Abstract
Ticks and fleas are arthropods widely distributed around the world involved in the transmission of various vector-borne diseases (VBDs), including Brazilian Spotted Fever (BSF), Baggio-Yoshinari Syndrome and the plague, with outstanding consequences for the public health. The aim of this study was to investigate the presence of Rickettsia spp., Borrelia spp. and Yersinia pestis in arthropods collected from dogs, cats and horses living in the state of Pernambuco, Northeastern Brazil. From January 2017 to April 2019, ectoparasites were collected, morphologically identified and molecularly analysed through PCR and sequencing. In total 401 specimens were collected from 86 animals, being 68% (n = 273) and 32% (n = 128) from rural and urban areas, respectively. The most commonly detected species were the ticks Dermacentor nitens, Amblyomma sculptum, Rhipicephalus sanguineus sensu lato (s.l.), Rhipicephalus microplus, and Amblyomma ovale, and the fleas Ctenocephalides felis and Ctenocephalides canis. DNA of Rickettsia felis was detected in D. nitens collected from horses, and C. felis, and R. sanguineus s.l. collected from dogs. All samples scored negative for Borrelia spp. and Y. pestis DNA. This study provides valuable data on ectoparasite fauna from domestic animals and identifies the circulation of a zoonotic pathogen (i.e., R. felis) in the population of the arthropods assessed. Therefore, preventive measures should be adopted in order to reduce the risk of occurrence of neglected VBD caused by this pathogen in animal and human hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of emerging and re-emerging diseases affecting humans originate from zoonotic pathogens (Zanela 2016) transmitted by vectors (e.g., mosquitoes, ticks and fleas) (Ewald 1983). In fact, the involvement of blood-sucking vectors in ancient epidemics, as that caused by plague, has been speculated for a long time (Simond 1898) and currently the development of molecular techniques has opened a new chapter on the study of these diseases, revealing unprecedented information on the interaction between host, vector and parasite at the molecular level.
In Brazil the role of ticks as vectors of pathogens such as Rickettsia spp. (Moraes-Filho 2017; Aguirre et al. 2018) and the role of fleas as vectors of Yersinia pestis (Linardi and Guimarães 2000; Linardi 2017) is well documented. Accordingly, zoonoses such as the Brazilian Spotted Fever (BSF) and plague have acquired a great importance over time. BSF is a disease of public health concern caused by Rickettsia rickettsii and transmitted mainly by Amblyomma ticks (Szabó et al. 2013; Moraes-Filho 2017). Clinically, this parasitic condition is characterized by fever, joint pain and general vasculitis (Del Fiol et al. 2010). In recent years, several cases have been reported, especially from the Southeast and South regions of the country (Oliveira et al. 2016). At the same time, there is speculation that the prevalace of the infection in the Northeastern region is underestimated, the first fatal case having been documented only in 2016 (Oliveira et al. 2018). It is important to highlight the current importance of Rickettsia felis, which has been considered an emerging rickettsial pathogen and whose distribution overlaps the occurence area of Ctenocephalides felis fleas (Brown and Macaluso 2016). The spreading of this pathogen represents a threat to the human population due to the lack of host specificity for the cat flea (Pérez-Osorio et al. 2008).
On the other hand, the plague caused by Y. pestis is responsible for a severe, acute and progressing febrile illness, with significant mortality rates and clinically characterized by three clinical conditions (bubonic, pneumonic and septicemic diseases) (Brasil 2008). It is important to note that in some Brazilian regions this disease is still a threat (CDC 2019) due to the existence of two natural foci—Foco do Nordeste and Foco da Serra dos Órgãos—located in areas with specific ecological and geographical conditions (Brasil 2017). Although the last human case in Brasil occurred in 2005 (Tavares et al. 2012), the rich fauna of rodents and fleas allow the circulation of Y. pestis in these foci.
Another important disease is caused by spirochetes within the Borrelia burgdorferi complex, which are primarily transmitted by ticks of the genus Ixodes. In Brazil, this disease is known as the Baggio-Yoshinari Syndrome (BYS) and the main clinical sign observed in patients is the Erythema migrans, often associated with arthritis (Yoshinari et al. 2010; Pritt et al. 2016). Until now, only few cases have been notified (Yoshinari et al. 2007; Carranza-Tamayo et al. 2012; Rosa Neto et al. 2014), and although the clinical suspect exists since 1987 (Talhari et al. 1987) the isolation of the Borrelia species was only achieved in 2010 (Talhari et al. 2010; Santos et al. 2011).
