Abstract
The efficacy of Allium sativum and Cannabis sativa against Rhipicephalus microplus ticks was evaluated using the adult immersion and the larval packet test. In addition, an in silico approach was utilized by performing a docking study in order to identify the active ingredients from both plants. Results showed a comparatively high lethal effect of A. sativum and C. sativa on egg laying (index of egg laying = 0.26 and 0.24, respectively), egg hatching (33.5 and 37.1, respectively), and total larval mortality (100%, both), at 40 mg/mL. When applied to cattle which had been inoculated with larvae ticks, it was observed that a 45% solution of both herbal extracts significantly reduced the number of ticks by 96 h post treatment. We analyzed in silico 27 known active molecules from both plants and identified in the PubChem database to explore the hypothesis that the effect found on ticks was based on inhibition of acetylcholinesterase (AChE). Vitamin E and cannabidiol are the most potent AChE inhibitors with docking scores of -15.85 and -14.38, respectively. Based on these findings, we conclude that A. sativum and C. sativa may potentially be used for the control of R. microplus, and should be further investigated as a potential supplement to or replacement of synthetic acaricides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks and tick-borne diseases represent a serious economic threat to the global livestock industry. Globally, cattle ticks cause the loss of approximately 3 billion cattle hides annually, and the economic impact of ticks and tick-borne diseases has been estimated at 13.9–18.7 billion USD (Karim et al. 2017). The cattle tick Rhipicephalus (Boophilus) microplus is the principal vector of Babesia bovis, Babesia bigemina, and Anaplasma marginale, which cause babesiosis and anaplasmosis worldwide, also in Pakistan (Bhat et al. 2017).
For the last 6 decades, acaricides such as macrocyclic lactones, pyrethroids and organophosphates have been used to manage tick populations (Abbas et al. 2014). However, the indiscriminate use of these chemicals has resulted in the emergence of acaricide resistance in tick populations (Abbas et al. 2014). The detrimental effects of these acaricides on various tick species and concerns regarding contamination of meat and milk have led to increased interest in novel and less noxious methods to manage tick populations. Herbal remedies have historically been utilized to control veterinary ectoparasites, especially in developing countries (Martins et al. 2016), and the increasing demand for less toxic bio-insecticides has renewed interest in the use of these remedies. In the traditional agricultural community of Pakistan, numerous herbs are used by farmers in an effort to control ticks in dairy animals, but with a few exceptions, their efficacy has yet to be tested in a formal scientific setting (Zaman et al. 2012).
Garlic, Allium sativum (Amaryllidaceae), has been effectively used by farmers in Brazil, Canada, India and Pakistan for tick management (Lans et al. 2007; Sindhu et al. 2010; Silva et al. 2014; Jagadeeswary et al. 2014). Naturally occurring compounds in this herb with potential therapeutic uses include diallyl thiosulfinate (allicin), allyl methyl thiosulfinate, methyl allyl thiosulfinate, alliin, ajoene, diallyl disulphide, deoxyalliin, and diallyl trisulfide. Among these compounds, allicin is thought to be the most effective in controlling tick infestations (Martins et al. 2016).
Cannabis sativa (Cannabaceae) is known as hemp, marijuana, or ganja and has been used as an insect repellent. The terpenes, ketones, and ester compounds which are predominant in the leaf glands give a characteristic odour to this species which may be partially responsible for its use as an insect repellent (Bonini et al. 2018). Cannabis leaves contain numerous volatile compounds such as limonene and several pinenes, and approximately 75% of these volatile compounds have been demonstrated to be effective insect repellents (Elzinga et al. 2015). Methyl ketones occurring in C. sativa have also been demonstrated effective and are widely used to control crop pests (Andre et al. 2016). Given their demonstrated efficacy against insects and other arthropod pests of plants, it is possible that a synergistic action of these compounds may make C. sativa effective against arthropod pests of livestock as well.
