Abstract
Tick blood meals are stored and digested in their midguts. Blood digestion is complex, and many proteins are involved. Study of the tick-derived proteins in the midgut content may aid in the discovery of active molecules that would be useful for anti-tick vaccines. We analyzed the midgut content proteomes of partially engorged female Haemaphysalis flava, fully engorged female H. flava, and hedgehog serum using liquid chromatography tandem-mass spectrometry and label-free quantitation. In this study, high-confidence protein profiling of tick midgut content was determined. Based on the search against our in-house transcriptome database, the 28 high-confidence proteins were identified. Of these, 17 were identified as tick-derived, and the rest were of unspecified origin (proteins that could not be differentiated as host-derived or tick-derived proteins). The function of these midgut content proteins identified here may involve nutrient transportation, anti-coagulation, erythrocyte lysis, detoxification, lipid metabolism, and immunization. The presence of hemoglobin suggested that the red blood cells were lysed in the gut lumen. The midgut contents contain a large amount of fibrinogen and it has the ability to clot immediately. The midgut contained mostly host-derived proteins, and these host proteins provide rich nutrients for tick development and reproduction. However, some intracellular proteins were also identified, suggesting the possibility of shedding of the midgut epithelium and ingestion of saliva during feeding. This finding advances our understanding of the digestive mechanism and will be useful in the screening of vaccine antigens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haemaphysalis flava (Ixodidae) is a public health pest that feeds on cattle, horses, pigs, dogs, cats, hedgehogs, pandas, wild boars, birds, and humans (Chae et al. 2017; Choi et al. 2014; Yoon et al. 1996). It carries many pathogens including thrombocytopenia syndrome virus (Jo et al. 2019), Kabuto Mountain virus (Ejiri et al. 2018), Rickettsia species (Zhang et al. 2018), tick-borne encephalitis virus, Borrelia spp., Cercopithifilaria spp. (Nematoda: Onchocercidae), Anaplasma and Bartonella spp. (Yun et al. 2016; Ishiguro et al. 2000; Uni et al. 2013; Kang et al. 2016). It has a geographic distribution in Asia that includes China, Japan, and Sri Lanka (Doi et al. 2018; Ejiri et al. 2018; Jo et al. 2019; Kang et al. 2018; Zheng et al. 2019; Chen et al. 2010).
To screen for anti-tick antigens that may help control H. flava and reduce the diseases it transmits, Xu et al. (2015, 2016a) characterized its midgut and salivary gland transcriptome next-generation sequencing technology. Liu et al. (2018a, b) studied the protein components in the midgut contents and feces of H. flava by liquid chromatography tandem-mass spectrometry (LC–MS/MS) and label-free quantitation (LFQ). Additionally, some full-length cDNAs were cloned and gene expression levels were observed (Xu et al. 2016b, 2019; Liu et al. 2016). For instance, the recombinant heat shock protein 70 (HSP70) and enolase were expressed in vitro, and their anti-coagulant activities were studied (Liu et al. 2017; He et al. 2019).
In a previous study, the peptides were used to search the public database so that some proteins may be missed and only 131 unique peptides and 102 proteins were identified, fifteen of these proteins were high-confidence proteins (unique peptides ≥ 2). Here, it is the first time that the peptides were used to search the in-house protein libraries, the midgut and salivary gland transcriptome databases of H. flava published previously by Xu et al. (2015, 2016a). The proteome of host serum was not performed in the study of Liu et al. (2018a), so that the host-derived proteins could not be excluded. The in-house transcriptome databases were utilized and the LC–MS/MS of host serum was performed to clarify their presence in the midgut contents.
Materials and methods
Ethical approval
All animal experiments were approved and overseen by Institutional Animal Care and Use Committee at HUNAU (No. 43321503). Animals were protected from unnecessary suffering throughout the experiments.
Host and tick
Six hedgehogs (Erinaceus europaeus) (600 ± 50 g) were purchased from Xinyang, Henan, China (32° 13′ N, 114° 08′ E) and kept in our laboratory at the Hunan Agricultural University (Changsha, Hunan province, China). Three hedgehogs were placed into the experiment group and three into the control group. Thirty unfed adult H. flava were placed on hedgehogs in the experimental group. Hedgehogs in the control group were not infected with ticks.
Ticks were harvested from the hedgehogs when they were partially or fully engorged according to visual inspection. We randomly chose three female ticks with similar weights (180 ± 20 mg) as the partially engorged female H. flava (PEF) group and three female ticks (350 ± 20 mg) as the fully engorged female H. flava (FEF) group.
Collection of midgut contents and the host blood sample
The PEF and FEF were dissected immediately after harvest and the midgut contents from three PEF and three FEF (about 30 μL) were carefully squeezed into three clean tubes with 500 μL 4% sodium citrate-physiological saline, respectively. Three biological replications were performed in the following experiment. The tubes were centrifuged at 5000 rpm for 15 min to remove the blood cells. A 50 μL sample of the supernatant was transferred into clean tubes. Hedgehog blood from the control group of hedgehogs was also collected, and the serum was separated by centrifugation.