Recently, global warming has facilitated and increased contact between humans and vectors in part due to the spreading of these arthropods or their growing abundance in endemic areas (Estrada-Peña et al. 2012). As a matter of fact, the risk of infection by vector-borne pathogens has increased worldwide (Ogden and Lindsay 2016; Semenza and Suk 2018; Sonenshine 2018; Petersen et al. 2019) and (re) emerging infections such as BSF, BYS and plague are still a real threat. Therefore, the aim of this study was to investigate the presence of Rickettsia spp., Borrelia spp. and Y. pestis in ectoparasites collected from dogs, cats and horses living in the state of Pernambuco, Northeastern Brazil.
Materials and methods
Study area and ethical aspects
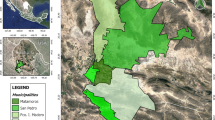
This study was conducted in various municipalities (Fig. 1) of the state of Pernambuco, Northeastern Brazil. The region is situated at a mean altitude of 842 m above sea level, with a semi-arid climate and an annual temperature mean of 22 °C (ranging from 17 to 30 °C), rainfall mean of 147 mm (ranging from 25 to 295 mm) and a relative air humidity of about 90%. It is an ecological area defined as high altitude swamp which is characterized by an oasis of humid vegetation surrounding the Caatinga. Therefore, it presents favorable natural conditions for the establishment and development of vector populations (Rodrigues et al. 2008; Santos et al. 2014). In addition, it is inserted in a natural foci (i.e., Foco do Nordeste) of risk for the occurrence of plague.
The Ethics Committee for Animal Experimentation of the ‘Universidade Federal Rural de Pernambuco’ approved all procedures herein performed (approval number: 94/2018).
Sampling and morphological identification
From January 2017 to April 2019 ectoparasites were collected from dogs, cats and horses living in urban and rural zones. Samples obtained in urban areas were from domiciled animals living inside the urban perimeters of each municipality. Conversely, those obtained in rural areas were from farms of bovine milk production, which is one of the most important economical activities of the region. Animals were selected by convenience irrespective of their sex, age or breed.
Each animal was physically examined for a period of 5 min. The presence of arthropods was assessed through the examination of the following body regions: head, ears, breast-neck, thorax, abdomen, fore and back limbs, inter-digital areas (dogs and cats), axilla, tail and inguinal area. Ectoparasites were removed with the aid of tweezers, washed in saline solution (0.9% NaCl) and placed in plastic vials containing 70% ethanol until laboratory analysis. Specimens were quantified, separated according to life stage/sex, and then identified morphologically by using dichotomous keys (Linardi and Guimarães 2000; Aragão and Fonseca 1961; Guimarães et al. 2001; Barker and Murrell 2004). All animals sampled did not use ectoparasiticide compounds over the previous 4 months before sampling.
Pools (n = 131) containing 1–3 individuals were prepared and kept at − 20 °C until molecular analysis. The segregation of pools was based on the species and life stage of ectoparasites. In addition, each pool included samples merely from a single animal.
DNA extraction, PCR amplification and sequencing
Genomic DNA was extracted from pools using a protocol previously described (Ramos et al. 2015). Each pool was tested for DNA detection of Rickettsia spp., Borrelia spp. and Y. pestis using the primers reported in Table 1. All reactions included positive and negative controls. Amplified products were revealed through electrophoresis using 1.5% agarose gel, stained with GelRed (Biotium) and viewed under an UV transilluminatior. Amplicons were purified using ExoSAP-IT (Thermo Fisher Scientific), according to manufacturer’s instructions, and sequenced in both directions by the Sanger method (Sanger et al. 1977) using an automatic sequencer ABI 3130 Genetic Analyser (Applied Biosystems). The DNA sequences identity was defined through comparison with others from the GenBank using the BLASTn search tool (Altschul et al. 1990).
Data analysis
A descriptive analysis was performed to obtain absolute and relative frequencies. In addition, the differences of species collected in rural and urban areas were analysed by using the χ2 test (α = 0.05). All analyses were carried out using the statistical software BioEstat v.5.3 (Ayres et al. 2007).