Acetylcholinesterase (AChE) is a vital enzyme in the nervous system of animals including all arthropods, where it hydrolyses the neurotransmitter acetylcholine into choline and acetate. Organophosphate (OP) and carbamate acaricides, which are widely used against ticks and biting flies (Temeyer et al. 2013), work by inhibiting AChE. However, a naturally occurring mutation in invertebrates produces a variant of AChE which is not sensitive to OP pesticides, and the overuse of OP acaricides has resulted in positive selection of this mutation, which has in turn resulted in diminishing efficacy of OP in controlling ectoparasite populations (Temeyer et al. 2013). Previous studies exhibit AChE inhibition caused by essential oils and terpenoids contained in the alchoholic fraction obtained from C. sativa (Benelli et al 2018, McPartland and Sheikh 2018) and A. sativum extracts (Chaubey, 2017).
In order to shed light on which naturally occurring compounds may be used to develop novel treatments for the control of tick infections, the present research is an in silico study using computational simulations in order to explore the efficacy of plant-based anti-tick agents. The goal of this study was to evaluate the acaricidal activity of A. sativum and C. sativa extracts on the larvae, nymphs and adults of R. microplus and to identify which naturally occurring compounds serve as AChE inhibitors. The present study may help develop novel methods to prevent infection by ticks and the propagation of tick-borne diseases by identifying which compounds are detrimental to ticks using a molecular docking approach.
Materials and methods
Plant material and extract preparation
Areal plants and roots of A. sativum and C. sativa were collected from the district of Mardan, located in the Khyber Pakhtunkhwa (KPK) province of Pakistan in the area around 34° 11′ 54.6″ N and 72° 01′ 37.4″ E. The plant material was identified by a taxonomist of the Botany Department at Abdul Wali Khan University Mardan and deposited in the university’s herbarium via accession number AN-12735. Plant material was thoroughly washed and dried for 14 days at room temperature (30–40 °C) and subsequently pulverised using a plant grinder (Albrigi Inhrba). Powdered material (50 g) of each species was dissolved in 100 mL of methanol. This solution was continuously agitated for 10 days in a shaking incubator and was then filtered through muslin cloth and Whatman filter paper no. 1. To remove the methanol and obtain a semisolid extract solution, the filtrate was placed in a rotary evaporator (Re-LA100 100L rotary evaporator, Labfreez Instruments, Hunan, China). Consequently, the high viscosity solution was dried in a water bath at 45 °C and the powder yield was weighed in a balance. Each plant extract was evaluated at a range of concentrations (40, 20, 10, 5 and 2.5 mg/mL) prepared from the stock solution of 50 g of plants in 100 mL of methanol.
Ticks
Adult engorged female R. microplus (n = 300) were collected from various cattle farms in Mardan district, KPK. All samples were brought to the Parasitology laboratory, Department of Zoology, Abdul Wali Khan University Mardan, and identified by morphological characteristics following Walker et al. (2007). After identification, the ticks were divided into two groups. For adult immersion testing (AIT), one group (200 ticks) was placed in biological oxygen demand (BOD) incubators at 10 °C and the second group (100 ticks) was placed in a separate incubator to enable optimal conditions for oviposition (10% KOH, 28 ± 1 °C and 85 ± 5% RH). Larvae that emerged from the eggs (2 weeks following oviposition) were subsequently used for larval packet test (LPT).
Acaricides used for detection of resistance in ticks
The adult R. microplus collected in the field and their larvae were tested for acaricide resistance against commercial grades of cypermethrin (Ecofleece, Prix Pharmaceutica) and trichlorfon (Neguvon, Bayer). Stock solutions in methanol were prepared for each acaricide. Two-fold serial dilutions of these stock solutions were prepared in distilled water such that the following concentrations were attained for each acaricide tested: 6.25, 12.5, 25, 50 and 100 ppm. All dilutions and the control group (distilled water) were tested in triplicate with the LPT described below.
Bioassays
The following parameters were evaluated to assess the acaricidal effects of the methanol extracts of A. sativum and C. sativa: egg laying index, egg hatching rate, and larval mortality rate. The results for each plant extract were expressed as the percentage change between the control group and the group treated with the plant extract.