Samples (50 μL) were mixed with 30 μL SDT buffer (4% sodium dodecyl sulfate, 1 mM DL-Dithiothreitol (DTT), 150 mM Tris–HCl pH 8.0) and boiled for 5 min. The undissolved debris was removed after centrifugation at 14,000×g for 15 min. Supernatant samples were quantified with a bicinchoninic acid (BCA) protein assay kit (Bio-Rad, Berkeley, CA, USA) and were visualized using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Protein digestion
Protein digestion was carried out according to the filter-aided sample preparation (FASP) procedure described by Wiśniewski et al. (2009). DL-Dithiothreitol was added to samples (100 μg) up to the final concentration at 100 mM, boiled for 5 min, and then cooled to room temperature. Thereafter, to remove detergent, DL-Dithiothreitol and other low-molecular-weight components, the 200 μL UA buffer (8 M urea, 150 mM Tris–HCl pH 8.0) was added, and then centrifuged with 10 kDa ultrafiltration centrifuge tubes at 14,000 rpm for 15 min. The filtrate was then discarded and the process was repeated. Then 100 μL UA buffer containing 50 mM iodoacetamide was added to block reduced cysteine residues. The samples were then incubated for 30 min in darkness, and then centrifuged at 14,000 rpm for 10 min. The filtrates were washed three times with 100 μL UA buffer and then three times with 100 μL 100 mM NH4HCO3. Eventually, the resultant samples were digested in trypsin buffer (4 μL Trypsin in 40 μL NH4HCO3) with incubation for 16 h at 37 °C. The peptide filtrates were collected and the peptide content was estimated crudely by UV light at 280 nm before mass spectrometry.
Mass spectrometry
Each product of enzymatic hydrolysis was desalted on C18 Cartridges (Empore™ SPE Cartridges C18; Sigma, Santa Clara, CA, USA) and concentrated using a vacuum centrifuge concentrator. Then 40 µl of 0.1% (v/v) trifluoroacetic acid was added for acidification or protonation. A total of 2 µg of concentrated peptide products were loaded onto a C18-reversed phase column (Agilent Technologies, Wilmington, DE, USA), which were balanced by the buffer A (2% acetonitrile and 0.1% formic acid). Peptides were separated on the EASY nLC100 HPLC system (Proxeon Biosystems; Thermo Fisher Scientific, Waltham, MA, USA) at a flow rate of 300 nL/min with a linear gradient of buffer B (80% acetonitrile and 0.1% formic acid) from 0 to 45% for 55 min, 45 to 100% for 2 min, and 100% for 3 min.
After separation by HPLC, a data-dependent top-10 method that used the most abundant precursor ions from the survey scan (300–1800 m/z) was dynamically chosen for HCD fragmentation and used to acquire MS data. The MS and MS/MS scan at 200 m/z was set as 17,500 and 70,000 for the survey scans acquisition, respectively. The maximum ion injection times were 10 ms for MS and 60 ms for MS/MS. Normalized collision energy for dissociating ions was 30 eV, and the isolation window was 2 m/z. Precursor ions were chosen after a dynamic exclusion duration of 40 s, and the under fill ratio was defined as 0.1%.
MS data analysis and label-free quantitation (LFQ) with MaxQuant
MaxQuant software (v.1.3.0.5) was used for the analysis and the detection of peptides. The MS data of midgut contents from PEF and FEF were searched against the protein libraries deduced from Unigenes of the midgut transcriptomes and salivary gland transcriptome of H. flava (GSE67247 and GSE69721 in NCBI GEO) (Xu et al. 2015, 2016a, b), respectively, and the MS data of host serum were searched against the NCBI database (https://www.ncbi.nlm.nih.gov/taxonomy/?term=Erinaceinae). The main search parameters included: a mass deviation filter of 6 ppm; enzyme set to trypsin; a maximum missed cleavage set at 2; and a mass tolerance of 20 ppm. The fixed modification was set as carbamidomethyl (C), and the variable modification was oxidation (M), acetyl (Protein N-term). Peptide and protein false discovery rate (FDR) was set at 0.01.
Label-free quantification was carried out in MaxQuant as the description by Luber et al. (2010). Label-free quantification intensity was obtained based on the normalized spectral protein intensity. The label-free quantity of each protein of PEF and FEF were compared, significantly different expressed proteins in abundance were observed if the ratio between PEF and FEF intensity was > 1.5 or < 0.67, and the P value was < 0.05.
Annotation of proteins and analysis of protein origin
The high-confidence peptide sequences (unique peptides ≥ 2) were searched against the Ixodoidea dataset downloaded from UniProt (190,995 protein sequences downloaded on 12/07/2018, https://www.UniProt.org), and then the same peptide sequences were grouped based on sequence alignment. The coverage of unique peptides was the method used to exclude hedgehog-derived proteins and to identify tick-derived proteins.