Results
In total 401 ectoparasites (male = 96; female = 259; and nymphs = 46) were collected from 86 animals (cats = 8; dogs = 22; horses = 56) during the whole study period (Table 2), 68% (n = 273) from rural areas and 32% (n = 128) from urban areas. In partircular, fleas predominated in urban areas whereas ticks were more common in rural zones (χ2 = 56.94, p < 0.0001).
Two flea species (Ctenocephalides canis and C. felis) and five tick species (Amblyomma ovale, A. sculptum, Dermacentor nitens, Rhipicephalus microplus and R. sanguineus s.l.) were identified. Table 2 summarizes the results of molecular examination according to the arthropod species. Out of all positive samples only (D. nitens from a horse) was collected from an animal from a rural area. All scored negative for Borrelia spp. and Y. pestis DNA.
The sequences derived from the amplicons obtained in PCR for Rickettsia spp. showed identity > 99% with R. felis sequences available in the GenBank. The DNA sequences obtained in the present study were deposited in the GenBank under the access numbers shown in Table 2.
Discussion
This study confirms the presence of R. felis in ectoparasites collected from dogs and horses in the study area. All species of ticks and fleas reported in this study have already been described as infesting cats, dogs and horses in tropical regions (Ehlers et al. 2019). The climatic conditions observed in these areas favour the establishment of these arthropods in vertebrate hosts (Kumsa et al. 2019). Most of the ectoparasites collected were ticks from horses, including A. sculptum, which is the vector of R. rickettsii, the etiological agent of BSF (Moraes-Filho 2017). Amblyomma ovale was collected from a dog living in an urban area. This tick species is frequently reported in wild carnivores, occasionally sharing the same environment with domestic dogs (Labruna et al. 2000). It has already been demonstrated that the proximity among animals, ectoparasites and humans may be considered a risk due to the possibility of sharing pathogens with each other (Esch and Petersen 2013).
The presence of R. felis in C. felis and R. sanguineus s.l. collected from dogs, and in D. nitens collected from horses is important due to the possibility of transmission to vertebrate hosts, including human beings (Pacheco et al. 2011; Angerami et al. 2012). The detection of R. felis DNA in these invertebrates does not confirm the vector role of these arthropods, but it indicates the circulation of this pathogen in the area of study. It is known that C. felis is recognized as the most relevant vector of R. felis due to its ability to infect progeny by transovarian transmission (Azad et al. 1992). The absence of R. rickettsi was an interesting finding. Although a fatal case of BSF has already been reported in Northeastern Brazil (Oliveira et al. 2018), this kind of infection in vertebrate hosts and arthropods in this area has been poorly investigated and data are scarse. Also, the presence of R. felis does not exclude the possibility of detection of other rickettsial organisms, rather it may suggest the predominance of this pathogen in invertebrates in the study area.
From an epidemiological perspective, the detection of this emerging vector-borne pathogen in urban areas is interesting and follows a similar trend reported in other regions of the world (Raoult et al. 2001). The disease in humans is called flea-born spotted fever and the symptoms of infection range from non-specific flu-like illness to severe multisystemic disease with generalized vasculitis (Teoh et al. 2016). In Brazil, these clinical signs are also observed in diseases caused by other rickettsial organisms (murine typhus and Q fever) and dengue, which makes diagnosis difficult (Oliveira et al. 2002). This suggests that infections caused by R. felis are underestimated, therefore their real impact on public health remains unknow.
Unfortunately, in this study animals were not investigated for detection or exposure to these pathogens. To have information about the real condition of animals it would be important to corroborate our findings. The non detection of Y. pestis and Borrelia spp. DNA does not confirm the absence of both pathogens in this area, but indicates that these invertebrates most likely have not established contact with vetebrate hosts involved in the epidemiological cycle of these organisms.
In conclusion, the data herein reported indicates the circulation of a zoonotic pathogen (i.e., R. felis) in the population of arthropods assessed. Therefore, preventive measures should be adopted in order to reduce the risk of occurrence of neglected vector-borne disease caused by this pathogen in animal and human hosts.
