 in human PBMC. MC-induced apoptosis was mediated by the intrinsic rather than the extrinsic death pathway. Thus, MC caused loss of the mitochondrial membrane potential in MM6 cells and evoked release of cytochrome c from mitochondria. Interestingly, Jurkat cells deficient in caspase-9 were resistant to MC-induced cell death and no processing of PARP or caspase-8 was evident. In cell lines deficient in either CD95 (Fas, APO-1) signalling, FADD or caspase-8, MC was still able to potently induce cell death and PARP cleavage. Conclusively, MC induces apoptosis in cancer cell lines, with marginal cytotoxicity for non-transformed cells, via the mitochondrial cytochrome c/Apaf-1/caspase-9 pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis is a natural process by which cells undergo cell death to control cell number and proliferation as part of normal development [1]. Deregulation of apoptosis may disrupt the delicate balance between cell proliferation and cell death, and is therefore considered a hallmark of most or even all types of cancer [2, 3]. In fact, in many types of cancer, the expression of anti-apoptotic proteins is up-regulated or pro-apoptotic proteins have inactivating mutations which leads to an uncontrolled growth of the tumour. Accordingly, drugs that induce apoptotic cell death might be effective against many cancers [3, 4]. It is of interest that natural compounds play a dominant role in cancer chemotherapeutics with about 74% of anticancer compounds being either natural products, or natural product-derived [5].
At least two major pathways of apoptotic cell death can be distinguished: the extrinsic pathway that signals through so-called death receptors, and the intrinsic pathway involving mitochondria [6]. The first is triggered through the death receptors including CD95 (Fas, APO-1), tumour necrosis factor (TNF) receptor-1, TRAIL receptor-1 and -2, ultimately leading to the recruitment of the adaptor protein FADD and subsequent activation of caspase-8 which in turn activates caspase-3. The intrinsic pathway is characterized by a loss of mitochondrial membrane potential (ΔΨ m ) and the release of cytochrome c from mitochondria that interacts with the adaptor protein Apaf-1 recruiting caspase-9 which then activates caspase-3 [7]. Hence, both pathways converge to a final common route inducing the activation of downstream effector caspases that cleave regulatory and structural proteins, culminating in the death of the cell [8]. The components of these apoptotic pathways as well as regulatory proteins (e.g. Bcl-2 family members, or inhibitor of apoptosis proteins (IAPs) that inhibit caspases) may represent potential cancer drug targets [3]. Indeed, many anti-cancer agents used in clinical therapy intervene with apoptotic signalling, either with the intrinsic or the extrinsic pathway [9].
Myrtle (Myrtus communis L., Myrtaceae) is a shrubby plant, widely distributed in the Mediterranean area and is used as a culinary spice and as a folk medicine. It is one of the oldest medical plants applied as antiseptic and anti-inflammatory agent. Nevertheless, only a small number of studies have been performed that addressed the pharmacological effects of the plant or its specific ingredients. For example, myrtle extracts have been reported to be efficient as antibacterial [10], antihyperglycemic [11, 12] and analgetic [13] applicants, however, there is yet no clinical use of the plant or its characteristic ingredients for treating any disease.
In its leaves, myrtle contains myrtucommulone (MC) and semi-myrtucommulone (S-MC), unique, nonprenylated acylphloroglucinols (Fig. 1a) that may act as antioxidants [14] and possess antibacterial activities [15]. Recently, we showed that MC potently suppresses eicosanoid biosynthesis by direct targeting cyclooxygenase-1 and 5-lipoxygenase (5-LO), and blocks the release of human leukocyte elastase and the formation of reactive oxygen species associated with inhibition of agonist-induced Ca2+ mobilisation [16]. No other biological or pharmacological actions of MC have been reported thus far. In this study we show for the first time that MC induces apoptotic cell death in different cancer cells with low cytotoxicity in non-transformed cells, suggesting a potential for its use as anti-cancer drug.
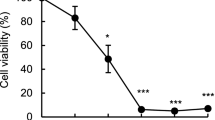
Effects of myrtucommulone on the cell viability of HL-60 and MM6 cells, and of normal human PBMC. (a) Chemical structure of MC, S-MC, and IBP-C. (b) Concentration-response curves of MC-induced cell death. HL-60, MM6 and human PBMC were seeded in standard growth medium containing 10% FCS at 0.2 × 106/ml and incubated with MC at the indicated concentrations or vehicle (DMSO, control) for 24 h. Cell numbers and cell viability was assessed by light microscopy and trypan blue exclusion (right panel) or by MTT assay (left panel). (c) Effects of MC, S-MC, IBP-C (30 μM, each), and daunorubicin (DR, 1 μM) on cell viability. HL-60 or MM6 cells were seeded as above and incubated with the compounds or vehicle (DMSO, control) for 24 h and cell numbers and cell viability was assessed. Data are given as mean + S.E., n = 4. *P < 0.05; **P < 0.01
Materials and methods
Materials
MC and S-MC were isolated from myrtle leaves as described previously [15]. The isobutyrophenone core (IBP-C) was synthesized from S-MC. The compounds were dissolved in dimethyl sulfoxide (DMSO) and kept in the dark at −20°C, and freezing/thawing cycles were kept to a minimum. Antibodies against caspase-3 (Cat. No. 9662, detecting the full length protein and a 17 kDa fragment), caspase-8 (Cat. No. 9746, detecting full length protein as well as 43- and 41-kDa fragments), caspase-9 (Cat. No. 9502, detecting full length protein as well as 17-, 35-, and 37-kDa fragments), poly(ADP-ribose)polymerase (PARP) and cytochrome c were from New England Biolabs (Beverly, MA). Staurosporine was from Calbiochem (La Jolla, CA), FasL was from MBL (Naka-ku Nagoya, Japan). All other chemicals were obtained from Sigma (Deisenhofen, Germany), unless stated otherwise.