Adult immersion test (AIT)
To assess the acaricidal effectiveness of the two plant extracts, modified AITs were performed (Drummond et al. 1973; Sharma et al. 2012). Engorged adult female R. microplus were washed thoroughly with purified water and dried on filter paper. Two hundred ticks were randomly selected and measured per replicate. A dose–response assay was performed using 2.5–40 mg/mL concentrations of the methanolic extracts, and for each concentration, 5 ticks for group with three replicates were fully immersed in the preparation for 5 min. Similarly, the negative control group (N = 5) was immersed in distilled water for 5 min. After immersion, the ticks were retained in separate clean Petri dishes and kept in an incubator at 28 ± 1 °C and 85 ± 5% RH. Mortality in adult ticks was assessed daily for 15 days post treatment based on lack of mobility and pedal reflex following exposure to light following the methods outlined by Shyma et al. (2014).
Egg laying index (IE) was measured following the methods of Sabatini et al. (2001). Briefly, each female was weighed prior to oviposition, and the eggs laid by each female were weighed following oviposition. The average weights of the females and eggs were then used to calculate IE as: mean weight of eggs laid/mean weight of females. After being weighed, eggs were retained in glass tubes and allowed to hatch for 21 days. The percentage inhibition of oviposition (% IO) was calculated as: (IEcontrol group − IEtreated group) × 100 / IEcontrol group.
Larval packet test (LPT)
The preparations of methanolic plant extracts were also used in LPT assays to assess the acaricidal effects on tick larvae. One mL of each dilution (2.5–40 mg/mL) of each plant extract was evenly distributed on a portion of filter paper. The filter paper was air-dried and then folded to form a packet containing 100 R. microplus larvae (14 days old). These closed packets were incubated at 28 ± 1 °C and 85 ± 5% RH for 24 h. Immediately thereafter dead larvae were counted and the larval mortality rate was calculated (Luguru et al. 1984). All dilutions of both plant extract and the control group (distilled water) were tested in triplicate.
In vivo experiment for herbal extracts
In vivo experiments using the ear bag method were also conducted in order to evaluate the efficacy of C. sativa and A. sativum plant extracts (synergistic assay) on animals, as described by Ghosh et al. (2007) and Zaman et al. (2012). Briefly, three herbal extracts were prepared at 15, 35 and 45% by diluting the methanolic extracts in distilled water. Twelve tick-free calves of similar height, weight and breed (Zebu-Sahiwal) were selected from a herd, separated, and placed in tick-free sheds. The sheds were regularly maintained and checked for the presence of ticks. All sheds had walls free of crevices and were placed in fresh air for proper aeration. The ears of each animal were shaved and inoculated with around 100 unfed 14-day-old R. microplus larvae. Ear bags were then tied closed to the pinna (outer ear) in order to facilitate tick attachment. Attachment of the larvae was observed after 24 h. Following confirmation of successful attachment (with at least 40 ticks attached), herbal extract suspension was topically applied to three randomly selected animals for each dilution of each plant extract. For six consecutive days, diluted plant extracts were poured on a clean cloth which was then wiped over the infected area twice (see Kaaya et al. 1995). A control group of animals was treated with pure distilled water using the same procedure. For each animal, the ticks were counted 24 h after each treatment exposure.
Identification of inhibitor enzyme
Protein sequences of AChE were retrieved from the database of the National Center for Biotechnology Information (NCBI) (www.ncbi.nlm.nih.gov) in FASTA format. The retrieved FASTA files were then converted into 3D structures. The Phyre2 Protein Homology/Analogy Recognition Engine v.2.0 (https://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index) was used to evaluate homology between sequences. The structures of all protein sequences were modeled using the Phyre2 Engine, downloaded and saved as PDB files.
PubChem
A search was conducted using the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) to identify compounds from of A. sativum and C. sativa which potentially act as AChE inhibitors. In total, 27 compounds were identified.
Molecular docking
For each of the 27 compounds identified, structures were drawn using ChemDraw software, and the structures were saved in mol format. A molecular docking simulation was then performed for each of these compounds against AChE using Molecular Operating Environment (MOE) software. Isolated metabolites were then checked in literature, and those not present are suggested for docking. Following the docking simulation, the docked files were analyzed in MOE. Energy minimization and 3D protonation were performed using MOE, and protein–ligand interaction, 2D structures, and 3D structures analyzed in MOE were saved.