References
Aguirre AAR, Garcia MV, Costa IND, Csordas BG, Rodrigues VDS, Medeiros JF, Andreotti R (2018) New records of tick-associated spotted fever group Rickettsia in an Amazon savannah ecotone, Brazil. Tick Tick-Borne Dis 9(4):1038–1044. https://doi.org/10.1016/j.ttbdis.2018.03.015
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Angerami RN, Câmara M, Pacola MR, Rezende RCM, Duarte RMR, Nascimento EMM, Colombo S, Santos FCP, Leite RM, Katz G, Silva LJ (2012) Features of Brazilian spotted fever in two different endemic areas in Brazil. Tick Tick-Borne Dis 3(5–6):346–348. https://doi.org/10.1016/j.ttbdis.2012.10.010
Aragão H, Fonseca F (1961) Notas de Ixodologia. VII. Lista e chave para os representantes da fauna ixodológica brasileira. Mem Inst Oswaldo Cruz 59:15–130. https://doi.org/10.1590/S0074-02761961000200001
Ayres M, Ayres Júnior M, Ayres DL, Santos AA (2007) BIOESTAT—Aplicações estatísticas nas áreas das ciências bio-médicas. Ong Mamiraua, Belém, PA
Azad AF, Sacci JB Jr, Nelson WM, Dasch GA, Schmidtmann ET, Carl M (1992) Genetic characterization and transovarial transmission of a typhus-like rickettsia found in cat fleas. Proc Natl Acad Sci USA 89:43–46. https://doi.org/10.1073/pnas.89.1.43
Barker SC, Murrell A (2004) Systematic and evaluation of ticks with a list of valid genus and species names. J Parasitol 129:15–36. https://doi.org/10.1017/s0031182004005207
Brasil (2008) Manual de vigilância e controle da peste. Ministério da Saúde, Brasília
Brasil (2017) Guia de Vigilância em Saúde: volume 3/1ª. ed. atual. Ministério da Saúde, Brasília
Brown LD, Macaluso KR (2016) Rickettsia felis an emerging flea-borne rickettsiosis. Curr Trop Med Rep 3(2):27–39. https://doi.org/10.1007/s40475-016-0070-6
Carranza-Tamayo CO, Costa JNG, Bastos WM (2012) Lyme disease in the state of Tocantins, Brazil: report of the first cases. Braz J Infect Dis 16(6):586–589. https://doi.org/10.1016/j.bjid.2012.07.013
Centers for Disease Control and Prevetion – CDC (2019) Plague worldwide—plague cases by Country 2010–2015. https://www.cdc.gov/plague/maps/index.html Acessed 20 Apr 2020
Del Fiol FS, Junqueira FM, Rocha MCP, Toledo MI, Barberato Filho S (2010) A febre maculosa no Brasil. Rev Panam Salud Pública 27(6):461–466. https://doi.org/10.1590/S1020-49892010000600008
Ehlers J, Poppert S, Ratovonamana RY, Ganzhorn JU, Tappe D, Krüger A (2019) Ectoparasites of endemic and domestic animals in southwest Madagascar. Acta Trop 196:83–92. https://doi.org/10.1016/j.actatropica.2019.05.008
Esch KJ, Petersen CA (2013) Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin Microbiol Rev 26(1):58–85. https://doi.org/10.1128/CMR.00067-12
Estrada-Peña A, Ayllón N, De La Fuente J (2012) Impact of climate trends on tick-borne pathogen transmission. Front Physiol 3:64. https://doi.org/10.3389/fphys.2012.00064
Ewald PW (1983) Host-parasite relations vectors and the evolution of disease severity. Annu Rev Ecol Evol Syst 14:465–485. https://doi.org/10.1146/annurev.es.14.110183.002341
Guimarães JH, Tucci CE, Barros-Battesti DM (2001) Ectoparasitos de Importância Veterinária. Plêide/FAPESP, São Paulo, SP
Hinnebusch J, Schwan TG (1993) New method for plague surveillance using polymerase chain reaction to detect Yersinia pestis in fleas. J Clin Microbiol 31(6):1511–1514
Kumsa B, Abiy Y, Abunna F (2019) Ectoparasites infesting dogs and cats in Bishoftu, central Oromia. Ethiopia Vet Parasitol Reg Stud Rep 15:100263. https://doi.org/10.1016/j.vprsr.2019.100263
Labruna MB, Homem VSF, Heinemann MB, Neto JSF (2000) Ticks (Acari: Ixodidae) associated with rural dogs in Uruará, Eastern Amazon, Brazil. J Med Entomol 37:774–776. https://doi.org/10.1603/0022-2585-37.5.774
Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH (2004) Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol 42(1):90–98. https://doi.org/10.1128/JCM.42.1.90-98.2004
Linardi PM (2017) Checklist dos Sifonápteros (Insecta) do Estado do Mato Grosso. Brasil Iheringia Sér Zool 107:e2017148. https://doi.org/10.1590/1678-4766e2017148
Linardi MP, Guimarães LR (2000) Sifonápteros do Brasil. Museu de Zoologia, São Paulo, SP
Moraes-Filho J (2017) Febre maculosa brasileira. Rev Edu Cont Med Vet Zoo CRMV-SP 15:38–45
Ogden NH, Lindsay LR (2016) Effects of climate and climate change on vectors and vector-borne diseases: ticks are different. Trends Parasitol 32(8):646–656. https://doi.org/10.1016/j.pt.2016.04.015
Oliveira RP, Galvão MAM, Mafra CL, Chamone CB, Calic SB, Silva SU, Walker DH (2002) Rickettsia felis in Ctenocephalides spp. fleas, Brazil. Emerg Infect Dis 8(3):317–319. https://doi.org/10.3201/eid0803.010301
Oliveira SV, Pereira SVC, Pinna FV, Fonseca LX, Serra-Freire NM, Cardoso KM, Borsoi ABP, Amorim M, Caldas EP, Gazeta GS (2016) Vigilância de ambientes da febre maculosa: explorando as áreas silenciosas do Brasil. Rev Pan-Amazônica Saúde 7(3):65–72. https://doi.org/10.5123/S2176-62232016000300008
Oliveira SV, Costa RMF, Ferreira G, Pereira SVC, Amorim M, Monteiro MFM, Alves LC, Gazeta GS (2018) Fatal case of spotted fever in a patient from Northeastern Brazil. Rev Inst Med Trop São Paulo 60:e21. https://doi.org/10.1590/S1678-9946201860021
Pacheco RC, Moraes-Filho J, Guedes E, Silveira I, Richtzenhain LJ, Leite RC, Labruna MB (2011) Rickettsial infections of dogs, horses and ticks in Juiz de Fora, southeastern Brazil, and isolation of Rickettsia rickettsii from Rhipicephalus sanguineus ticks. Med Vet Entomol 25(2):148–155. https://doi.org/10.1111/j.1365-2915.2010.00915.x
Pérez-Osorio CE, Zavala-Velázquez JE, Arias-León JJ, Zavala-Castro JE (2008) Rickettsia felis as emergent global threat for humans. Emerg Infect Dis 7:1019–1023. https://doi.org/10.3201/eid1407.071656
Petersen LR, Beard CB, Visser SN (2019) Combatting the increasing threat of vector-borne disease in the United States with a national vector-borne disease prevention and control system. Am J Trop Med Hyg 100(2):242–245. https://doi.org/10.4269/ajtmh.18-0841
Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, Petersen JM (2016) Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infec Dis 16:556–564. https://doi.org/10.1016/S1473-3099(15)00464-8
Ramos RAN, Campbell BE, Whittle A, Lia RP, Montarsi F, Parisi A, Dantas-Torres F, Wall R, Otranto D (2015) Occurrence of Ixodiphagus hookeri (Hymenoptera: Encyrtidae) in Ixodes ricinus (Acari: Ixodidae) in Southern Italy. Tick Tick-Borne Dis 6(3):234–236. https://doi.org/10.1016/j.ttbdis.2015.01.001
Raoult D, La Scola B, Enea M, Fournier P-E, Roux V, Fenollar F, Galvão MA, de Lamballerie X (2001) A flea-associated rickettsia pathogenic for humans. Emerg Infect Dis 7:73–81. https://doi.org/10.3201/eid0701.010112
Rezende J, Lopes FA, Alves FCG, Bruno AR, Moreno SE, Costa IP (2016) Detection of Borrelia burgdorferi sensu lato in Mato Grosso do Sul. Brazil JSM Trop Med Res 1:1003
Rodrigues PCG, Chagas MGS, Silva FBR, Pimentel RMM (2008) Ecologia dos Brejos de Altitude do agreste pernambucano. Rev Geogr 25(3):20–34
Rosa Neto NS, Gauditano G, Yoshinari NH (2014) Chronic lymphomonocytic meningoencephalitis, oligoarthritis and erythema nodosum: report of Baggio-Yoshinari syndrome of long and relapsing evolution. Rev Bras Reumatol 54(2):148–151. https://doi.org/10.1016/j.rbr.2014.03.010
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74(12):5463–5467. https://doi.org/10.1073/pnas.74.12.5463