Cells and cell culture
Jurkat-A3 cells, caspase-8-deficient Jurkat cells, and FADD-deficient Jurkat cells were provided by Dr. John Blenis, Boston, MA; caspase-9-deficient Jurkat cells and caspase-9-deficient Jurkat cells re-transfected with caspase-9 have been described previously [17]; PC-3 (androgen-independent prostate carcinoma), LNCaP (androgen-dependent prostate carcinoma), H9 (cutaneous T-cell lymphoma), DLD-1 (colorectal adenocarcinoma), HL-60 (acute promyelocytic leukaemia), Jurkat (acute T-cell leukaemia) and Jurkat DD3 (mutated in CD95), were obtained from the American Type Culture Collection (ATCC). KFR (rhabdomyosarcoma) and UKF-NB-3 (neuroblastoma) cells had been established from patients as described previously [18, 19]. Mono Mac 6 (MM6, acute monocytic leukaemia) cells were kindly provided by Dr. H.W. Ziegler-Heitbrock, Leicester, UK.
Human peripheral blood mononuclear cells (PBMC) were freshly isolated from leukocyte concentrates of human healthy donors obtained from the Blood Center at Tuebingen University Hospital. In brief, venous blood was taken from healthy adult volunteers and subjected to centrifugation at 4,000g for 20 min at 20°C for preparation of leukocyte concentrates. PBMC were promptly isolated by dextran sedimentation and centrifugation on Nycoprep cushions (PAA Laboratories, Linz, Austria). After washing in PBS pH 7.4, PBMC were resuspended in RPMI 1640 medium with supplements. Human foreskin fibroblasts were isolated as described previously [20]. All cells were cultured in respective media supplemented with 10% fetal calf serum (FCS, Roche Molecular Biochemicals, Mannheim, Germany), 100 μg/ml streptomycin, and 100 units/ml penicillin and grown at 37°C in a 5% CO2 atmosphere. For harvesting, cells were centrifuged (200g, 10 min, rt) and finally resuspended as indicated, respectively.
Determination of cell growth and cell death by trypan blue exclusion
Cells were seeded at 0.2 × 106 cells per ml growth medium and treated with various compounds or vehicle (DMSO) for the indicated times. Cells were harvested, washed with PBS and counted under a light microscope using the “Bürker” hemocytometer after mixing with trypan blue (1:1, vol/vol). As trypan blue is a cell impermeable dye staining chromatin, dead cells appeared blue under the microscope.
Determination of cell viability by MTT assay
Cell viability was assessed using the MTT assay [21] as described before [22]. In brief, cell lines were incubated at 37°C and 5% CO2 atmosphere with the indicated additives for 5 days. PBMC were treated for 40 h and in some experiments, MM6 cells were treated only for 24 h. DMSO was used as solvent never exceeding 0.5% (vol/vol). Then, MTT reagent was added for 4 h. Thereafter, 100 μl SDS solution (20% SDS in a 1:1 (vol by vol) dimethylformamide/water solution) was added for 4 h. Plates were read on a multiwell scanning spectrophotometer (Victor3 plate reader, PerkinElmer, Rodgau-Juegesheim, Germany) at a wavelength of 620 nm and a reference wavelength of 690 nm. Induction of cell death was determined as the relative reduction of the optical density.
SDS-PAGE and Western blot
Cells (4 × 106) were resuspended in 50 μl PBS, mixed with the same volume of 2 × SDS/PAGE sample loading buffer (20 mM Tris–HCl, pH 8, 2 mM EDTA, 5% (m/vol) SDS, 10% (vol/vol) β-mercaptoethanol), and boiled for 5 min at 95°C. Aliquots (20 μl) corresponding to equivalents of 0.8 × 106 cells were mixed with 4 μl glycerol/0.1% bromophenol blue (1:1, vol/vol) and proteins were separated by SDS-PAGE. After electroblotting to nitrocellulose membrane (GE Healthcare, Munich, Germany) and blocking with 5% non-fat dry milk for 1 h at room temperature, membranes were washed and incubated with primary antibodies overnight at 4°C. The membranes were washed and incubated with 1:1000 dilution of alkaline phosphatase-conjugated IgG for 3 h at room temperature. After washing, proteins were visualized with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate.
Nucleosome formation
Cells were treated with the test compounds or vehicle (DMSO) for 24 h as described above. 0.2–0.5 × 106 cells were then evaluated for the release of histone/DNA complexes using a Cell Death Detection ELISA Kit (Roche Molecular Biochemicals, Mannheim, Germany). The principle of this test is the detection of mono- and oligonucleosomes in the cytoplasmic fractions using monoclonal anti-histone and peroxidase-coupled anti-DNA antibodies. The amount of nucleosomes is quantified by photometrical determination at 405 nm with 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) as a substrate of the peroxidase activity retained in the immunocomplexes. The assay was performed according to the manufacturer’s instructions. Data are expressed as apoptotic enrichment factor using the following formula: absorbance [10−3] of the sample (dying/dead cells)/absorbance [10−3] of the corresponding control (viable cells).
DNA gel electrophoresis (DNA ladder)
Apoptotic DNA fragments were isolated from HL-60 cells (1 × 107) treated with 1 ml DNAzol (Molecular Research Center, Cincinnati, OH). The DNA was precipitated with 100% ethanol, washed twice with 95% ethanol, resuspended in 200 μl water and mixed with gel loading buffer (30% glycerol/0.25% xylencyanol FF/0.25% bromophenol blue), and separated on a 2% agarose gel containing 0.01% ethidiumbromide together with 100 bp DNA marker.