Statistical analysis
Statistical analysis was conducted using Ldp Line and GraphPad Prism (v.7) and dose mortality data were fit using a probit model (Finney 1952). The lethal concentrations LC50 and LC90 for trichlorfon and cypermethrin were estimated using the probit model. For both plant extracts, IE values were tested against the control group using one-way ANOVA (SPSS software). The dose–response mortality data were used to estimate the slope of the log-transformed dose–response curve, as well as the LC50, LC90, and their respective confidence intervals (95% CI) for each plant extract using Ldp Line software. In the in-vivo test the reduction of ticks was calculated on each animal during the period of 6 days post treatment exposure to herbal plant extract compared with negative control using distilled water. One-way ANOVA with Dunnett’s multiple comparison test was conducted to compare means among control and treated groups. For all tests α = 0.05.
Results
Acaricides
The mortality of ticks increased with increasing doses of cypermethrin and trichlorofon (Fig. 1a). LC50 and LC90 values of the two commercial acaricides were extracted from the regression equations and were shown to not differ significantly, based on overlap of the 95% confidence intervals (Table 1).
Efficacy of plant extracts
For both A. sativum and C. sativa (Table 2), IE generally decreased, and % IO and larval mortality generally increased in response to higher concentrations of the plant extracts. For all concentrations of both plant extracts, IE was significantly lower compared to the controls, whereas % IO and larval mortality were significantly higher, with 100% mortality achieved at 20 and 40 mg/mL (Table 2).
For larval mortality, estimated LC50 and LC90 values, respectively, were 2.52 and 6.95 mg/mL for A. sativum and 2.74 and 8.34 mg/mL for C. sativa (Table 3, Fig. 1b). For % IO, estimated LC50 and LC90 values, respectively, were 94 and 2510 mg/mL for A. sativum and 83 and 1532 mg/mL for C. sativa (Table 4, Fig. 2).
In vivo analysis
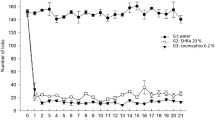
Three calves per replicate were used to detect the efficacy of herbal extract on tick infestation. There were 100 larvae confined in each bag which was attached to the ear of calves but only 40 were successful to get attached in and around the ear region. At 96 h (4 days) post exposure, the number of live ticks treated with 30 and 45% concentrations was significantly lower than in the control group, but not when treated with 5 or 15% (Fig. 3). At 144 h (6 days) post exposure, calves treated with the 15, 30 and 45% concentrations had significantly fewer live ticks compared to the control group, whereas the number of ticks at 5% concentration was not significantly lower than that of the control (Fig. 3).
Survival (no. ticks) of infested Rhipicephalus microplus on calves treated with 5–45% of methanol extracts of Allium sativum and Cannabis sativa combined or pure water (control) over time. The asterisks indicate significant differences compared to the control group at the same time point (one-way ANOVA followed by Dunnett’s multiple comparison test: P < 0.05)
In silico analysis
A selection of 27 phytochemical compounds from A. sativum and C. sativa were used as ligands and docked in MOE with active sites of AChE to test for inhibition. The most potent AChE inhibitor in A sativum was vitamin E, with a docking score of − 15.8534, whereas the most potent AChE inhibitor in C. sativa—and second-most potent AChE inhibitor overall—was cannabidiol, with a docking score of − 14.3793 (Table 5). When modeled in MOE, the amino acid in AChE to which vitamin E binds was found to be Glu255 with a bond length of 2.96 Å and energy of − 2.9 kcal/mol (Fig. 4a). Similarly, cannabidiol was found to bind to Val123 with a bond length of 2.57 Å and energy of − 2.2 kcal/mol (Fig. 4b). Of the other 25 compounds tested, all but delta-9-tetrahydrocannabivarin bound to AChE, with docking scores ranging from − 5.6624 (acenaphthol) to − 13.9115 (cannabinol) (Table 5).
Discussion
Due to poverty and other constraints, most of the farming community in rural Pakistan consists of small livestock holders. In this community, native plants and their derivatives are frequently used as treatments against parasitic infections, particularly against ticks (Zaman et al. 2012). The current study on A. sativum and C. sativa was undertaken to evaluate their efficacy as alternatives to commercial acaricides, for resistance has evolved in strains of R. microplus.
