Abstract
The southern cattle tick Rhipicephalus microplus is a major problem for the cattle industry in tropical and subtropical areas worldwide. Chemical products are commonly applied to control it; however, their indiscriminate use has resulted in the appearance of resistant lineages. In the last decades, plants have been used as an alternative to conventional acaricidal drugs, as several plant compounds repel activity, decrease the reproductive potential and reduce the survival rate of ticks. For this reason, the in vitro efficacy of hexanic and hydroalcoholic extracts of Randia aculeata, Moringa oleifera and Carica papaya were evaluated against the larvae and engorged females of R. microplus. Larval packet tests and adult immersion tests were performed with seven concentrations of each of the extracts. The extracts obtained with hydroethanolic solution (polar solvent) exhibited a higher acaricidal activity than extracts prepared with n-hexane (non-polar solvent). Hydroethanolic extracts of R. aculeata seed and shell showed the highest larvicidal activity against R. microplus (100 and 91% mortality, respectively) at a concentration of 100 mg/mL. Randia aculeata (seed and shell), M. oleifera and C. papaya treatments at the same concentration (100 mg/mL) also resulted in adult mortality of 85, 75, 66 and 55%, respectively. The adult immersion test showed that hydroethanolic extracts derived from R. aculeata seed significantly reduced the index of egg laying and increased the percentage inhibition of oviposition of female ticks at a concentration of 100 mg/mL. These results indicate that the tested extracts exhibit acaricidal activity and could be considered as potential agents for the development of alternative natural acaricides against R. microplus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The southern cattle tick Rhipicephalus microplus is one of the most widely distributed ticks that constitutes a problem for the cattle industry in tropical and subtropical regions. This species causes the loss of approximately $30 billion annually worldwide (Estrada-Peña et al. 2006; Grisi et al. 2014). In massive infestations, this tick produces negative effects on its hosts by generating anaemia, tick worry, hide damage, injection of toxins, and the transmission of pathogens like Anaplasma marginale, Babesia bovis and Babesia bigemina (Miraballes and Riet-Correa 2018). Ticks are controlled with synthetic acaricides, such as organophosphates, carbamates, organochlorines, amidines, pyrethroids, fipronil, fluazuron, and macrocyclic lactones; however, this practice has resulted in the development of acaricidal resistance in several populations, environmental chemical contamination, effects on non-target species and risks to human health (Banumathi et al. 2017). An alternative for controlling tick infestations in cattle is the use of natural compounds. The main research interest has focused on components synthetized by the metabolism of plants that take part in the plant’s defense mechanisms against pests and pathogens. These metabolites—including steroids, alkaloids, terpenes, flavonoids, phenylpropanoids, amides and lignans—stand out as promising bioactive plant molecules against the emerging acaricidal resistance (Adenubi et al. 2018). Further studies suggest that extracts can be used alone or in combination with chemical compounds to enhance control methods (Singh et al. 2018; Khan et al. 2019). Moringa oleifera, commonly known as the horse radish tree, is a commercial crop and a widely distributed multipurpose tree (Khan et al. 2017). The glucosinolates and their breakdown products—such as isothiocyanates, thiocyanates, nitriles and thiocarbamates—are characteristic metabolites of the Moringa tree, which have an important role in the control of insect pests and nematodes. Several studies have documented the insecticidal activity of the Moringa species (Manzoor et al. 2015; Nwankwo et al. 2015; Dougoud et al. 2019).
Carica papaya belongs to the Caricaceae, a small family with four genera. This fruit is widely recognized as an important source of medicinal and insecticidal agents and is used in the treatment of various ailments due to its antimalarial, anti-inflammatory, hypoglycaemic and wound healing properties (Vij and Prashar 2015). Kovendan (2012) found larvicidal and pupicidal activity against the chikungunya vector Aedes aegypti, in a methanol extract of the leaf. During phytochemical screening, C. papaya leaves have been shown to contain many active components, such as papain, chymopapain, cystatin, alpha-tocopherol, ascorbic acid, flavonoids, cyanogenic glucosides and glucosinolates (Ekaiko et al. 2015).