Results and discussion
Protein profiling with SDS-PAGE
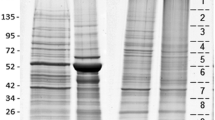
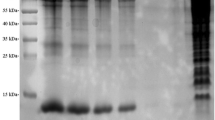
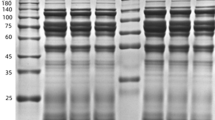
Protein concentrations of midgut contents from PEF, FEF, and the hedgehog serum were determined by the BCA method and were, respectively, 54.79 ± 4.68, 19.90 ± 1.47, and 23.56 ± 2.06 μg/μL. The protein profiling of samples was determined by SDS-PAGE (Fig. 1). Bands at 25, 38, 63, 80, 90, 140, and 170 kDa were intense and common between tick midgut contents and hedgehog serum. The band at 50 kDa was visible in PEF and FEF but not in host serum samples.
Comparison between Haemaphysalisflava midgut contents and hedgehog serum. Marker: 10 μL protein marker; partially engorged female H. flava: 10 μL midgut content extract from partially engorged female H. flava (54.79 ± 4.68 μg/μL); fully engorged female H. flava: 10 μL midgut content extract from partially engorged female H. flava with concentration (19.90 ± 1.47 μg/μL); and host serum: 10 μL hedgehog serum (23.56 ± 2.06 μg/μL)
Annotation and the assembly of peptide sequences
The 174 unique peptides and 107 peptide sequences were identified by searching against the midgut transcriptomic library of H. flava (NCBI GEO: GSE69721), and 17 peptide sequences were confirmed as high-confidence proteins (Table 1). The 191 peptides and 84 peptide sequences were identified by a search of the salivary gland transcriptomic library of H. flava (NCBI GEO: GSE67247), and 21 peptide sequences were confirmed as high-confidence proteins (Table 1). These results showed that the corresponding mRNA of midgut content proteins present in the midgut or the salivary gland because the same gene may be expressed in the different tissues. However, it is unknown that the midgut content proteins may be derived from the midgut or salivary gland. Due to the variation among different species, more proteins may be identified by searching against the libraries from the H. flava transcriptomes than universal databases such as NCBI GenBank and the UniProt database.
The high-confidence peptide sequences were annotated and the peptide sequences deduced from the same transcript were merged (Table 2). A total of 28 high-confidence proteins were identified in the tick midgut contents. Only one (peptide sequence MG-2799 (SG-5002)) was present at the phase of fully engorged female and two (peptide sequence MG-631 (SG-4096) and peptide sequence MG-9922 (SG-5824)) present in the partially engorged phase. The rest were found in both. Most of peptide sequences had a high similarity to known proteins such as peptide sequence SG-12613, which shared 100% identity with Vitellogenin 1 (Vg 1), and peptide sequence MG-41014, which was similar (97.7%) to HSP70 protein (Table 2). However, the peptide sequences of SG-228, SG-9354 and SG-10717 had low similarity with the proteins of microplusin-like anti-bacterial peptide, reeler and soluble epoxide hydrolase.
Characterization of tick-derived protein and function analysis
The comparison of peptide sequences identified in H. flava and proteins identified in hedgehogs showed that 17 proteins were tick-derived proteins such as Vgs and HSP70. However, the others like polyubiquitin and heat shock protein 90 (HSP90) could not be determined to tick-derived proteins, because these proteins are highly conserved and the identified peptides were shared by tick and host protein sequences. Some of the 17 tick-derived proteins play important roles in tick development, and they are discussed here.
Vitellogenin (Vg)
Based on the sequence alignment, there was 100% identity between the peptide sequence MG-1058 and peptide sequence SG-12613, and these two were considered to be the same protein. Likewise, the peptide sequence MG-13914 and peptide sequence SG-195 were also considered to the same protein. These two proteins were annotated to the H. flava Vg 1 (GenBank number: MH178299) and Vg 2 (GenBank number: MH178300) (100% identity) (Table 2). Vgs were expressed in the tick midgut, fat body, and ovary (Boldbaatar et al. 2010; Raikhel and Dhadialla, 1992; Khalil et al. 2011) and were then transferred into the oocytes by receptor-mediated endocytosis with the hemolymph and finally converted to Vn. Vn could sequester, transport, and store heme for embryo development (Logullo et al. 2002). However, Vgs have also played a role in heme sequestration (Thompson et al. 2007; Boldbaatar et al. 2010) and transportation of carbohydrates, lipids and other nutrients (Kunke and Nordin 1985; Dhadialla and Raikhel 1990). Vgs were classed as transport proteins in a similar proteomic study by Tirloni et al. (2014), suggesting that Vgs in this experiment may function in nutrients transportation. The Vgs identified in the midgut content did not change in abundance significantly between the two fully engorged and partially engorged phages.
Heat shock protein 70 (HSP70)
HSP70 is a family of proteins with at least eleven members found in humans (Shrestha and Young 2016). It is not known how many HSP70 members occur in ticks, but six HSP70 cDNA sequences were found in H. flava, and five of these were released by NCBI GenBank. Here, three of these were identified in the midgut contents, namely: peptide sequence MG-26577 (SG-8235), peptide sequence MG-41014 and peptide sequence MG-1176 (SG-949). Two of these HSP70 identified here were considered to be tick-derived proteins, but the derivation of the other (peptide sequence MG-1176 (SG-949)) is difficult to resolve.