Resistance to synthetic acaricides and the need for biological agents to supplement or replace them is increasingly a global concern (Prichard and Tait 2001). The most common acaricides used in Pakistan against R. microplus are Ecofleece (Prix Pharmaceutica; active ingredient cypermethrin) and Neguvon (Bayer; a.i. trichlorfon) (Muhammad et al. 2008; Abbas et al. 2014). Despite the growing problem of acaricide-resistant ticks in Pakistan, only a handful of scientific studies have been conducted on this topic (Abbas et al. 2014). In this study, the experimentally derived LC50 values for both cypermethrin and trichlorfon were higher than the doses recommended on the label. This outcome aligns with previous studies conducted on the emergence of resistance to cypermethrin and trichlorfon in strains of R. microplus across the globe.
The in vitro and in vivo portions of this study demonstrate that the methanolic extract of A. sativum has acaricidal effects on two development stages of R. microplus. In a previous study, a 100 mg/mL methanolic extract of A. sativum produced the highest mortality rate in adult R. microplus (85%) whereas larval mean mortality was 69% (Shyma et al. 2014). Muraleedharan et al. (2008) observed that application of an aqueous extract of A. sativum caused 30% of ticks to fall from infected sheep. In addition to ticks, A. sativum is thought to negatively affect adult and larval stages of other arthropod pests, such as mosquitoes. It has also been suggested that A sativum has other antiparasitic, antifungal, anticancer and antioxidant effects (Mikaili et al. 2013). The results of our study also align with those of Shyma et al. (2014), who demonstrated the ability of A. sativum to cause larval mortality and inhibit egg hatching in R. microplus. In the current study, extracts from C. sativa caused a decrease of R. microplus larvae and inhibited egg production. For both A. sativum and C. sativa, both larval mortality and % IO increased with concentrations of methanolic extract.
To the best of our knowledge, the acaricidal effects of C. sativa have not previously been studied. However, C. sativa has been shown to be an effective agent against seven bacterial strains, as well as an effective antipsychotic agent when prescribed to patients suffering from anxiety (Basile et al. 2000; Zuardi et al. 2006; Appendino et al. 2008). The in vitro, in vivo and in silico methods used in the current study offer an interesting opportunity to investigate the acaricidal effects by combining various disciplines of assessment (Pavithra et al. 2016). The current study highlights the use of whole-plant extracts of A. sativum and C. sativa as acaricidal agents against R. microplus.
Part of the acaricidal properties of A. sativum and C. sativa may be attributed to the compounds vitamin E and cannabidiol, respectively, as the in silico analysis indicated that both are inhibitors of AChE. As such, it is likely that the cause of mortality in ticks exposed to vitamin E and cannabidiol is excessive impulse conduction caused by acetylcholine accumulation.
Although extracts of both plants are cost effective, easily manufactured, and have demonstrated potential as acaricidal agents, further research must be done before A. sativum and C. sativa extracts can be widely adopted in the field. To ensure the efficacy and safety of these plant extracts, it is essential to study their long-term stability and their sensitivity to extreme temperature and humidity differences expected in the field, as well as their possible side effects. Also the economic viability of A. sativum and C. sativa as acaricidal agents must be studied.
Conclusions
This study revealed that extracts of A. sativum and C. sativa have significant acaricidal effects on field collected resistant strains of R. microplus. A phytochemical in silico analysis identified 27 compounds contained in the alcoholic extracts of both plants, among these compounds we found that vitamin E and cannabidiol act as AChE inhibitors. As such, these plants may be used to develop new acaricidal treatments which are ecologically sustainable, cost effective, and effective against strains of R. microplus which have developed resistance to synthetic acaricides.