Flow cytometry analysis of fragmented DNA from apoptotic nuclei
The leakage of fragmented DNA from apoptotic nuclei was measured by the method of Nicoletti et al. [23]. Briefly, apoptotic nuclei were prepared by lysing cells in a hypotonic buffer (1% sodium citrate, 0.1% Triton X-100, 50 μg/ml propidium iodide) and subsequently analyzed by flow cytometry. Nuclei to the left of the 2N peak containing hypodiploid DNA were considered as apoptotic. All flow cytometry analyses were performed on a FACScalibur apparatus (Becton Dickinson, Heidelberg, Germany) using CellQuest analysis software.
Flow cytometry assay of mitochondrial membrane potential (ΔΨ m )
Cells (106 per ml) were incubated in culture medium with the indicated test compounds for 24 h, solvent (DMSO) was used as negative control. Cells were harvested, washed twice and resuspended in 1 ml PBS. JC-1 (1 μg/ml) was added and after 15 min at 37°C, cells were washed three times and resuspended in 1 ml PBS. Aliquots of 0.5 ml were analysed by flow cytometry, where JC-1 was excited at 488 nm. Orange fluorescence was detected through FL2 (575 nm) and green fluorescence through FL1 (530 nm). A log FL1 (x-axis) versus log FL2 (y-axis) dot blot was created. The percentage of cells that lost ΔΨ m versus cells possessing ΔΨ m was calculated using the cellQuest software.
Cytochrome c release
Cells were seeded at 4 × 106/ml culture medium and incubated with the test compounds or vehicle (DMSO). After 24 h cells were harvested, washed once in PBS and 107 cells were resuspended in 200 μl PBS. In order to permeabilize the plasma membrane, 20.3 μM digitonin was added and immediately vortexed for 10 s, incubated for another 30 s at RT and centrifuged at 20,000g at 4°C for 1 min. The supernatants (cytosolic fractions) were transferred to new tubes and mixed 1:1 (vol/vol) with 5% trichloroacetic acid. Precipitation of cytosolic proteins was performed at 4°C overnight. Proteins were pelleted by centrifugation at 20,000g at 4°C for 30 min and resuspended in 25 μl PBS. Aliquots of 5 μl were used for determination of protein concentration using Roti-Nanoquant (Roth, Karlsruhe, Germany). Equal amounts of protein were mixed 1:1 (vol/vol) with 2 × SDS/PAGE sample loading buffer and analyzed for cytochrome c by SDS-PAGE and Western Blot using an anti-cytochrome c-antibody.
Statistical analysis
Statistical evaluation of the data was performed by one-way ANOVAs for independent or correlated samples followed by Tukey HSD post-hoc tests. Where appropriate, Student´s t test for paired and correlated samples was applied. A P value of <0.05 (*) or <0.01 (**) was considered significant.
Results
Myrtucommulone reduces cell viability of cancer cell lines
Eight different types of human or rat cancer cell lines were utilized for determination of the effects of MC on cell viability, assessed by the MTT assay. The EC50 values of MC for reduction of viable cells of each cell type are summarized in Table 1. MC consistently reduced cell viability within 5 days in all cancer cell lines investigated. The EC50 values ranged between approx. 3.1 and 8.9 μM, with HL-60 and MM6 cells being the most sensitive cell lines. Of interest, non-transformed human foreskin fibroblasts (after 5 days) or isolated human PBMC (after 40 h) were hardly sensitive towards MC and the EC50 values were determined above 20 or 50 μM MC, respectively.
Myrtucommulone, but not semi-myrtucommulone or the isobutyrophenone core of MC induces cell death in leukaemic HL-60 and MM6 cells
Since the leukaemic cell lines were rather sensitive towards MC, HL-60 and MM6 cells were selected for further determinations. Freshly isolated human PBMC from healthy donors were used in order to analyse the effects of MC on primary non-transformed cells. In agreement with the data obtained after 5 days, MC concentration-dependently reduced the number of viable MM6 and HL-60 cells capable to exclude trypan blue as assessed by light microscopy (EC50 values of approx. 8 μM, Fig. 1b, right panel), and decreased cell viability (analysed by MTT assay, EC50 values of approx. 3–7 μM, Fig. 1b, left panel) within 24 h of treatment. Of interest, for primary human PBMC, MC was significantly less efficient in both experimental settings with EC50 values >60 μM. Next, the effects of S-MC and the IBP-C on cell viability were analyzed and compared to the efficiency of MC. The well-recognized anti-tumour agent daunorubicin [24] was utilized as reference compound. Cells were treated with the test compounds for 24 h and numbers of viable cells were analyzed by trypan blue staining and light microscopy. In contrast to MC or daunorubicin (1 μM), the structural analogues S-MC and IBP-C (30 μM, each) were not or at least hardly effective (Fig. 1c). Similarly, S-MC or IBP-C (30 μM, each) failed to induce cell death of MM6 after 24 h as detected by MTT assay (not shown).
Myrtucommulone induces cleavage of poly(ADP-ribose)polymerase (PARP) and activation of caspase-3, -8, and -9 in cancer cell lines
Next, the influence of MC on certain caspases, including caspase-3, -8, and -9, important mediators of apoptotic cell death [8], was determined. Furthermore, cleavage of the 116 kDa nuclear PARP, which is one of the main targets of caspase-3, undergoing cleavage to an 89 kDa fragment during apoptosis [25], was investigated. HL-60 and MM6 cells were incubated with 30 μM of IBP-C, S-MC and MC, and after 24 h, cells were harvested and analyzed for PARP cleavage and caspase processing.