The basic functions of HSP70 are involved in protein folding and refolding, preventing protein aggregation, and solubilizing proteins (Hartl and Hayer-Hartl 2002). HSP70 also has anticoagulation and immunological properties (Bolhassani and Rafati 2008). HSP70 can inhibit thrombosis without bleeding risk in mice (Allende et al. 2016, 2017). Tick HSP70 elicited fibrinogenolytic activities (Vora et al. 2017). He et al. (2019) showed that H. flava recombinant HSC70 could delay prothrombin time and thromboplastin time in vitro. Silencing of RH-Hsc70 inhibited tick blood feeding and significantly decreased the tick engorgement ratio (Wang et al. 2019). These data suggested that HSP70 may have potential value for the development of drugs or vaccines to control tick and tick-borne diseases (Vora et al. 2017; Bolhassani and Rafati 2008).
Serine protease
Red blood cells are lysed in the gut lumen, and serine protease plays an important role in this process (Sojka et al. 2013; Miyoshi et al. 2007). The recombinant serine protease from Haemaphysalis longicornis was potently able to hydrolyze the substrates Bz-(DL)-Arg-pNA and Z-Ala-Ala-Leu-pNA (Miyoshi et al. 2004a, 2007). The serine proteases mRNAs of Rhipicephalus appendiculatus were expressed in tick midgut (Mulenga et al. 2003). The peptide sequence MG-3859 (SG-13671) shared 73.5% similarity to the H. longicornis serine protease published by Miyoshi et al. (2004b), suggesting that the serine protease of H. flava midgut content may be involved in the degradation of host erythrocyte membranes.
Aldehyde dehydrogenase
The release of heme, free iron radicals, H2O2, and other stress-inducing molecules results in significant oxidative stress during blood digestion. These molecules are scavenged by enzymes, such as aldehyde dehydrogenase. Anderson et al. (2008) studied the mialome in D. variabilis midguts and speculated that aldehyde dehydrogenase might function in the detoxification of aldehydes and be involved in oxidoreductase activity.
Fatty acid-binding protein
Fatty acid-binding proteins belongs to the calycins superfamily. Fatty acid-binding protein can bind hydrophobic ligands, such as fatty acids and pigments (Bernier and Jollès, 1987; Ockner, 1990). Some members of this family are secreted and are involved in the metabolism and transportation of lipids (Bernier and Jollès, 1987; Ockner, 1990; Glatz and van der Vusse 1990). The difference of fatty acid-binding protein content between PEF and FEF was small. However, there are no studies on tick fatty acid-binding proteins and their function remains unknown.
ML domain-containing protein
Horácková et al. (2010) reported that a member of ML (MD-2-related lipid-recognition) domain protein family, which contain an MD-2-related lipid-recognition (ML) domain, was strongly induced by a blood meal. It appeared to involve a tick’s immune response because it had the ability to interact with immunoglobulin E. Herein, the peptide sequence MG-5701 annotated to the Ml domain-containing protein also contained an ML domain, implied that the protein from H. flava may be related to tick immunization. The peptide sequence MG-5701 showed a significant increase (PEF: FEF = 3.38, Table 3) in abundance in the FEF compared to the PEF.
Serine protease-like, ATP synthase, actin and metalloexopeptidase
The peptide sequence MG-291 (SG-20125) was annotated to the serine protease-like protein. However, it showed a low similarity with serine protein in amino acid levels. In addition, it is probable that the midgut content samples contained the secretome with intracellular proteins such as actin The peptide sequence MG-896 (SG-3575) shared 80% similarity with the putative metalloexopeptidase based on the alignment results online. The information related to the metalloexopeptidase was from the transcriptome (de Castro et al. 2016; Garcia et al. 2014). There is no published information on the function of ATP synthase (subunit alpha), serine protease-like protein, metalloexopeptidase.
These proteins, including heat shock protein 70, aldehyde dehydrogenase, ATP synthase and actin, are intracellular proteins. It is unknown whether these proteins were secreted into the midgut lumen or originated from the breakdown of epithelium cells.
Host proteins in Haemaphysalis flava midgut contents
The identified peptides were used to blast against the NCBI database (NCBI, Erinaceinae 29978, 13/06/2018). The 2805 peptides and 372 proteins were obtained, and 299 proteins of those were considered as high-confidence proteins. The tick midgut stores the blood meal so that the 10 proteins with the highest content in the tick midgut were host-derived, and they are listed in the Table 4.
In these host-derived proteins, the hemoglobin subunit beta, hemoglobin subunit alpha and carbonic anhydrase 2 were the main components of red blood cells, and the serum albumin, serotransferrin, apolipoprotein A-I, complement C3, alpha-2-macroglobulin-like, haptoglobin and fibrinogen alpha chain were important components of the host serum.
Hemoglobin and serum albumin
During tick feeding, the host blood was imbibed to the midgut lumen. The midgut will greatly enlarge, meanwhile, the proliferation of undifferentiated reserve cells to digestive and secretory cells. The host blood will be lysed in the midgut lumen and the components of host red blood cells will be released from host erythrocytes. Therefore, a large amount of hemoglobin, a major component of tick blood meals (Graça-Souza et al. 2006), was detected in the tick midgut contents. After the hemoglobin was released, the hemoglobin was phagocytosed by digestive cells and digested in acidic vesicles of the tick gut cells.