References
Abbas RZ, Zaman MA, Colwell DD, Gilleard J, Iqbal Z (2014) Acaricide resistance in cattle ticks and approaches to its management: the state of play. Vet Parasitol 203(1–2):6–20
Andre CM, Hausman JF, Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front Plant Sci 7:19. https://doi.org/10.3389/fpls.2016.00019
Appendino G, Gibbons S, Giana A, Pagani A, Grassi G, Stavri M, Rahman MM (2008) Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. J Nat Prod 71:1427–1430
Basile A, Sorbo S, Giordano S, Ricciardi L, Ferrara S, Montesano D, Castaldo Cobianchi R, Vuotto ML, Ferrara L (2000) Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 71(1):110–116
Benelli G, Pavela R, Lupidi G, Nabissi M, Petrelli R, Ngahang Kamte SL, Cappellacci L, Fiorini D, Sut S, Dall’Acqua S, Maggi F (2018) The crop-residue of fiber hemp cv. Futura 75: from a waste product to a source of botanical insecticides. Environ Sci Pollut Res 25:10515–10525
Bhat S, Singh N, Singh H, Rath S (2017) Molecular prevalence of Babesia bigemina in Rhipicephalus microplus ticks infesting cross-bred cattle of Punjab, India. Parasite Epidemiol Control 2:85–90
Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M, Mastinu A (2018) Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227:300–315. https://doi.org/10.1016/j.jep.2018.09.004
Chaubey MK (2017) Study of insecticidal properties of Garlic, Allium sativum (Alliaceae) and bel, Aegle marmelos (Rutaceae) essential oils against Sitophilus zeamais L. (Coleoptera: Curculionidae). J Entomol 14:191–198. https://doi.org/10.3923/je.2017.191.198
dos Santos Silva F, Albuquerque UP, Júnior LMC, da Silva LA, do Nascimento ALB, Monteiro JM, (2014) An ethnopharmacological assessment of the use of plants against parasitic diseases in humans and animals. J Ethnopharmacol 155:1332–1341
Drummond R, Ernst S, Trevino J, Gladney W, Graham O (1973) Boophilus annulatus and B. Microplus: laboratory tests of insecticides. J Econ Entomol 66:130–133
Elzinga S, Fischedick J, Podkolinski R, Raber J (2015) Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat Prod Chem Res 3:181
Finney DJ (1952) Probit analysis: a statistical treatment of the sigmoid response curve. Cambridge University Press, Cambridge, UK
Gaddaguti V, Ch SR, Bhogireddy NR, Venkat B, Rao VRT, Rao AP (2015) Pharmacognostic and preliminary phytochemical evaluation of Ocimum basilicum l. Var. Pilosum (willd.) benth. And o. Tenuiflorum var. Cim-ayu. Methods 9: 11-12
Galay RL, Umemiya-Shirafuji R, Mochizuki M, Fujisaki K, Tanaka T (2015) Iron metabolism in hard ticks (acari: Ixodidae): The antidote to their toxic diet. Parasitol Int 64:182–189
Ghosh S, Azhahianambi P, Yadav MP (2007) Upcoming and future strategies of tick control: a review. J Vec Borne Dis 44(2):79
Jagadeeswary V, Sudarshan Reddy M, Satyanarayan K (2014) Ethno-veterinary practices used by tribals of chittoor district, andhra pradesh, india. Indian J Anim Res 48(3):251–257
Jyoti J, Kour S (2015) Assessing the cultural intelligence and task performance equation: mediating role of cultural adjustment. Int J Cross Cult Manag 22:236–258. https://doi.org/10.1108/CCM-04-2013-0072
Karim S, Budachetri K, Mukherjee N, Williams J, Kausar A, Hassan MJ, Adamson S, Dowd SE, Apanskevich D, Arijo A (2017) A study of ticks and tick-borne livestock pathogens in Pakistan. Plos Neglect Trop Dis 11:e0005681
Kaaya GP, Mwangi EN, Malonza MM (1995) Acaricidal activity of Margaritaria discoidea (Euphorbiaceae) plant extracts against the ticks Rhipicephalus appendiculatus and Amblyomma variegatum (Ixodidae). Int J Acarol 21(2):123–129
Lans C, Turner N, Khan T, Brauer G, Boepple W (2007) Ethnoveterinary medicines used for ruminants in british columbia, canada. J Ethnobiol Ethnomed 3:11
Luguru SM, Banda DS, Pegram RG (1984) Susceptibility of ticks to acaricides in zambia. Trop Anim Health Prod 16:21–26