Using a cleavage-specific PARP antibody, a marked generation of the 89 kDa PARP fragment was observed after treatment of HL-60 (Fig. 2a) and MM6 cells (Fig. 2b) with MC. In parallel, the amounts of the full-length form of caspase-3, -8, and -9 were reduced, accompanied by the appearance of the typical processed caspase (active) fragments. Neither IBP-C nor S-MC led to PARP cleavage or caspase activation at concentrations up to 30 μM (Fig. 2a). Concentration-response studies in MM6 (Fig. 2b) and HL-60 cells (data not shown) indicated that 3–10 μM MC were sufficient to induce substantial processing of PARP, caspase-3, -8, and -9, comparable to the effect by 1 μM daunorubicin. Importantly, MC (up to 30 μM) caused only moderate processing of PARP, caspase-3, -8 and -9 in PBMC, while DR (3 μM) was efficient (Fig. 2c).
Mytrucommulone induces processing of PARP, caspase-3, -8, and -9 in cancer cell lines. Cells were seeded in standard growth medium at 0.2 × 106/ml and incubated with the test compounds at the indicated concentrations or vehicle (DMSO) for 24 h. Cells were then harvested, total cell lysates were prepared and analyzed for processing of PARP (89 kDa), caspase-3, -8, and -9 after separation using SDS-PAGE by Western blotting. Arrows indicate the inactive full-length form of the caspases and/or the active caspase fragments, respectively. Comparable results were obtained in at least two additional experiments. (a) Effects of MC, S-MC and the IBP-C (30 μM, each) in HL-60 cells. (b) Concentration-response for MC in MM6. (c) Analysis of caspase-3, -8, and -9 as well as cleavage of PARP in human PBMC
Next, the time-course of MC-induced caspase activation and PARP cleavage was assessed. The numbers of viable HL-60 cells slightly decreased within the first 6 h after cell splitting, regardless of the treatment (Fig. 3a). However, after 12 h, untreated HL-60 cells started to proliferate, whereas MC (and daunorubicin) reduced proliferation which was accompanied by PARP cleavage and processing of caspase-3, -8, and -9 (Fig. 3b). No activation of caspases or cleavage of PARP was evident within the first 6–12 h of treatment with MC. Similarly, an onset of PARP and caspase processing after 12 h was found for MM6 cells (not shown).
Time course of myrtucommulone-induced apoptosis. HL-60 or MM6 cells were seeded in standard growth medium at 0.2 × 106/ml and incubated with 10 μM MC, 1 μM daunorubicin (DR) or vehicle (DMSO). (a) The numbers of viable HL-60 cells were determined after the indicated times. Data are given as mean + S.E., n = 4. (b) HL-60 cells were harvested after the indicated times and analyzed for processing of PARP (89 kDa), caspase-3, -8, and -9 as described in legend to Fig. 2. (c) MM6 cells were incubated with MC (10 μM), leukotriene B4 (LTB4, 1 μM) and 5(S)-hydroxyeicosatetraenoic acid (5(S)-HETE, 1 μM) as indicated. After 24 h cells were harvested and processing of PARP and caspase-3 was assessed. Comparable results for all experiments given were obtained in at least two additional experiments
5-LO products stimulate proliferation and survival of various cancer cells, whereas inhibitors of the 5-LO pathway induce apoptotic cell death [26]. Since MC potently inhibits 5-LO [16] it appeared reasonable that MC could induce apoptosis by suppression of 5-LO. However, neither the MC-induced reduction of the numbers of viable cells nor the cleavage of PARP or caspase-3 was reversed by the 5-LO products 5(S)-hydroxyeicosatetraenoic acid or leukotriene B4 (Fig. 3c).
Induction of nucleosome release and degradation of DNA by myrtucommulone
Apoptotic cell death is characterized by the release of histone-bound DNA fragments into the cytoplasm and the generation of a typical DNA ladder. Thus, the release of mono- and oligonucleosomes in the cytoplasm of HL-60 cells treated with MC was determined. As shown in Fig. 4a, MC, but not S-MC or the IBP-C, potently induced the formation of nucleosomes comparable to daunorubicin within 24 h, and this effect of MC was concentration-dependent (Fig. 4b). Furthermore, the leakage of fragmented DNA from apoptotic nuclei was measured according to Nicoletti et al. [23]. In MM6 cells, MC caused a concentration-dependent induction of apoptotic nuclei (starting at 3 μM of MC) appearing as a broad hypodiploid DNA peak that is discriminable from the narrow peak of MM6 cells with intact diploid DNA content (Fig. 4c). In contrast, in PBMC such a broad hypodiploid DNA peak first occurred at 60 μM MC, but not at lower MC concentrations.