During tick development and reproduction, the ultimate source of amino acids used for the de novo protein synthesis is hemoglobin and serum albumin (Sojka et al. 2016). The hemoglobin and serum albumin rank highest in the midgut content proteins (Table 4).
Fibrinogen
The high level of the fibrinogen in tick midgut contents suggests that the midgut contents have the ability to clot immediately.
Serotransferrin
Laskay et al. (2013) monitored the mammalian proteins present in nymphal ticks and detected transferrin on the 29th day. Mori et al. (2014) reported that host-derived transferrin occurred in all developmental stages. Tick-derived transferrin was detected in the midgut and the ovary of adult females, but not in the eggs and the larvae, indicating that host-derived transferrin was not transferred to offspring. In addition, host-derived transferrin was located in the midgut digestive cells and the oocytes of engorged adult female ovaries, Mori et al. (2014) suggested that host-derived transferrin may be the main iron source in the ovary.
Alpha-2-macroglobulin and the complement C3
The alpha-2-macroglobulin and the complement C3 evolved from a common ancestor (Samonte et al. 2002). Both play an important role in innate immunity. The rest of the proteins including carbonic anhydrase 2, apolipoprotein A-I and haptoglobin were the serum components. It is unknown whether these proteins be digested and utilized by ticks.
Unspecified proteins (proteins that could not be differentiated as host-derived or tick-derived proteins)
The origin of polyubiquitin, EF-α, HSP90, tubulin alpha chain, tubulin beta chain, GTP-binding nuclear protein, H2B, valosin-containing protein, and GAPDH has not been determined because their peptides shared by the host and ticks. Intracellular proteins, such as polyubiquitin, EF-α, HSP90, tubulin alpha chain, tubulin beta chain, were detected in tick midgut contents, suggesting a breakdown of the midgut epithelium or the release of host red blood cells.
Compared to PEF, the content of tubulin alpha chain, tubulin beta chain, and GTP-binding nuclear protein was up-regulated and the histone H2B was down-regulated in FEF.
Quantitation of HSP70 and H2B
The HSP70 (Peptide sequence MG-26577 (SG-8235), peptide sequence MG-41014) identified as a tick-derived protein was not only detected in tick midgut contents but also in hedgehog serum. This may be because HSP70 is highly conserved. The peptide sequence MG-26577 (SG-8235) and peptide sequence MG-41014 were similar to hedgehog HSP70 (A0A1S3AMB4) and shared 75.2 and 78.1% identity. Based on the detected peptides used to identify HSP70, four (STAGDTHLGGEDFDNR, TTPSYVAFTDTER, SQIHDIVLVGGSTR, and VEIIANDQGNR) were peptides common to ticks and hedgehogs. Only IINEPTAAALAYGLDK of H. flava is different with IINEPTAAAIAYGLDK of hedgehogs by a single amino acid. As the leucine and the isoleucine had the same molecular weight, the peptides matched to HSP70 were not specific. Therefore, the HSP70 was detected in host serum (Table 3).
Although the quantitative results showed that histone H2B was detected only in tick midgut contents. However, the evidence that the HAVSEGTK and LLLPGELAK identified in histone H2B were in the sequence of both the H. flava H2B protein and also in hedgehogs (UniProt accession number: A0A1S3WR78) cannot determine whether histone H2B was a tick-derived protein. Histone H2B was detected in tick midgut contents but not in host serum, probably because H2B is released extracellularly when blood cells are broken, or neutrophil extracellular traps are formed (Yang et al. 2016).
Conclusion
In-house protein libraries, inferred from the midgut and salivary gland transcriptomes of H. flava, were used in conjunction with the proteome to identify the midgut content proteins and to observe mRNA expression of midgut content proteins in midgut tissue and salivary gland tissue. The host serum was used as control group for excluding host protein interference with identification of the midgut content proteins. The profiles of high-confidence proteins of the midgut content were discovered. However, the midgut contents of H. flava were more complex than anticipated, and had a high percentage of host-derived proteins. These proteins present in the midgut content, such as Vgs, HSP70, serine protease, are associated with diverse biological functions in ticks including the transportation, anticoagulation, degradation of host erythrocyte membranes, detoxification, lipid metabolism and immunization. For example, the HSP70 involved as an anticoagulant. Vgs may play a role in transportation and the serine protease might be related to erythrolysis. These proteins play an important role in the digestion of tick blood meals.
Hemoglobin and carbonic anhydrase 2 released from the red blood cells lysed in gut lumen are basal elements of red blood cells (Sojka et al. 2013). A high content of host-derived proteins was detected in the midgut contents and these host proteins were mainly used as nutrients for tick development and oviposition. The study of tick midgut contents may help illuminate the mechanism of tick digestion and to aid in the screening for anti-tick antigens.
In the midgut contents, many highly conserved proteins were identified and the origin of these proteins could not be differentiated. These proteins can be intracellular and even nuclear and the shedding of the midgut epithelium and the ingestion of saliva may contribute to the midgut contents. Surprisingly, some proteins involved in the anti-haemostatic, anti-inflammatory and immunomodulatory functions such as serpin and lipocalin (Chmelar et al. 2011; Prevot et al. 2006; Sangamnatdej et al. 2002), which play an important role in tick saliva anti-haemostatic and anti-inflammatory activity (Radulović et al. 2014; Kazimírová and Štibrániová 2013), were not found in this study.
