Abstract
We determined how conidia of arthropod-pathogenic fungi on leaves affected the behavior of two predators—Orius majusculus (Hemiptera: Anthocoridae) and Phytoseiulus persimilis (Acari: Phytoseiidae)—when offered a choice between preying on two-spotted spider mites, Tetranychus urticae (Acari: Tetranychidae), in the presence or absence of infective conidia of Metarhizium brunneum (Ascomycota: Hypocreales) and Neozygites floridana (Entomophthoromycota: Neozygitaceae). The results indicate no significant relation between the presence of conidia and predator behavior. The only indication of interference is between the generalists O. majusculus and M. brunneum, with a trend towards more time spent feeding and more prey encounters turning into feeding events on leaf discs without conidia than on leaf discs with conidia. Our results show that the presence of fungal conidia does not alter the preying behavior of the predators, and using predators and fungi together is not limited by any interference between organisms in the short term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arthropod predators and arthropod-pathogenic fungi are important natural enemies of pests used in biological pest control (Hajek and Eilenberg 2018). Arthropod predators encounter arthropod-pathogenic fungi while foraging on plants for prey (Roy and Pell 2000) or when searching for mates (Trandem et al., 2015). Such fungi can affect predators directly through infection or indirectly by competition for prey (Roy and Pell 2000) or by reducing prey quality (Seiedy et al. 2012). Predator behavior can possibly be affected by the perceived threat from a fungus present as conidia on infected, dead target arthropods or as conidia on leaves.
Detailed studies on behavioral effects in systems combining one fungus species and one predator species have shown interesting results. The two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), infected with its specialist biotrophic fungus Neozygites floridana (Entomophthoromycota: Neozygitaceae), can induce behavioral responses in predators (Trandem et al. 2016; Wekesa et al. 2007). This is also possible of prey infected with generalist fungal species, where avoidance often has been reported (Alma et al. 2010; Meyling and Pell 2006; Roy et al. 1998; Wu et al. 2016). Seiedy et al. (2012) showed that prey handling time in the Tetranychid specialist predator Phytoseiulus persimilis (Acari: Phytoseiidae) (McMurtry and Croft 1997) increased, while feeding rate decreased when the predator was presented with their target prey, T. urticae, infected with mesotrophic generalist entomopathogenic fungus Beauveria bassiana (Ascomycota: Hypocreales). The generalist predator Orius albidipennis (Hemiptera: Anthocoridae) responded in a similar way to Thrips tabaci (Thysanoptera: Thripidae) infected with the mesotrophic generalist entomopathogenic fungus Metarhizium anisopliae sensu lato (Ascomycota: Hypocreales); their searching time increased and their feeding time decreased (Pourian et al. 2011). The outcome of predator-fungus interactions in a more natural environment may be significant for successful biological control. In the field, Fischhoff et al. (2017) and Rauch et al. (2017) documented that the mesotrophic generalist Metarhizium brunneum (Ascomycota: Hypocreales) (Boomsma et al. 2014) aimed for pest control, did not reduce non-target arthropod abundance and diversity significantly. Actually, the interactions between a predator and a fungus may even prove beneficial for biological control attempts. A study by Azevedo et al. (2017) revealed that the combined use of M. brunneum and the specialist predatory gall midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae) positively influenced aphid control compared to when either natural enemy was used alone. Though the combined use significantly reduced the number of predatory midges, the same treatment still suppressed the aphid population more than either control agent used alone.