Effect of myrtucommulone on the release of mono- and oligonucleosomes, DNA fragmentation and leakage of DNA fragments from apoptotic nuclei. (a) Nucleosome release. HL-60 cells were seeded in standard growth medium and incubated with 30 μM MC, S-MC and IBP-C, respectively, or with 1 μM daunorubicin (DR). After 24 h, cells (0.5 × 106) were evaluated for nucleosome formation using the Cell Death Detection ELISA. The amount of nucleosomes was photometrically quantified; data, expressed as apoptotic enrichment factor, are given as mean of three independent experiments + S.E. (b) Concentration-response study of MC on nucleosome release. HL-60 cells were seeded in growth medium at 0.2 × 106/ml and incubated with MC at the indicated concentrations. After 24 h, cells were evaluated for nucleosome formation as described above. Data are given as mean + S.E. *P < 0.05; **P < 0.01. (c) Analysis of fragmented DNA from apoptotic nuclei. MM6 cells or human PBMC were treated with MC at the indicated concentrations or with 1 μM staurosporine (control) for 24 h. Apoptotic nuclei were prepared and subsequently analyzed by flow cytometry. Apoptotic nuclei to the left of the 2N peak contain hypodiploid DNA. Data are given as mean + S.E. *P < 0.05; **P < 0.01; ***P < 0.001. (d) DNA fragmentation. HL-60 cells were seeded in growth medium at 0.2 × 106/ml and incubated with 10 μM MC, 10 μM CHX or DMSO (vehicle). After 24 h, DNA was extracted and separated on a 2% agarose gel. Similar results were obtained in two additional experiments
Next, HL-60 cells were evaluated for degraded DNA by gel electrophoresis. A typical apoptotic DNA ladder could be detected in samples containing genomic DNA from cells treated with 10 μM MC or 10 μM cycloheximide (CHX, positive control) (Fig. 4d), but not in samples containing vehicle (DMSO)-treated cells. Together, these data support the concept that MC induces apoptosis in cancer cells.
Myrtucommulone causes loss of the mitochondrial membrane potential (ΔΨ m ) and release of cytochrome c from mitochondria
Induction of apoptosis via the intrinsic pathway, induced for example by genotoxic stress, is often characterized by a loss of the ΔΨ m and the release of cytochrome c from mitochondria that in turn activates caspase-9 [27]. The ΔΨ m of MM6 cells and for comparison of PBMC was detected by the fluorescent dye JC-1 using flow cytometry analysis. As shown in Fig. 5a, treatment of MM6 cells with MC caused a concentration-dependent loss of the ΔΨ m with an EC50 of approx. 5 μM, whereas in PBMC (that are much less sensitive to MC-induced cell death) only a moderate reduction of the ΔΨ m was observed at the highest concentration (30 μM) tested. Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP), a protonophore and uncoupler of oxidative phosphorylation in mitochondria was used as positive control. The effects of MC on cytochrome c release from mitochondria of MM6 (and HL-60) as well as of PBMC were investigated. No release of cytochrome c was detected for vehicle (DMSO)-treated cells or cells stimulated with 3 μM MC for 24 h, however, treatment of cells with 10 or 30 μM MC induced a substantial cytochrome c release in both cell types (Fig. 5b). Significant cytochrome c release was observed when CHX was used in MM6 cells, whereas only a moderate effect was obtained in HL-60 cells (Fig. 5b).
Myrtucommulone induces loss of the mitochondrial membrane potential (ΔΨ m ) and causes cytochrome c release in cancer cell lines. (a) Loss of ΔΨ m . MM6 cells or freshly isolated PBMC were incubated with MC at the indicated concentrations for 24 h. Then, the ΔΨ m was analysed in both cell types by FACS using the dye JC-1 as described in Materials and Methods. Results are shown as the distribution of cells that lost ΔΨ m (area R3) versus cells possessing ΔΨ m (area R2) (lower panel) and as the percentage of the loss of ΔΨ m of treated cells compared to vehicle (DMSO)-treated control cells (100%, upper panel). Respective plots are representative for at least 4 determinations and data are given as mean + S.E., = 4. (b) Release of cytochrome c. HL-60 or MM6 cells were incubated with the indicated concentrations of MC, 50 μM CHX or vehicle (DMSO, control) for 24 h. Cells were harvested and permeabilized with digitonin as described and cytochrome c released into the cytosol was analyzed by Western blotting. Comparable results were obtained in at least three additional experiments
Induction of cell death by myrtucommulone requires caspase-9, but not death receptors, FADD, or caspase-8
Using various cell lines with deficiencies of key regulators of apoptosis, we aimed to assess if components of the extrinsic pathway of apoptotic cell death, involving death receptors (CD95, TNF receptor-1, TRAIL receptor-1 and -2), FADD and caspase-8, mediates MC-induced cell death. To investigate the role of death receptors, we first took advantage of the neuroblastoma cell line UKF-NB-3 which are resistant to typical death receptor stimuli [19, 22]. MC potently reduced viability (MTT assay) of UKF-NB-3 cells, whereas TNF, CD95 ligand, TRAIL failed in this respect (Fig. 6a). For control, TNF, CD95 ligand, TRAIL, and MC caused cell death of T-lymphocytic Jurkat cells. Similarly, as assessed by trypan blue staining, MC caused cell death in Jurkat cells deficient in FADD, and in the Jurkat cell line DD3, which is deficient in CD95-mediated signalling [28] implying that death receptor/FADD signalling is dispensable for MC-reduced cell viability (Fig. 6b). Moreover, Jurkat cells lacking caspase-8 were susceptible to MC. Of interest, however, Jurkat cells deficient in caspase-9 were partially resistant to the effects of MC on cell viability, and PARP cleavage was not apparent. Re-transfection of caspase-9−/− cells with caspase-9 recovered the ability of MC to reduce cell viability. It should be noted that the reduced numbers of caspase-9−/− Jurkat cells by MC (depicted from Fig. 6b) is not due to reduced cell viability, confirmed by analysis of apoptotic nuclei, but probably due to reduced cell proliferation. Thus, in caspase-9−/− Jurkat cells treated with 30 μM MC, no increase in the numbers of apoptotic nuclei was detectable, while in caspase-9-retransfected cells MC caused a clear leakage of fragmented DNA (Fig. 6c). In parallel, MC induced PARP cleavage in those cell lines which were susceptible to MC, but PARP was not processed in caspase-9-deficient Jurkat cells in which MC failed to reduce cell viability (Fig. 6b). Moreover, in caspase-9−/− Jurkat cells, caspase-8 was not processed in response to MC, while FasL caused caspase-8 cleavage (Fig. 6d).