References
Allende M, Molina E, Guruceaga E, Tamayo I, González-Porras JR, Gonzalez-López TJ, Toledo E, Rabal O, Ugarte A, Roldán V, Rivera J, Oyarzabal J, Montes R, Hermida J (2016) Hsp70 protects from stroke in atrial fibrillation patients by preventing thrombosis without increased bleeding risk. Cardiovasc Res 110(3):309–318
Allende M, Molina E, Lecumberri R, Sanchez-Arias JA, Ugarte A, Guruceaga E, Oyarzabal J, Hermida J (2017) Inducing heat shock protein 70 expression provides a robust antithrombotic effect with minimal bleeding risk. Thromb Haemost 117(9):1722–1729
Anderson JM, Sonenshine DE, Valenzuela JG (2008) Exploring the mialome of ticks: an annotated catalogue of midgut transcripts from the hard tick, Dermacentor variabilis (Acari: Ixodidae). BMC Genomics 9:552
Bernier I, Jollès P (1987) (1987) A survey on cytosolic non-enzymic proteins involved in the metabolism of lipophilic compounds: from organic anion binders to new protein families. Biochimie 69(11–12):1127–1152
Boldbaatar D, Umemiya-Shirafuji R, Liao M, Tanaka T, Xuan X, Fujisaki K (2010) Multiple vitellogenins from the Haemaphysalis longicornis tick are crucial for ovarian development. J Insect Physiol 56(11):1587–1598
Bolhassani A, Rafati S (2008) Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccines 7(8):1185–1199
Chae JB, Kang JG, Kim HC, Chong ST, Lee IY, Shin NS, Chae JS (2017) Identification of tick species collected from wild boars and habitats of wild boars and domestic pigs in the Republic of Korea. Korean J Parasitol 55(2):185–191
Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J (2010) Ticks (acari: ixodoidea: argasidae, ixodidae) of China. Exp Appl Acarol 51(4):393–404
Chmelar J, Oliveira CJ, Rezacova P, Francischetti IMB, Kovarova Z, Pejler G, Kopacek P, Ribeiro JMC, Mares M, Kopecky J, Kotsyfakis M (2011) A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood 117(2):736–744
Choi CY, Kang CW, Kim EM, Lee S, Moon KH, Oh MR, Yamauchi T, Yun YM (2014) Ticks collected from migratory birds, including a new record of Haemaphysalis formosensis, on Jeju Island. Korea. Exp Appl Acarol 62(4):557–566
de Castro MH, de Klerk D, Pienaar R, Latif AA, Rees DJ, Mans BJ (2016) De novo assembly and annotation of the salivary gland transcriptome of Rhipicephalus appendiculatus male and female ticks during blood feeding. Ticks Tick Borne Dis 7(4):536–548
Dhadialla TS, Raikhel AS (1990) Biosynthesis of mosquito vitellogenin. J Biol Chem 265:9924–9933
Doi K, Kato T, Hayama SI (2018) Infestation of introduced raccoons (Procyon lotor) with indigenous ixodid ticks on the Miura Peninsula, Kanagawa Prefecture. Japan. Int J Parasitol Parasites Wildl 7(3):355–359
Ejiri H, Lim CK, Isawa H, Yamaguchi Y, Fujita R, Takayama-Ito M, Kuwata R, Kobayashi D, Horiya M, Posadas-Herrera G, Iizuka-Shiota I, Kakiuchi S, Katayama Y, Hayashi T, Sasaki T, Kobayashi M, Morikawa S, Maeda K, Mizutani T, Kaku K, Saijo M, Sawabe K (2018) Isolation and characterization of Kabuto Mountain virus, a new tick-borne phlebovirus from Haemaphysalis flava ticks in Japan. Virus Res 244:252–261
Garcia GR, Gardinassi LG, Ribeiro JM, Anatriello E, Ferreira BR, Moreira HN, Mafra C, Martins MM, Szabó MP, de Miranda-Santos IK, Maruyama SR (2014) The sialotranscriptome of Amblyomma triste, Amblyomma parvum and Amblyomma cajennense ticks, uncovered by 454-based RNA-seq. Parasit Vectors 7:430
Glatz JF, van der Vusse GJ (1990) Cellular fatty acid-binding proteins: current concepts and future directions. Mol Cell Biochem 98(1–2):237–251
Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol 36(4):322–335
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295(5561):1852–1858
He XM, Liu L, Cheng TY (2019) HSC70 from Haemaphysalis flava (Acari: Ixodidae) exerts anticoagulation activity in vitro. Ticks Tick Borne Dis 10(1):170–175
Horácková J, Rudenko N, Golovchenko M, Grubhoffer L (2010) Der-p2 (Dermatophagoides pteronyssinus) allergen-like protein from the hard tick Ixodes ricinus – a novel member of ML (MD-2-related lipid-recognition) domain protein family. Parasitology 137(7):1139–1149
Ishiguro F, Takada N, Masuzawa T, Fukui T (2000) Prevalence of Lyme disease Borrelia spp in ticks from migratory birds on the Japanese mainland. Appl Environ Microbiol 66(3):982–986
Jo YS, Kang JG, Chae JB, Cho YK, Shin JH, Jheong WH, Chae JS (2019) Prevalence of severe fever with thrombocytopenia syndrome virus in ticks collected from national parks in Korea. Vector Borne Zoonotic Dis 19(4):284–289