Entomopathogenic fungi and arthropod predators are now often combined to control a complex of pests in a crop. It is therefore important to understand whether their biology and behavior will have a synergistic, antagonistic or no effect on each other. We therefore performed a comparative study on fungus-induced changes in predator behavior and chose the entomopathogenic fungi M. brunneum and N. floridana that belong to different orders of arthropod pathogenic fungi and are very different in their biology (Boomsma et al. 2014). Metarhizium brunneum is a mesotrophic fungus in the Hypocreales that produces large quantities of small, dry conidia in long chains, which are passively detached from dead hosts and can be readily suspended in water. It can be grown on artificial media (sabouraud dextrose agar (SDA), rice etc.), is produced commercially in large quantities, and is used as the active ingredient in several microbial control products and may be used against T. urticae. Neozygites floridana is a biotrophic fungus in the Entomophthoromycota and a specialist of T. urticae. It actively discharges larger non-infective primary conidia. These primary conidia then produce secondary sticky infective conidia on long capillary tubes, so called capilliconidia (Keller 1997). It only takes one capilliconidium to kill a spider mite (Oduor et al. 1995), and one Tetranychus cadaver may throw more than 2000 primary conidia (Wekesa et al. 2010), which germinate into infective secondary capilliconidia. Neozygites floridana is an important natural enemy of T. urticae and may be produced in vivo on T. urticae but not yet for commercial use, though there has been some success of in vitro production by Leite et al. (2000) and an in vivo production patent was also made several years ago (Kennedy and Smitley 1988). We chose to expose these two very different fungal species to the specialist spider-mite predator P. persimilis and the generalist predator Orius majusculus (Hemiptera: Anthocoridae) (Fathipour and Maleknia 2016) to evaluate their behavioral changes in searching and time feeding on prey when presented with the following leaf disc choice combinations: (1) M. brunneum conidia vs. no conidia, (2) N. floridana conidia vs. no conidia. As target prey for predators we used the pest mite T. urticae.
Materials and methods
Fungi, plants and arthropods
Colonies of T. urticae were obtained from a laboratory culture kept on strawberry plants in a plexiglass cage, in a climatically controlled room at 21 °C, 60% RH and 16 h L: 8 h D. The predatory bugs, O. majusculus, were provided by the company EWH Bioproduction (Denmark) in bottles containing 500 individuals of all stages mixed with buckwheat. The predatory mites, P. persimilis, were provided by LOG (Norway) and by EWH Bioproduction (Denmark) in 100 mL bottles containing 2000 adult mites mixed with vermiculite.
The in vitro culture of M. brunneum, isolate KVL 99-112 (i.e. F52, BIPESCO 5) was grown on Sabouraud Dextrose Agar (SDA) at room temperature in darkness for approximately 25 days before harvesting the conidia for the experiments.
The in vivo culture of N. floridana, Brazilian isolate ESALQ 1420, was produced as described in Castro et al. (2013). Similar leaf disc methods have also been used by Oduor et al. (1995). The following procedure was used: adult female T. urticae were inoculated with conidia of N. floridana on bean plants (Phaseolus vulgaris cv. Masai). After 8–9 days, N. floridana infected T. urticae had died and dry non-sporulating cadavers were collected, wrapped in a cotton cloth and stored in Eppendorf tubes at 5 °C until used in the experiment within 30 days.
Preparation of leaf discs with fungal spores
Leaf discs were made from strawberry leaves from the same plant for each observation day. Due to the differences in biology between M. brunneum, and N. floridana, the preparation of leaf discs with fungal conidia that was used in the choice experiment was conducted in two ways.
For M. brunneum, leaf discs were inoculated by dipping them in a conidial suspension. This was done as follows: the M. brunneum isolate was taken out of the freezer and transferred to sabouraud dextrose agar (SDA), cultured for 19–25 days at ambient laboratory conditions (21–25 °C; 20–35% RH) placed in a plastic box (22 × 16 × 7 cm) and wrapped with aluminum foil for darkness. Conidia of M. brunneum from SDA were then harvested with a sterile spatula, in sterile water with 0.05% Tween 80 to suspend the hydrophilic fungal conidia in water. The suspension was filtered through a 3-layer cotton cloth and adjusted to 1 × 107 conidia/mL with a Neubauer Improved hemocytometer. Strawberry leaf discs (diameter 15 mm) were then dipped in the M. brunneum conidial suspension before being air-dried on a tissue paper with the abaxial side up. Leaf discs with conidia were placed in Petri dishes with water agar (1.5%) at 6 °C overnight to be used in choice experiments the next day. Conidial viability of conidia suspensions were established by a standard germination test (Inglis et al. 2012), and only suspensions with > 95% germination were used in the experiment.
Since N. floridana is a biotrophic fungus, it is difficult to produce conidia from other substrates than the host (T. urticae) itself. Therefore, three N. floridana-killed T. urticae cadavers, dorsal side up, were evenly distributed onto one strawberry leaf disc and placed in darkness for 24 h at 20 °C and 90% RH for primary conidia to discharge and germinate to form infective capilliconidia (secondary conidia). For each leaf disc, sporulation of all cadavers and an even distribution of conidia were assured by observing each leaf disc under a compound microscope (X80) prior to the observations. Spore-producing cadavers were carefully removed to obtain similar conditions as for leaf discs with M. brunneum, i.e. presence of conidia only, before the introduction of healthy T. urticae (see below) and the predator species. All leaf discs, including those receiving no spores, were dipped in 0.05% Tween 80 as described above to correct for possible effects of Tween 80.