Induction of apoptosis by MC in cell lines defective in select apoptotic signalling pathways or molecules. (a) Cell viability in response to ligands of death receptors. UKF-NB-3 (death receptor-resistant) or Jurkat cells were treated with either TNFα, TRAIL, CD95 ligand (100 ng/ml, each) or MC (30 μM). After 5 days, cell viability was assessed by MTT assay as described. Data are given as mean + S.E., n = 3. **P < 0.01. (b) Reduction of cell viability and PARP cleavage by MC in select Jurkat cell lines. Normal Jurkat cells or Jurkat cell lines deficient in either CD95 signalling (DD3), FADD, caspase-8, caspase-9 or caspase-9 and then retransfected with caspase-9 were incubated with 3, 10, and 30 μM MC, or vehicle (DMSO). Cells were harvested after 48 h and cell viability was determined light microscopy and trypan blue staining as described in the Methods section. In the absence of test compound (vehicle-treated), the numbers of viable cells for each cell line increased within 48 h from 0.2 × 106/ml to 0.70 × 106/ml for normal Jurkat, 0.89 × 106/ml for DD3, 0.92 × 106/ml for FADD−/−, 0.58 × 106/ml for casp-8−/−, 0.49 × 106/ml for casp-9−/−, and 0.81 × 106/ml for casp-9−/− retransfected, respectively. For analysis of PARP cleavage, cells were harvested after 16 h, total cell lysates were prepared and analyzed for processing of PARP (89 kDa) using Western blotting. Comparable results were obtained in at least three additional experiments. (c) Analysis of fragmented DNA from apoptotic nuclei. Jurkat cells (casp-9−/- or casp-9−/- retransfected with casp-9) were treated with MC at the indicated concentrations, or with 1 μM staurosporine or 100 ng/ml FasL (controls, each) for 24 h. Apoptotic nuclei were prepared and subsequently analyzed by flow cytometry. Apoptotic nuclei to the left of the 2N peak contain hypodiploid DNA. Data are given as mean + S.E., n = 4; **P < 0.01; ***P < 0.001. (d) Processing of caspase-8. Jurkat cells deficient in caspase-9 were treated with the indicated agents for 16 h and were then analyzed for cleavage of caspase-8 by WB. β-actin was used as control to normalize protein levels. Similar results were obtained in two additional experiments
Discussion
Despite the increasing understanding of the biological processes of cancer development, there is still a strong need for novel and effective pharmacological strategies for intervention with this disease. Pharmacological agents that induce apoptosis might be effective against many cancers by inducing death in cancer cells [3]. One common and effective strategy for developing novel chemotherapeutics is the evaluation of natural compounds. In fact, a great number of clinically active drugs that are used in cancer therapy are either natural products or are based on natural products. Established plant-derived therapeutics include vinblastine, vincristine, etoposide, teniposide, paclitaxel, doxetaxel, and camptothecin (reviewed in [29, 30]).
We show here for the first time that the naturally occurring MC, contained exclusively in the leaves of the herb myrtle (Myrtus communis L.), induces apoptosis in various cancer cell lines of human or rat, but lacks substantial cytotoxicity for normal non-malignant human cells such as foreskin fibroblasts or PBMC. This feature implies a promising potential of MC as chemotherapeutic for cancer treatment with a low risk of side effects, usually related to the unspecific cytotoxicity of many conventional cancer therapeutics. MC decreased cell viability after five days exposure, as assessed by MTT assay in eight of eight cancer cell lines tested. Moreover, MC caused cell death in the leukaemic HL-60 and MM6 cell line, as assessed by light microscopy and trypan blue exclusion within 24 h after exposure, but not before 12 h. Cell death may occur by many ways such as necrosis, autophagy, mitotic catastrophe, senescence and apoptosis [31]. For MC-induced cell death, apoptosis seems to be way of how cancer cells die. This is supported by the following findings: first, MC caused cleavage of PARP, an established marker of apoptosis, and induced activation of caspase-3, -8, and -9 that typically mediate and execute apoptotic cell death. Second, MC led to release of (mono- or oligo-)nucleosomes into the cytoplasm, caused DNA laddering as well as leakage of degradated DNA from apoptotic nuclei, well-recognized processes typically occurring during apoptosis [32]. Third, MC caused a loss of the ΔΨ m and evoked the release of cytochrome c into the cytoplasm, a characteristic for numerous stimuli that cause apoptosis via the intrinsic pathway involving mitochondria [7]. Note that in contrast to cancer cells, MC up to 30 μM failed to cause substantial PARP-cleavage, processing of caspase-3, -8 and -9, induction of apoptotic nuclei, and loss of the ΔΨ m in primary non-transformed PBMC, correlating to the moderate susceptibility of these cells to MC-induced cell death. Finally, MC failed to evoke apoptosis in a Jurkat variant lacking caspase-9, implying that a select apoptotic signalling pathway involving caspase-9 is required to evoke cell death by MC. Since neither the IBP-C nor S-MC showed comparable apoptotic effects in our assays, we conclude that defined structural requirements may be responsible for MC-induced apoptosis induction, rather than unspecific actions of the acylphloroglucinol backbone.