Kang JG, Ko S, Kim HC, Chong ST, Klein TA, Chae JB, Jo YS, Choi KS, Yu DH, Park BK, Park J, Chae JS (2016) Prevalence of Anaplasma and Bartonella spp. in ticks collected from Korean water deer (Hydropotes inermis argyropus). Korean J Parasitol 54(1):87–91
Kang JG, Chae JB, Cho YK, Jo YS, Shin NS, Lee H, Choi KS, Yu DH, Park J, Park BK, Chae JS (2018) Molecular detection of Anaplasma, Bartonella, and Borrelia theileri in Raccoon dogs (Nyctereutes procyonoides) in Korea. Am J Trop Med Hyg 98(4):1061–1068
Kazimírová M, Štibrániová I (2013) Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2013.00043
Khalil SM, Donohue KV, Thompson DM, Jeffers LA, Ananthapadmanaban U, Sonenshine DE, Mitchell RD, Roe RM (2011) Full-length sequence, regulation and developmental studies of a second vitellogenin gene from the American dog tick, Dermacentor variabilis. J Insect Physiol 57(3):400–408
Kunke JG, Nordin JH (1985) Yolk proteins. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon, New York, pp 83–111
Laskay UA, Breci L, Vilcins IM, Dietrich G, Barbour AG, Piesman J, Wysocki VH (2013) Survival of host blood proteins in Ixodes scapularis (Acari: Ixodidae) ticks: a time course study. J Med Entomol 50(6):1282–1290
Liu L, Cheng TY, He XM (2018a) Proteomic profiling of the midgut contents of Haemaphysalis flava. Ticks Tick Borne Dis 9(3):490–495
Liu L, Cheng TY, Yan F (2016) Expression pattern of subA in different tissues and blood-feeding status in Haemaphysalis flava. Exp Appl Acarol 70(4):511–522
Liu L, Cheng T-Y, Yang Y (2017) Cloning and expression pattern of a heat shock cognate protein 70 gene in ticks (Haemaphysalis flava). Parasitol Res 116(6):1695–1703
Liu L, Liu YS, Liu GH, Cheng TY (2018b) Proteomics analysis of faecal proteins in the tick Haemaphysalis flava. Parasit Vectors 11(1):89
Logullo C, Moraes J, Dansa-Petretski M, Vaz IS, Masuda A, Sorgine MH, Braz GR, Masuda H, Oliveira PL (2002) Binding and storage of heme by vitellin from the cattle tick, Boophilus microplus. Insect Biochem Mol Biol 32(12):1805–1811
Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, Akira S, Wiegand M, Hochrein H, O'Keeffe M, Mann M (2010) Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 32(2):279–289
Miyoshi T, Tsuji N, Islam KM, Kamio T, Fujisaki K (2004a) Enzymatic characterization of a cubilin-related serine proteinase from the hard tick Haemaphysalis longicornis. J Vet Med Sci 66(10):1195–1198
Miyoshi T, Tsuji N, Islam MK, Kamio T, Fujisaki K (2004b) Cloning and molecular characterization of a cubilin-related serine proteinase from the hard tick Haemaphysalis longicornis. Insect Biochem Mol Biol 34(8):799–808
Miyoshi T, Tsuji N, Islam MK, Huang X, Motobu M, Alim MA, Fujisaki K (2007) Molecular and reverse genetic characterization of serine proteinase-induced hemolysis in the midgut of the ixodid tick Haemaphysalis longicornis. J Insect Physiol 53(2):195–203
Mori H, Galay RL, Maeda H, Matsuo T, Umemiya-Shirafuji R, Mochizuki M, Fujisaki K, Tanaka T (2014) Host-derived transferrin is maintained and transferred from midgut to ovary in Haemaphysalis longicornis ticks. Ticks Tick Borne Dis 5(2):121–126
Mulenga A, Misao O, Sugimoto C (2003) Three serine proteinases from midguts of the hard tick Rhipicephalus appendiculatus; cDNA cloning and preliminary characterization. Exp Appl Acarol 29(1–2):151–164
Ockner RK (1990) Historic overview of studies on fatty acid-binding proteins. Mol Cell Biochem 98(1–2):3–9
Prevot P-P, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E (2006) Anti-hemostatic effects of a serpin from the saliva of the tick. J Biol Chem 281(36):26361–26369
Radulović ŽM, Kim TK, Porter LM, Sze S-H, Lewis L, Mulenga A (2014) A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC Genomics 15(1):518
Raikhel AS, Dhadialla TS (1992) Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 37:217–251
Samonte IE, Sato A, Mayer WE, Shintani S, Klein J (2002) Linkage relationships of genes coding for alpha2-macroglobulin, C3 and C4 in the zebrafish: implications for the evolution of the complement and Mhc systems. Scand J Immunol 56(4):344–352
Sangamnatdej S, Paesen GC, Slovak M, Nuttall PA (2002) A high affinity serotonin- and histamine-binding lipocalin from tick saliva. Insect Mol Biol 11(1):79–86