Randia aculeata, commonly known as crucetillo, is a member of the Rubiaceae family and is used to counteract the effects of snake bites and other venomous animals, as well as for treatment of cancer, diabetes, inflammation and pain (Gallardo-Casas et al. 2012). Phytochemical analysis of R. aculeata has reported the presence of tannins (Torres-Fajardo et al. 2019) and alkaloids (Soto et al. 2001). On the other hand, Frame et al. (1998) reported the anti-Mycobacterium tuberculosis properties of R. aculeata. Possible anti-tick activity of Moringa oleifera root, C. papaya leaves, and R. aculeata seed and shell extracts have not been reported previously. The aim of this study was to evaluate the in vitro acaricidal activity of hexanic and hydroethanolic extracts of M. oleifera root, C. papaya leaves, and R. aculeata seed and shell on R. microplus larvae and engorged females.
Materials and methods
Biological material: plant material and extraction
Carica papaya leaves and R. aculeata fruits were acquired from local markets in the municipality of Veracruz and Paso de Ovejas, Veracruz, Mexico, in September 2018. Each fruit of R. aculeata was separated to obtain the seeds and shell. Plant materials were cleaned and air dried under shade in a well-ventilated place for 7 days at room temperature, then pulverized with a Hamilton-Beach mixer grinder. The M. oleifera root powder (from primary and secondary roots) was obtained from trees cultivated and processed as previously described by Alvarez-Roman et al. (2020). All extracts were prepared at 1:10 (w/v) ratio by adding the solvent n-hexane or hydroethanolic solution (EtOH–H2O, 80:20) to the powdered plant material. The extraction was carried out by maceration at room temperature. Each 24 h for 3 days, the contents were allowed to settle; the solvent was then collected, and a Whatman No. 1 filter paper was used to remove the solid material. The residue was re-extracted by adding the same volume of solvent. The extracts were reduced under vacuum using a Buchi Roto-Vapor at 26 °C. Finally, the concentrated extracts were lyophilized to remove traces of the solvent, collected in glass tubes and kept refrigerated at 4 °C in air-tight containers until required for biological assays.
Preparation of stock and test concentrations
Initially, a stock solution of Triton X-100 2% (Sigma–Aldrich) was prepared in absolute ethanol (ETH-TX 2%). The stock solution was then water diluted up to 1% ethanol in 0.02% Triton X-100 solution (diluent), and extracts were dissolved up to 100, 50, 25, 12.5, 6.25, 3.12 and 1.56 mg/mL.
Tick preparation
Engorged females were collected from naturally infested cattle that had not received acaricidal treatment for 20–30 days, on a farm of the municipality of Saltabarranca, Veracruz, Mexico. After collection, ticks were identified by morphological characteristics following Walker et al. (2007). The ticks were then immersed in a 2% sodium hypochlorite solution (for use in subsequent molecular analyses), dried and selected according to integrity, motility and degree of engorgement. The larvae used in the larval packet test (LPT) came from engorged females. These ticks were attached dorsally in Petri dishes and placed at 27 ± 1 °C and 80% RH for egg laying. After 18 days of oviposition, the egg masses were removed, placed in plastic tubes and kept at the same temperature and humidity conditions previously described until hatching of the larvae. Bioassays were conducted in sextuplicate for each concentration of the extracts, in both the larval and engorged female tests.
Larval packet test (LPT)
Bioassays were performed according to FAO (2004). The packets were made of filter paper and impregnated with 1 mL of each plant extract concentration. Packets were also impregnated with 1 mL of diluent, 1% ethanol and 0.02% Triton X-100 (negative control), and amitraz at a concentration of 0.0002% (positive control). Approximately 100 larvae were placed in each packet (six per concentration), which was immediately sealed with paper clips and put in a BOD chamber at 27 ± 1 °C and > 80% RH, where they remained for 24 h. After this interval, the packets were opened, and the live and dead larvae were counted in each replicate to calculate mortality. Larval mortality was corrected using Abbott's formula (1925).