Though the nature of the target of MC that initializes apoptosis is unknown, our data suggest that MC signals via the intrinsic pathway, rather than via a death-receptor/FADD/caspase-8-mediated signalling route. Thus, MC induced a loss of ΔΨ m , release of cytochrome c, and required caspase-9 for induction of cell death and PARP cleavage. These events typically signal within the intrinsic pathway [7] acting on downstream effector caspases including caspase-3, -6, and -7. Although MC failed to cause PARP cleavage and to induce cell death in caspase-9 deficient Jurkat cells, it clearly reduced cell numbers, implying that MC either interferes with proliferation or induces alternate forms of cell death in a caspase-9 independent manner.
MC also clearly caused processing of caspase-8 that usually occurs in response to ligation of death receptors such as CD95 or the TNFα receptor involving FADD [6]. Since active caspase-8 is capable of inducing the release of cytochrome c from mitochondria involving cleavage of Bid to t-Bid [33], MC could also affect mitochondria via such a route. However, MC potently induced cell death and PARP-cleavage in Jurkat cells deficient in either CD95, FADD or caspase-8 that are critical elements of the extrinsic pathway. Also, MC was highly effective against UKF-NB-3 neuroblastoma cells that are resistant to typical death receptor ligands like TNFα, CD95 ligand or TRAIL, again pointing to the intrinsic pathway as relevant route. Finally, in caspase-9-deficient cells, MC failed in caspase-8 activation, though FasL (acting via the extrinsic pathway) was effective. Together, the intrinsic pathway involving mitochondrial events and caspase-9 virtually contributes to MC-evoked cell death, whereas the extrinsic pathway involving death receptors, FADD and caspase-8 seems less important.
MC is a direct and potent inhibitor of 5-LO suppressing the formation of leukotrienes and 5(S)-hydroxyeicosatetraenoic acid [16], mediators that depending on the cell type stimulate cell growth and activate typical survival pathways (e.g. Akt and extracellular signal-regulated kinases). Pharmacological or genetic inhibition of the 5-LO pathway attenuated proliferation, induced apoptosis of cancer cells in vitro and in vivo (for review see [26]), and reduced mitochondrial uncoupling and cytochrome c release [34]. Although supplementation of 5-LO products was sufficient to prevent 5-LO inhibitor-induced apoptosis [35–37], addition of leukotriene B4 or 5(S)-hydroxyeicosatetraenoic acid did not affect the actions of MC. Accordingly, inhibition of 5-LO product formation as a molecular mode of action underlying the MC-induced apoptosis can be rather excluded.
Certainly, the elucidation of the pathways and the respective targets of MC involved in apoptosis are of interest. Possible upstream routes and central candidates for mitochondria-mediated apoptosis that are targeted by typical cancer agents include the pro-apoptotic protein p53 and Bcl-2 family of proteins. p53 plays a major role in cellular response to DNA damage, and when activated leads to cell cycle arrest and DNA repair or to apoptosis [38]. p53 protects cells against cancer and many apoptosis inducing chemotherapeutics depend on functional p53 [39], and most human cancers have either mutations or defects in the p53 pathway [40]. HL-60, PC3 and Jurkat cells that are deficient in functional p53 [41, 42] were highly susceptible for MC, suggesting that MC induces apoptosis in a p53-independent manner. The BH3-only proteins act as sentinels for cell stress, damage or infection thereby initiating mitochondrial outer membrane permeabilisation by oligomerisation of Bax and/or Bak in the mitochondrial outer membrane forming channels that permit cytochrome c to escape from the mitochondria [7]. Since the BH3-only proteins target pro-apoptotic as well as anti-apoptotic Bcl-2 proteins [43], an orchestrated interplay of these proteins is of importance for normal cell proliferation and cell death, whereas an influence on a select BH3-only protein may cause an imbalance of these processes. MC may also govern the function of a pro-apoptotic Bcl-2 member or, on the other hand, may suppress an anti-apoptotic family member. Alternatively, MC may directly act on the mitochondria, hence decreasing the loss of ΔΨ m and/or allowing cytochrome c to escape from this organelle. Current experiments in our lab address the proximal signalling steps in MC-induced loss of ΔΨ m and cytochrome c release.
Conclusion
The natural ingredient MC from myrtle acts as a strong inducer of apoptosis selectively for cancer cells with lower cytotoxicity for normal non-transformed cells. Obviously, mitochondrial events are determinants in MC-induced cell death and caspase-9 is a critical element in the transduction of the apoptotic signal, whereas the extrinsic pathway involving death receptors, FADD and caspase-8 are less important. As mentioned before, very little is known in scientific research about the properties of myrtle extracts and only a few studies have addressed the pharmacological actions of myrtle-specific ingredients. Therefore, it will be an exciting challenge to further characterize the pharmacology of myrtle and in particular the pharmacological actions of MC with respect to apoptotic cell death.