Shrestha L, Young JC (2016) Function and chemotypes of human Hsp70 chaperones. Curr Top Med Chem 16(25):2812–2828
Sojka D, Franta Z, Horn M, Caffrey CR, Mareš M, Kopáček P (2013) New insights into the machinery of blood digestion by ticks. Trends Parasitol 29(6):276–285
Sojka D, Pytelková J, Perner J, Horn M, Konvičková J, Schrenková J, Mareš M, Kopáček P (2016) Multienzyme degradation of host serum albumin in ticks. Ticks Tick Borne Dis 7(4):604–613
Thompson DM, Khalil SM, Jeffers LA, Sonenshine DE, Mitchell RD, Osgood CJ, Michael RR (2007) Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem Mol Biol 37(4):363–374
Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates JR 3rd, Termignoni C, Pinto AF, Vaz Ida Jr S (2014) Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS ONE 9(4):e94831
Uni S, Bain O, Fujita H, Matsubayashi M, Fukuda M, Takaoka H (2013) Infective larvae of Cercopithifilaria spp (Nematoda: Onchocercidae) from hard ticks (Ixodidae) recovered from the Japanese serow (Bovidae). Parasite 20:1
Vora A, Taank V, Dutta SM, Anderson JF, Fish D, Sonenshine DE, Catravas JD, Sultana H, Neelakanta G (2017) Ticks elicit variable fibrinogenolytic activities upon feeding on hosts with different immune backgrounds. Sci Rep 7:44593
Wang F, Gong H, Zhang H, Zhou Y, Cao J, Zhou J (2019) Molecular characterization, tissue-specific expression, and RNA knockdown of the putative heat shock cognate 70 protein from Rhipicephalus haemaphysaloides. Parasitol Res 118(5):1363–1370
Wiśniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362
Xu XL, Cheng TY, Yang H, Yan F, Yang Y (2015) De novo sequencing, assembly and analysis of salivary gland transcriptome of Haemaphysalis flava and identification of sialoprotein genes. Infect Genet Evol 32:135–142
Xu XL, Cheng TY, Yang H, Liao ZH (2016a) De novo assembly and analysis of midgut transcriptome of Haemaphysalis flava and identification of genes involved in blood digestion, feeding and defending from pathogens. Infect Genet Evol 38:62–72
Xu XL, Cheng TY, Yang H (2016b) Enolase, a plasminogen receptor isolated from salivary gland transcriptome of the ixodid tick Haemaphysalis flava. Parasitol Res 115(5):1955–1964
Xu L, Liu L, Cheng T-Y (2019) Cloning and expression profile of glyceraldehyde-3-phosphate dehydrogenase in (Acari: Ixodidae). J Med Entomol 56(2):569–575
Yang H, Biermann MH, Brauner JM, Liu Y, Zhao Y, Herrmann M (2016) New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Front Immunol 7:302
Yoon SY, Kim KH, Suhr KB, Cho BK, Nam IH, Lee WK, Lee JH, Park JK (1996) A case of tick bite caused by Haemaphysalis flava. Korean J Dermatol 34(2):326–330
Yun SM, Lee YJ, Choi W, Kim HC, Chong ST, Chang KS, Coburn JM, Klein TA, Lee WJ (2016) Molecular detection of severe fever with thrombocytopenia syndrome and tick-borne encephalitis viruses in ixodid ticks collected from vegetation, Republic of Korea, 2014. Ticks Tick Borne Dis 7(5):970–978
Zhang X, Geng J, Du J, Wang Y, Qian W, Zheng A, Zou Z (2018) Molecular identification of Rickettsia species in Haemaphysalis ticks collected from Southwest China. Vector Borne Zoonotic Dis 18(12):663–668
Zheng W, Xuan X, Fu R, Tao H, Xu R, Liu Y, Liu X, Jiang J, Wu H, Ma H, Sun Y, Chen H (2019) Preliminary investigation of ixodid ticks in Jiangxi Province of Eastern China. Exp Appl Acarol 77(1):93–104
Acknowledgments
This work was financially supported by the Double First–class Construction Project of Hunan Agricultural University (SYL201802016) and the Natural Science Foundation of Hunan Province, China (2018JJ2167). We thank LetPub (www.letpub.com) for linguistic assistance during manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and Hunan Agricultural University Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10493_2019_457_MOESM7_ESM.xlsx
Table. S7. Quantitative analysis of tick midgut content proteins by blasting Haemaphysalis flava midgut transcriptome (XLSX 36 kb)
10493_2019_457_MOESM8_ESM.xlsx
Table. S8. Quantitative analysis of tick midgut content proteins by blasting Haemaphysalis flava salivary gland transcriptome (XLSX 35 kb)
Rights and permissions
About this article
Cite this article
Feng, LL., Cheng, TY. A survey of proteins in midgut contents of the tick, Haemaphysalis flava, by proteome and transcriptome analysis. Exp Appl Acarol 80, 269–287 (2020). https://doi.org/10.1007/s10493-019-00457-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-019-00457-2