Adult immersion test (AIT)
The adult immersion test (AIT) was performed as described in the literature (Drummond et al. 1973; FAO 2004; Sharma et al. 2012). Each replicate (six per concentration) consisted of a group of 10 females, with homogeneous weight, immersed for 5 min in 10 mL extract solution at each of the concentrations evaluated. The controls were amitraz at a concentration of 0.0002% (positive control) and diluent, 1% ethanol and 0.02% Triton X-100 (negative control). After immersion, the engorged females were dried on a paper towel and mounted dorsally in Petri dishes with two-sided tape. The plates were kept in the BOD chamber at 27 ± 1 °C and > 80% RH. Ticks were examined with a stereoscope, and mortality counts were recorded daily. Ticks were confirmed dead based on signs of haemorrhagic skin lesions, cuticular darkness and lack of Malpighian tube movement. After 14 days, the eggs were weighed and transferred to tubes, which were identified and sealed. Tubes were then placed in the incubator under the same conditions for larval hatching and were read visually after 16 days of incubation. Reading was performed by a single technician who had no knowledge about the treatment, to avoid biased estimation according to the procedure described by Drummond et al. (1973) and Figueiredo et al. (2018).
The egg production index (EPI), the reduction in oviposition (RO), reproduction efficiency index (REI) and the efficiency of the extract (EP) were calculated according to the following formulas:
EPI (%) = (weight of eggs/weight of engorged female) × 100 (Bennett 1974),
RO (%) = [(EPI control group − EPI experimental group)/EPI control group] × 100 (Roulston et al. 1968),
REI = (egg mass weight × % egg hatching/engorged females weight) × 20,000 (Drummond et al. 1973), and.
EP (%) = [(REI control − REI treated)/REI control] × 100 (Drummond et al. 1973).
Statistical analysis
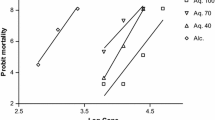
Data were analyzed by one-way ANOVA, followed by Tukey test to separate means, using STATISTICA v.10 software (α = 0.05). The lethal concentrations (LC50 and LC90) were calculated by probit analysis (Finney 1962) using Stata Graphics v.18. For all analyses of variance and probit, the assumptions of normality and homogeneity of variance were checked and no data transformation was required.
Results
Larval packet test (LPT)
All extracts showed an acaricidal effect against the larvae of R. microplus. Especially, the hydroethanolic extract of R. aculeata seed at 1.56–100 mg/mL had the highest acaricidal effect (93–100%), followed by R. aculeata shell (91.2%), M. oleifera root (77.7%) and C. papaya leaves (75.5%) at 100 mg/mL (Table 1). For the hexanic extracts at 100 mg/mL, the R. aculeata seed also showed the highest acaricidal effect (82.2%), followed by R. aculeata shell (81.2%), M. oleifera root (78.2%) and C. papaya leaves (61.7%). Mortality was significantly different after larvae ticks were exposed to extracts in a dose dependent manner, compared with the control groups exposed to diluent (Table 1). Ascending concentrations of tested extracts were evaluated to obtain lethal doses that were then subjected to probit analysis. The LC50 and LC90 for hydroethanolic and hexanic extracts are shown in Table 2.
Adult immersion test (AIT)
Mortality caused by the hydroethanolic extracts (Table 3) and hexanic extracts (Table 4) of R. aculeata, M. oleifera and C. papaya varied from 55–85.5% to 0–75%, respectively, when tested at 1.56–100 mg/mL. Hatching of the eggs was blocked only by (the higher concentrations of) hydroethanolic extracts of R. aculeata seed. Other extracts were only partially able to block the hatching; however, newly hatched larvae did not survive and died within a few hours. All concentrations of hydroethanolic extract tested caused significant mortality of adult engorged ticks and reduction in the mass of eggs laid, when compared to the control. Consequently, there was a significant reduction in the reproduction efficiency index. Adult mortality caused by the hydroethanolic extract of the R. aculeata seed varied from 17.5 to 85.5%—and mortality by R. aculeata shell extracts varied from 15 to 75%—when tested at concentrations ranging from 1.56 to 100 mg/mL, respectively. Moringa oleifera root extracts at 50 and 100 mg/mL had lower efficacy, and caused 55–66% mortality.