Martins N, Petropoulos S, Ferreira IC (2016) Chemical composition and bioactive compounds of garlic (Allium sativum) as affected by pre-and post-harvest conditions: A review. Food Chem 211:41–50
McCoy KD, Leger E, Dietrich M (2013) Host specialization in ticks and transmission of tick-borne diseases: a review. Front Cell Infect Mi 3:57. https://doi.org/10.3389/fcimb.2013.00057
McPartland JP, Zahra Sheikh Z (2018) A review of Cannabis sativa-based insecticides, miticides, and repellents. J Entomol Zool Stud 6(6):1288–1299
Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S (2013) Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci 16:1031–1048
Muhammad G, Naureen A, Firyal S, Saqib M (2008) Tick control strategies in dairy production medicine. Pak J Zool 28:43
Muraleedharan K, Sahadev A, Mallikarjuna G, Gopinathan N, Nagaraju S (2008) Screening of certain plants for controlling ticks in goats and sheep. Indian J Anim Res 17:44–49
Pavithra S, Preetha S, Sujatha P, Srinivasan M, Karthikeyan A, Balachandran C, Venkateswaran K, Kumarasamy P (2016) A computational approach to use the active principles in custard apple (Annona squamosa), as an ectoparasiticide by using potential target acetylcholinesterase of rhipicephalus sanguineus (brown dog tick). In: Advances in Electrical, Electronics, Information, Communication and Bio-Informatics (AEEICB), 2nd International Conference on, 2016. IEEE: 442–444
Prichard R, Tait A (2001) The role of molecular biology in veterinary parasitology. Vet Parasitol 98:169–194
Sabatini G, Kemp D, Hughes S, Nari A, Hansen J (2001) Tests to determine lc50 and discriminating doses for macrocyclic lactones against the cattle tick, boophilus microplus. Vet Parasitol 95:53–62
Sharma AK, Kumar R, Kumar S, Nagar G, Singh NK, Rawat SS, Dhakad M, Rawat A, Ray D, Ghosh S (2012) Deltamethrin and cypermethrin resistance status of Rhipicephalus (Boophilus) microplus collected from six agro-climatic regions of india. Vet Parasitol 188:337–345
Shyma K, Gupta J, Ghosh S, Patel K, Singh V (2014) Acaricidal effect of herbal extracts against cattle tick Rhipicephalus (boophilus) microplus using in vitro studies. Parasitol Res 113:1919–1926
Sindhu ZD, Zafar I, Nisar Khan M, Jonsson NN, Muhammad S (2010) Documentation of ethnoveterinary practices used for treatment of different ailments in a selected hilly area of Pakistan. Int J Agric Biol 12(3):353–358
Temeyer KB, Ap T, Brake DK, Li AY, de León AAP (2013) Acetylcholinesterases of blood-feeding flies and ticks. Chem Biol Interact 203:319–322
Walker AR, Bouattour, Camicas J, Estrada-Peña L, Horak A, Latif IG, Pegram AA, Preston, PM (2007) Ticks of domestic animals in africa: a guide to identification of species: 149–164
Zaman MA, Iqbal Z, Abbas RZ, Khan MN, Muhammad G, Younus M, Ahmed S (2012) In vitro and in vivo acaricidal activity of a herbal extract. Vet Parasitol 186:431–436
Zuardi AW, Crippa JAS, Hallak JEC, Moreira FA, Guimaraes FS (2006) Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res 34:421–429
Acknowledgments
The authors extend their appreciation to the researchers supporting Project Number (RSP-2019/56) King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
MAZ, NN designed the study. AK, SN and SA drafted the manuscript. HN and NN collected the samples and performed data analysis. NN, NN and AK provided comments while AME and NK offered final editions. All authors read and approved the final paper for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there is no conflict of interest among them.
Ethical approval
Ethical approval was provided from animal health care committee of Department of Zoology, Abdul Wali Khan University Mardan.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nasreen, N., Niaz, S., Khan, A. et al. The potential of Allium sativum and Cannabis sativa extracts for anti-tick activities against Rhipicephalus (Boophilus) microplus. Exp Appl Acarol 82, 281–294 (2020). https://doi.org/10.1007/s10493-020-00540-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00540-z