Abbreviations
- CHX:
-
Cycloheximide
- FADD:
-
Fas-associated death domain
- IBP-C:
-
Isobutyrophenone core
- 5-LO:
-
5-Lipoxygenase
- MC:
-
Myrtucommulone
- ΔΨ m :
-
Mitochondrial membrane potential
- MM6:
-
Mono Mac 6
- PARP:
-
Poly(ADP-ribose)polymerase
- PBMC:
-
Peripheral blood mononuclear cells
- S-MC:
-
Semi-myrtucommulone
- TNF:
-
Tumour necrosis factor
References
Danial NN, Korsmeyer SJ (2004) Cell death: critical control points. Cell 116:205–219
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5:876–885
Nicholson DW (2000) From bench to clinic with apoptosis-based therapeutic agents. Nature 407:810–816
Tan G, Gyllenhaal C, Soejarto DD (2006) Biodiversity as a source of anticancer drugs. Curr Drug Targets 7:265–277
Nagata S (1997) Apoptosis by death factor. Cell 88:355–365
Green DR, Kroemer G (2004) The pathophysiology of mitochondrial cell death. Science 305:626–629
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
Galluzzi L, Larochette N, Zamzami N et al (2006) Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene 25:4812–4830
Al-Saimary IE, Bakr SS, Jaffar T et al (2002) Effects of some plant extracts and antibiotics on Pseudomonas aeruginosa isolated from various burn cases. Saudi Med J 23:802–805
Elfellah MS, Akhter MH, Khan MT (1984) Anti-hyperglycaemic effect of an extract of Myrtus communis in streptozotocin-induced diabetes in mice. J Ethnopharmacol 11:275–281
Onal S, Timur S, Okutucu B et al (2005) Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep Biochem Biotechnol 35:29–36
Levesque H, Lafont O (2000) Aspirin throughout the ages: a historical review. Rev Med Interne 21(Suppl 1):8s–17s
Rosa A, Deiana M, Casu V et al (2003) Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radic Res 37:1013–1019
Appendino G, Bianchi F, Minassi A et al (2002) Oligomeric acylphloroglucinols from myrtle (Myrtus communis). J Nat Prod 65:334–338
Feisst C, Franke L, Appendino G et al (2005) Identification of molecular targets of the oligomeric nonprenylated acylphloroglucinols from Myrtus communis and their implication as anti-inflammatory compounds. J Pharmacol Exp Ther 315:389–396
Samraj AK, Sohn D, Schulze-Osthoff K et al (2007) Loss of caspase-9 reveals its essential role for caspase-2 activation and mitochondrial membrane depolarization. Mol Biol Cell 18:84–93
Cinatl J, Herneiz P, Rabenau H et al (1994) Induction of myogenic differentiation in a human rhabdomyosarcoma cell line by phenylacetate. Cancer Lett 78:41–48
Kotchetkov R, Driever PH, Cinatl J et al (2005) Increased malignant behavior in neuroblastoma cells with acquired multi-drug resistance does not depend on P-gp expression. Int J Oncol 27:1029–1037
Michaelis M, Kohler N, Reinisch A et al (2004) Increased human cytomegalovirus replication in fibroblasts after treatment with therapeutical plasma concentrations of valproic acid. Biochem Pharmacol 68:531–538
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Michaelis M, Suhan T, Cinatl J et al (2004) Valproic acid and interferon-alpha synergistically inhibit neuroblastoma cell growth in vitro and in vivo. Int J Oncol 25:1795–1799
Nicoletti I, Migliorati G, Pagliacci MC et al (1991) A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139: 271–279
Zwelling LA, Altschuler E, Cherif A et al (1991) N-(5,5-diacetoxypentyl)doxorubicin: a novel anthracycline producing DNA interstrand cross-linking and rapid endonucleolytic cleavage in human leukemia cells. Cancer Res 51:6704–6707
Lazebnik YA, Kaufmann SH, Desnoyers S et al (1994) Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371:346–347
Werz O, Steinhilber D (2006) Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther 112:701–718
Budihardjo I, Oliver H, Lutter M et al (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290
Peterson EJ, Latinis KM, Koretzky GA (1998) Molecular characterization of a CD95 signaling mutant. Arthritis Rheum 41:1047–1053
Ghaemmaghami M, Jett JR (1998) New agents in the treatment of small cell lung cancer. Chest 113:86S–91S
Cragg GM, Newman DJ (2005) Plants as a source of anti-cancer agents. J Ethnopharmacol 100:72–79
Brown JM, Attardi LD (2005) The role of apoptosis in cancer development and treatment response. Nat Rev Cancer 5:231–237
Loo DT, Rillema JR (1998) Measurement of cell death. Methods Cell Biol 57:251–264
Luo X, Budihardjo I, Zou H et al (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481–490
Maccarrone M, Taccone-Gallucci M and Finazzi-Agro A (2003) 5-Lipoxygenase-mediated mitochondrial damage and apoptosis of mononuclear cells in ESRD patients. Kidney Int Suppl:S33–S36
Ding XZ, Iversen P, Cluck MW et al (1999) Lipoxygenase inhibitors abolish proliferation of human pancreatic cancer cells. Biochem Biophys Res Commun 261:218–223
Ghosh J, Myers CE (1997) Arachidonic acid stimulates prostate cancer cell growth: critical role of 5-lipoxygenase. Biochem Biophys Res Commun 235:418–423
Romano M, Catalano A, Nutini M et al (2001) 5-lipoxygenase regulates malignant mesothelial cell survival: involvement of vascular endothelial growth factor. FASEB J 15:2326–2336
Benchimol S (2001) p53-dependent pathways of apoptosis. Cell Death Differ 8:1049–1051
Lowe SW, Ruley HE, Jacks T et al (1993) p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74:957–967
Brown JM, Wouters BG (1999) Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res 59:1391–1399
Wolf D, Rotter V (1985) Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci USA 82:790–794
Rokhlin OW, Bishop GA, Hostager BS et al (1997) Fas-mediated apoptosis in human prostatic carcinoma cell lines. Cancer Res 57:1758–1768
Strasser A (2005) The role of BH3-only proteins in the immune system. Nat Rev Immunol 5:189–200
Author information
Authors and Affiliations
Corresponding author
Additional information
I. Tretiakova and D. Blaesius contributed equally to this work.
Rights and permissions
About this article
Cite this article
Tretiakova, I., Blaesius, D., Maxia, L. et al. Myrtucommulone from Myrtus communis induces apoptosis in cancer cells via the mitochondrial pathway involving caspase-9. Apoptosis 13, 119–131 (2008). https://doi.org/10.1007/s10495-007-0150-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-007-0150-0