Hexanic extracts of R. aculeata seed at 12.5–100 mg/mL caused 47.5–75% mortality and significantly inhibited reduction in oviposition (47.3–65.1%), when compared to the control ticks (Table 4). Hexanic extracts of C. papaya leaves did not cause mortality at any concentration. Adult ticks treated with higher concentrations of the various hexanic extracts—all except C. papaya extracts—laid egg masses that were significantly lower in weight than the egg masses of control ticks, in spite of the low adult mortality in the treated ticks. The LC50 and LC90 for hydroethanolic and hexanic extracts are shown in Table 5.
Discussion
Plants have an important role in traditional medicine, as it has been shown that they provide many metabolites that can intervene in the biological processes and the life cycle of ticks, and they are considered an important part of ethnoveterinary medicine (Zaman et al. 2012; Chagas et al. 2014). The potential use of plant extracts for the control of arthropods of veterinary importance has been reviewed by Ghosh et al. (2015) and Rosado-Aguilar (2017), and a few plants were identified as being promising against ticks. In the present study, the anti-tick potential of three more plants was identified, to contribute to the development of plant-based acaricides for the control of tick species.
Omoregie et al. (2018) found that an alcoholic extract of M. oleifera root showed the presence of bioactive compounds: alkaloids, tannins, flavonoids, steroids, triterpenoid glycosides, saponins and anthraquinones. These findings are similar to those reported by Sholapur and Patil (2013). In addition, the insecticidal activity of M. oleifera extract on termites (Nasutitermes cornige) has been reported by Paiva et al. (2010) and Muhammad (2012). Coelho et al. (2009) and Agra-Neto et al. (2014) documented that aqueous extracts from M. oleifera seeds delayed the larval development of A. aegypti, and a water-soluble seed lectin was able to kill A. aegypti larvae by promoting morphological alterations in the digestive tract, causing an imbalance in digestive enzyme activities. Oliveira et al. (2011) reported great insecticidal activity of the seed coagulant M. oleifera lectin (cMoL) on flour moth (Anagasta kuehniella). The insecticidal activity of this plant may be due to the presence of phenolic compounds such as β-amyrin, β-sitosterol, kaempferol and quercetin (Pontual et al. 2012), which have negative effects on insects, as they decrease fertility and shorten their life span (Dawkar et al. 2013).
Phytochemical analysis of methanolic extracts of C. papaya stems have shown the presence of alkaloids, tannins, flavonoids, carbohydrates and triterpenes (Rashed et al. 2013). Literature is scarce about the insecticidal activity of C. papaya leaf extract; however, Zobayer and Hasan (2013) reported great insecticidal activity against Aphis gossypii. Phytochemical analysis of methanol, ethanol and water extracts and fractions of C. papaya leaf revealed the presence of alkaloids, flavonoids, saponins, steroids and tannin (Yusha’u et al. 2009; Rashed et al. 2013). Larvicidal activity of C. papaya against the mosquitoes Culex quinquefasciatus and Anopheles stephensi was reported by Rawani et al. (2009). Methanol extract of C. papaya seeds caused 82.2% larval mortality and 93.3% adult mortality on R. microplus (Shyma et al. 2014). These higher acaricidal activity of the C. papaya seed extracts—compared to our study with hydroethanolic and hexane leaf extracts—may be due to a synergistic effect of the active components, or perhaps to more sensitive ticks. Other possible differences are related to the organospecific production profile of the plant or the solvent used, the phase of the plant at the time of collection, or the variety of C. papaya tested (Pandey et al. 2014).
Phytochemical analysis of R. aculeata revealed the presence of tannins (Torres-Fajardo et al. 2019), among other chemical constituents. Tannins have been shown to produce anthelminthic activities, and it is possible that tannin can bind to free proteins in the gastrointestinal tract of the host animal or to glycoproteins on the cuticle of the parasite, which may cause death (Niezen et al. 2002; Athanasiadou et al. 2001). Antoun et al. (1993) tested R. aculeata against Plasmodium falciparum without effect. To our knowledge, this is the first report of anti-tick activity of hydroalcoholic and hexane extracts of the seed and shell of R. aculeata. The acaricidal effect of hexane extracts in our study are not similar to previous studies, in which the non-polar fraction had higher anti-tick activity than the polar fraction. The acaricidal efficacy of this assay may be attributed to the organic solvents working better as the cuticle of the tick is mainly formed externally by waxes and internally by proteins (Cherry 1969); hence, the more non-polar a chemical compound, the greater its ability to penetrate the cuticle (Chagas et al. 2002).