Santos M, Ribeiro-Rodrigues R, Talhari C, Ferreira LCL, ZelgerB TS (2011) Presence of Borrelia burgdorferi “Sensu Lato” in patients with morphea from the Amazonic region in Brazil. Int J Dermatol 50(11):1373–1378. https://doi.org/10.1111/j.1365-4632.2011.05081.x
Santos LS, Barros Silva HP, Pereira ECG (2014) Cerrado em área disjunta em brejo de altitude no Agreste pernambucano. Brasil Bol Goiano Geogr 34(2):337–353
Semenza JC, Suk JE (2018) Vector-borne diseases and climate change: a European perspective. FEMS Microbiol Lett 1(2):365. https://doi.org/10.1093/femsle/fnx244
Simond PL (1898) La propagation de la peste. Ann Inst Pasteur 10:626–687
Sonenshine DE (2018) Range expansion of tick disease vectors in North America: implications for spread of Tick-borne disease. Int J Environ Res Public Health 15:478. https://doi.org/10.3390/ijerph15030478
Szabó MP, Pinter A, Labruna MB (2013) Ecology, biology and distribution of spotted-fever tick vectors in Brazil. Front Cell Infec Microbiol 3:27. https://doi.org/10.3389/fcimb.2013.00027
Talhari S, Schettini APM, Parreira VJ, Cruz RG, Melo IS, Talhari C (1987) Eritema crônico migrans/Doença de Lyme—Estudo de três casos. In: XLII Congresso Brasileiro de Dermatologia, Goiânia
Talhari S, Souza Santos MN, Talhari C, Lima Ferreira LC, Silva Jr RM, Zelger B, Massone C, Ribeiro RR (2010) Borrelia burgdorferi “sensu lato” in Brazil: occurrence confirmed by immunohistochemistry and focus floating microscopy. Acta Trop 115(3):200–204. https://doi.org/10.1016/j.actatropica.2010.02.017
Tavares C, Aragão AI, Leal NC, Leal-Balbino TC, de Oliveira MBM, de Oliveira Gonçalves Ferreira GM, de Almeida AMP (2012) Plague in Brazil: from now and then. Adv Exp Med Biol 954:69–77. https://doi.org/10.1007/978-1-4614-3561-7_10
Teoh YT, Hii SF, Graves S, Rees R, Stenos J, Traub RJ (2016) Evidence of exposure to Rickettsia felis in Australian patients. One Health 2:95–98. https://doi.org/10.1016/j.onehlt.2016.06.001
Yoshinari N, Spolidorio M, Bonoldi VL, Sotto M (2007) Lyme disease like syndrome associated lymphocytoma: first case report in Brazil. Clinics 62(4):525–526. https://doi.org/10.1590/S1807-59322007000400020
Yoshinari NH, Mantovani E, Bonoldi VLN, Marangoni RG, Gauditano G (2010) Brazilian lyme-like disease or Baggio-Yoshinari syndrome: exotic and emerging Brazilian tick-borne zoonosis. Rev Assoc Med Bras 56(3):363–369. https://doi.org/10.1590/s0104-42302010000300025
Zanela JRC (2016) Zoonoses emergentes e reemergentes e sua importância para saúde e produção animal. Pesq Agropec Bras 51(5):510–519. https://doi.org/10.1590/S0100-204X2016000500011
Acknowledgements
This article is based on the Master dissertation (Postgraduate Program in Animal Bioscience), developed at the Federal Rural University of Pernambuco, supported by a grant from the Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco (FACEPE). This work integrates the universal project (420184/2016-3) entitled ‘Diversity of Ixodids and Sifonapters in company animals and its relationship with pathogens of importance in Public Health in the northeast region of Brazil’, funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ).
Author information
Authors and Affiliations
Contributions
JCPdO and RANR conceived and designed this study. Material preparation and data collection was performed by JCPdO. Data analysis was conducted by AG, GAdC and LCA. Molecular analysis was performed by JCPdO, GHR and CAdNR. JCPdO and RANR wrote the original draft and all co-authors commented and contributed intellectually on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Oliveira, J.C.P., Reckziegel, G.H., Ramos, C.A.d. et al. Detection of Rickettsia felis in ectoparasites collected from domestic animals. Exp Appl Acarol 81, 255–264 (2020). https://doi.org/10.1007/s10493-020-00505-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00505-2