The current study revealed that the mean mortality of larvae and adult ticks increased with increasing dosage (concentration) and exposure time, after in vitro treatment. This result is in line with the findings of Fouche et al. (2017), in which the mortality effect of extracts was found to be dose and exposure time dependent. Our study also showed that hydroalcoholic extracts of R. aculeata seed and shell, C. papaya leaves and M. oleifera root induced significant acaricidal effects against R. microplus, compared with the negative control. Plants have a diversity of defense mechanisms to decrease insect attacks, both constitutive and inducible, while insects have evolved strategies to overcome these plant defenses. The mode of action and target site for insecticidal activities of plants have been studied often (Akhtar et al. 2009; Moreau et al. 2012; Ribeiro et al. 2012; Cardoso et al. 2020). Most of the work has been carried out by studying the effects of extracts and essential oils, their lethal doses and time to achieve lethal effects, but modes of action at a molecular level are generally unknown. Secondary plant metabolites, such as terpenoids and alkaloids, are reported as candidates for effective alternative insecticidal compounds. These metabolites can be linked to structural proteins, enzymes, receptors, ion-channels, nucleic acids and other cellular components, damaging the arthropod’s structure (Lopez et al. 2010). Phytochemicals can serve as a model for the development of chemically synthesized derivatives with enhanced activity (Kostyukovsky et al. 2002; O’Callaghan et al. 2019). Nevertheless, details of the specificity of the metabolites with insecticidal activity are scarcely known. In reality, it is likely that the mechanism will behave like a web, in which pathways and molecules will interact.
We can conclude that extracts of R. aculeata seed and shell, C. papaya leaf and M. oleifera root have in vitro acaricidal activity. In addition, seed and shell extracts of R. aculeata were shown to have a high acaricidal effect at various life stages of R. microplus, thus justifying the testing of these plants against other arthropods. Further in vivo studies including these plants should be undertaken to evaluate the effect on host animals. Furthermore, the identification of active ingredients presents in R. aculeata seed and shell that caused adult tick mortality, decreased egg production and the inhibition of egg hatching will be fruitful. These studies will further help to confirm factors that increase the acaricidal activity. The acaricidal properties of the tested extracts could make them a valuable component in the development of a sustainable strategy for arthropod pest management in agriculture and the cattle industry.
References
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Adenubi OT, McGaw LJ, Eloff JN, Naidoo V (2018) In vitro bioassays used in evaluating plant extracts for tick repellent and acaricidal properties: a critical review. Vet Parasitol 254:160–171
Agra-Neto AC, Napoleão TH, Pontual EV (2014) Effect of Moringa oleifera lectins on survival and enzyme activities of Aedes aegypti larvae susceptible and resistant to organophosphate. Parasitol Res 113:175–184
Akhtar Y, Shikano I, Isman MB (2009) Topical application of a plant extract to different life stages of Trichoplusia ni fails to influence feeding or oviposition behavior. Entomol Exp Appl 132:275–282
Álvarez-Román R, Silva-Flores PG, Galindo-Rodríguez SA, Huerta-Heredia AA, Vilegas W, Paniagua-Vega D (2020) Moisturizing and antioxidant evaluation of Moringa oleifera leaf extract in topical formulations by biophysical techniques. S African J Bot 129:404–411
Antoun MD, Gerena L, Milhous WK (1993) Screening of the flora of Puerto rico for potential antimalarial bioactives. Pharm Biol 31:255–258
Athanasiadou S, Kyriazakis I, Jackson F, Coop RL (2001) Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet Parasitol 99:205–219
Banumathi B, Vaseeharan B, Rajasekar P, Prabhu NM, Ramasamy P, Murugan K, Benelli G (2017) Exploitation of chemical, herbal and nanoformulated acaricides to control the cattle tick, Rhipicephalus (Boophilus) microplus–a review. Vet Parasitol 244:102–110
Bennett GF (1974) Oviposition of Boophilus microplus (Canestrini) (Acarida: Ixodidae). Influence of tick size on egg production. Acarologia 16:52–61
Cardoso A, Santos E, Lima A, Temeyer KB, Pérez de León AA, Costa LM (2020) Terpenes on Rhipicephalus (Boophilus) microplus: Acaricidal activity and acetylcholinesterase inhibition. Vet Parasitol. https://doi.org/10.1016/j.vetpar.2020.109090
Chagas AC, Passos WM, Prates HT, Leite RC, Furlong J, Fortes ICP (2002) Efeito acaricida de óleos essenciais e concentrados emulsionáveis de Eucalyptus spp em Boophilus microplus. Brazilian J Vet Res Anim Sci 39:247–253
Chagas AC, Domingues LF, Fantatto RR, Giglioti R, Oliveira MC, Oliveira DH, Mano RA, Jacob RG (2014) In vitro and in vivo acaricide action of juvenoid analogs produced from the chemical modification of Cymbopogon spp. and Corymbia citriodora essential oil on the cattle tick Rhipicephalus (Boophilus) microplus. Vet Parasitol 205:277–284
Cherry LM (1969) The production of cuticle wax by engorged females of the cattle tick, Boophilus Microplus (Canestrini). J Exp Biol 50:705–770
Coelho JS, Santos NDL, Napoleão TH, Gomes FS, Ferreira RS, Zingali RB, Coelho LCBB, Leite SP, Navarro DMAF, Paiva PMG (2009) Effect of Moringa oleifera lectin on development and mortality of Aedes aegypti larvae. Chemosphere 77:934–938
Dawkar VV, Chikate YR, Lomate PR, Dholakia BB, Gupta VS, Giri AP (2013) Molecular insights into resistance mechanisms of lepidopteran insect pests against toxicants. J Proteome Res 12:4727–4737
Dougoud J, Toepfer S, Bateman M (2019) Efficacy of homemade botanical insecticides based on traditional knowledge. A Review Agron Sustain Dev 39:37
Drummond RO, Ernest SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Econ Entomol 66:130–133
Ekaiko M, Stephen C, Emmanuel U, Chizaram E (2015) Antimicrobial screening and phytochemical analysis of Carica papaya leaf extracts. Stand Res J Microbiol Sci 2:1–4
Estrada-Peña A, Garcia Z, Sánchez HF (2006) The distribution and ecological preferences of Boophilus microplus (Acari:Ixodidae) in Mexico. Exp Appl Acarol 38:307–316
FAO (2004) Acaricide Resistance: Diagnosis, Management and Prevention. Guidelines Resistance Management and Integrated Parasite Control in Ruminants, Animal Production and Health Division, Agriculture Department, Food and Agriculture Organization of the United Nations, Rome, 25–77
Figueiredo A, Agnolon IC, Lopes LG, Giglioti R, Chagas AC (2018) Comparative study of hatching estimation methods of Rhipicephalus (Boophilus) microplus eggs. Vet Parasitol 264:35–38
Finney DJ (1962) Probit analysis, vol 78. Cambridge University Press, Cambridge, pp 388–390
Fouche G, Sakong BM, Adenubi OT (2017) Investigation of the acaricidal activity of the acetone and ethanol extracts of 12 South African plants against the adult ticks of Rhipicephalus turanicus. Onderstepoort j Vet Res 84:1–7
Frame AD, Ríos-Olivares E, De Jesús L, Ortiz D, Pagán J, Méndez S (1998) Plants from Puerto Rico with anti-Mycobacterium tuberculosis properties. P R Health Sci J 17:243–252
Gallardo-Casas CA, Guevara-Balcázar G, Morales-Ramos E (2012) Ethnobotanic study of Randia aculeata (Rubiaceae) in Jamapa, Veracruz, Mexico, and its anti-snake venom effects on mouse tissue. J Venom Anim Toxins Incl Trop Dis 18:287–294
Ghosh S, Tiwari SS, Kumar B (2015) Identification of potential plant extracts for anti-tick activity against acaricide resistant cattle ticks, Rhipicephalus (Boophilus) microplus (Acari:Ixodidae). Exp Appl Acarol 66:59–171
Grisi L, Leite RC, Martins JRS, Barros ATM, Andreotti R, Cançado PHD, León AAP, Pereira JB, Villela HS (2014) Reassessment of the potential economic impact of cattle parasites in Brazil. Rev Bras Parasitol Vet 23:150–156
Khan S, Basra SMA, Afzal I, Nawaz M, Rehman HU (2017) Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ Sci Pollut Res 24:27601–27612
Khan A, Nasreen N, Niaz S et al (2019) Acaricidal efficacy of Calotropis procera (Asclepiadaceae) and Taraxacum officinale (Asteraceae) against Rhipicephalus microplus from Mardan, Pakistan. Exp Appl Acarol 78:595–608
Kostyukovsky M, Gileadi C, Demchenko N, Shaaya E (2002) Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag Sci 58:1101–1106
Kovendan K, Murugan K, Naresh Kumar A, Vincent S, Hwang JS (2012) Bioefficacy of larvicdial and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res 110:669–678
Lopez MD, Pascual-Villalobos MJ (2010) Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind Crops and Prod 31:284–288
Manzoor M, Ali H, Muhammad A, Alam I, Khalid SH, Idrees A, Arif M (2015) Potential of Moringa (Moringa oleifera: Moringaceae) as plant growth regulator and bio-pesticide against wheat aphids on wheat crop (Triticum aestivum; Poaceae). J Biopest 8:120–127
Miraballes C, Riet-Correa F (2018) A review of the history of research and control of Rhipicephalus (Boophilus) microplus, babesiosis and anaplasmosis in Uruguay. Exp Appl Acarol 75:383–398
Moreau TL, Isman MB (2012) Combining reduced-risk products, trap crops and yellow sticky traps for greenhouse whitefly (Trialeurodes vaporariorum) management on sweet peppers (Capsicum annum). Crop Prot 34:42–46
Muhammad SN (2012) Effects of plant extracts on the behaviour and physiology of the Odontotermes obesus (Ramb.) (Isoptera: Termitidae). Dissertation. University of Agriculture, Faisalabad, Pakistan
Niezen JH, Charleston WAG, Robertson HA, Shelton D, Waghorn GC, Green R (2002) The effect of feeding sulla (Hedysarum coronarium) or lucerne (Medicago sativa) on lamb parasite burdens and immunity to gastrointestinal nematodes. Vet Parasitol 105:229–245
Nwankwo EN, Okonkwo NJ, Ogbonna CU, Akpom CJ, Egbuche CM, Ukonze BC (2015) Moringa oleifera and Annona muricataseed oil extracts as biopesticides against the second and fourth larval instar ofAedes aegypti L. (Diptera: Culicidae). J Biopest 8:56–61
O’Callaghan FE, Neilson R, MacFarlane SA (2019) Dynamic biospeckle analysis, a new tool for the fast screening of plant nematicide selectivity. Plant Methods 15:1–13
Oliveira CFR, Luz LA, Paiva PMG, Coelho LCBB, Marangoni S, Macedo MLR (2011) Evaluation of seed coagulant Moringa oleifera lectin (cMoL) as a bioinsecticidal tool with potential for the control of insects. Process Biochem 46:498–504
Omoregie M, Omoregie A, Iloba B (2018) Insecticidal potential of Moringa oleifera (Lamarck) root on workers of Macrotermes bellicosus (Smeathman). EIJST 7:9–16
Paiva PMG, Santana GMS, Souza IFAC, Albuquerque LP, Agra-Neto AC, Albuquerque AC, Luz LA, Napoleão TH, Coelho LCBB (2010) Effect of lectins from Opuntia ficus indica cladodes and Moringa oleifera seeds on survival of Nasutitermes corniger. Int Biodeter Biodegrad 65:982–989
Pandey A, Tripathi SM (2014) Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem 2:115–119
Pontual EV, Napoleão TH, Dias de Assis CR (2012) Effect of Moringa oleifera flower extract on larval trypsin and acetylcholinesterase activities in Aedes aegypti. Arch Insect Biochem Physiol 79:135–152
Rashed KN, da Cruz MG, Vieira GPG, Magalhaes LG, Cunha WR (2013) Evaluation of schistosomicidal and leishmanicidal activities from Carica papaya (Linn.) stem and phytochemical composition. J Herbal Med 2:239–243
Rawani A, Mallick HK, Ghosh A, Chandra G (2009) Larvicidal activities of three plants against filarial vector Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 105:1411–1417
Ribeiro VL, Vanzella C, Moysés S, Santos JC, Martins JR, Von Poser GL, Siqueira IR (2012) Effect of Calea serrata Less. n-hexane extract on acetylcholinesterase of larvae ticks and brain Wistar rats. Vet Parasitol 189:322–326
Rosado-Aguilar JA, Arjona-Cambranes K, Torres-Acosta JFJ (2017) Plant products and secondary metabolites with acaricide activity against ticks. Vet Parasitol 238:66–76
Roulston WJ, Schnitzerling HJ, Schuntner CA (1968) Acetylcholinesterase insensitivity in the Biarra strain of the cattle tick Boophilus microplus, as a cause of resistance to organophosphorus and carbamate acaricides. Aust J Biol Sci 21:759–768
Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad M, Rawat A, Ray D, Ghosh S (2012) Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of india. Vet Parasitol 188:337–345
Sholapur HPN, Patil BM (2013) Pharmacognostic and phytochemical investigations on the bark of Moringa oleifera Lam. Indian J Nat Prod Resour 4:96–101
Shyma KP, Gupta JP, Ghosh S, Patel K, Singh V (2014) Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (Boophilus) microplus using in vitro studies. Parasitol Res 113:1–8
Singh NK, Miller RJ, Klafke GM, Goolsby JA, Thomas DB, de Leon AAP (2018) In-vitro efficacy of a botanical acaricide and its active ingredients against larvae of susceptible and acaricide-resistant strains of Rhipicephalus (Boophilus) microplus Canestrini (Acari: Ixodidae). Ticks Tick-Borne Dis 9:201–206
Soto A, Castillo B, Delgado A, González A, Montenegro R (2001) Alkaloid screening of herbarium samples of Rubiaceae from Panama. Pharm Biol 39:161–169
Torres-Fajardo RA, Navarro-Alberto JA, Ventura-Cordero J (2019) Intake and selection of goats grazing heterogeneous vegetation: Effect of gastrointestinal nematodes and condensed tannins. Rangel Ecol Manag 72:946–953
Vij T, Prashar Y (2015) A review on medicina properties of Carica papaya Linn. Asian Pacific J Trop Dis 5:1–6
Walker AR, Bouattour Camicas J, Estrada-Peña L, Horak A, Latif IG, Pegram AA, Preston PM (2007) Ticks of domestic animals in africa: a guide to identifcation of species. Edinburgh Bioscience Reports, UK
Yusha’u M, Onuorah F, Murtala Y, (2009) In vitro sensitivity pattern of some urinary tract isolates to Carica papaya extracts. Bayero J Pure Appl Sci 2:75–78
Zaman MA, Iqbal Z, Abbas RZ, Khan MN, Muhammad G, Younus M, Ahmed S (2012) In vitro and in vivo acaricidal activity of an herbal extract. Vet Parasitol 186:431–436
Zobayer N, Hasan R (2013) Effects of manually processed bio-pesticides on crop production and pest managements in Okra (Abelmoschus esculentus (L.) Moench). J Nat Sci Res 3:112–116
Acknowledgements
We thank Cátedras-CONACyT project 1028 and project CONACYT-CB/2016-284813. JL Bravo-Ramos thanks CONACyT for scholarship 781463, Mexico. The authors would like to thank Daniel Zamudio Aguilar for generously contributing recollected tick strains to the current study, Dr. Francisco T. Barradas Piña for training in bioassays and Dr. D. Paniagua-Vega for granting the M. oleifera root powder.
Author information
Authors and Affiliations
Contributions
JLB-R wrote the manuscript. DR-S provided the biological material of Randia aculeata. DR-S, AF-P, DP-V, AC-R and MGS-O designed the project. JLBR, collected tick samples, identified tick species collected, and performed laboratory assays. MGSO, AFP performed extractions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflicts of interest among them.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bravo-Ramos, J.L., Flores-Primo, A., Paniagua-Vega, D. et al. Acaricidal activity of the hexanic and hydroethanolic extracts of three medicinal plants against southern cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Exp Appl Acarol 85, 113–129 (2021). https://doi.org/10.1007/s10493-021-00654-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00654-y