Experimental set-up of choice experiment
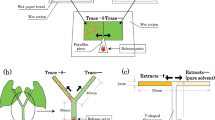
The experimental set-up is shown in Fig. 1. One strawberry leaf disc with fungal conidia and one strawberry leaf disc without fungal conidia were placed with a small gap between them, onto 1.5% water agar in a Petri dish (diameter 5 cm). The leaf discs were connected with a bridge of Parafilm (10 × 10 mm) as described by Asalf et al. (2011). Six young adult female T. urticae (for O. majusculus) or deutonymphs (for P. persimilis) were transferred to each leaf disc approximately 1 h before a predator was introduced. During the observation time, T. urticae remained on the leaf discs where they had been released, no webbing was observed, and only on rare occasions did they lay eggs. In such cases, eggs were removed before the introduction of the predator. The set up of the choice experiments was as follows: O. majusculus (4th or 5th nymphal stage) or an adult female P. persimilis were offered a choice between a leaf disc with (1) M. brunneum conidia vs. no conidia, or (2) N. floridana vs. no conidia. Petri dishes with two leaf discs without any fungal conidia served as the control. All predators were starved individually in plastic vials, with moist filter paper in a climate cabinet at 23 °C, 16 h L: 8 h D and 70% RH for 24 h prior to the start of the experiment.
Observation of behavior
Each predator was placed in the middle of the Parafilm bridge, allowing it to choose between the two leaf discs. The observation time per treatment was 15 min, starting immediately after the predator was released on the bridge. The following five behaviors were recorded: (1) walking (searching), (2) encountering prey (number of events, when prey was within a body length of the predator and the latter reacted to the presence of the prey), (3) feeding, (4) resting, (5) grooming. If feeding continued after the observation time of 15 min, observation continued until feeding stopped to obtain total feeding time per prey. Furthermore, the number of prey encounters was used to assess the success rate. Searching, resting and grooming time was recorded as it affects predation, especially if conidia attach to the body and legs of the predators.
Observations were made under an even light source. All treatments were replicated three times a day, between 9 am and 4 pm, with the sequence of treatments rotated between observation days (n = 9). Each observation was conducted in a new Petri dish with new leaf discs and a new predator. The position of the treated leaf disc (left/right of predator) was randomized. Observations with no feeding events were discarded and the experiment was continued until at least 20 replicates were obtained for each treatment. An average of four observations per day were discarded, mainly due to predator inactivity or it was disturbed by the water barrier surrounding the leaf discs.
Statistical analysis
Three response variables were analyzed separately for the two predators. (1) Number of prey encounter was analyzed with a Poisson regression with log-link including the logarithm of time spent searching as offset in order to correct for searching time. (2) Success of prey encounter turning into a feeding event was analyzed in a binomial regression with logit-link. (3) Feeding time per prey was analyzed in a normal regression after log-transformation. All analyses were done with conidia (none/N. floridana/M. brunneum) as fixed effect, and with arthropod id as random effect to correct for arthropods that searched on both leaves. If an overall effect of conidia was found, pairwise comparisons of the three levels (none/N. floridana/M. brunneum) were done with a Tukey correction for multiple testing. The statistical analyses were done in R v.3.2.2 (R Core Team 2015).
Results
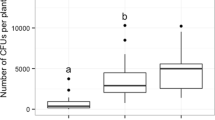
Both predators spent the majority of the observation time feeding, followed by time spent searching (Table 1). Little time was invested in resting (0.1–1.5% of total observation time) and grooming (0.1–1.1% of total observation time), and these behaviors were therefore not considered further.
For both predators no significant relations were found between the presence of conidia and number of prey encountered, the proportion of prey encounters resulting in a feeding event, and time spent feeding per prey. However, for O. majusculus, there was an almost significant effect of the presence of conidia on the success rate of the predators (p = 0.10), and time spent feeding per prey (p = 0.06). The odds ratio for a successful feeding event was 3.5 times larger (95% CI 0.9–13.2) on leaf discs with no conidia relative to leaf discs with M. brunneum (adjusted p = 0.07). The feeding time per prey was 1.8 times longer (95% CI 1.0–3.2) on leaf discs without conidia compared to leaf discs with M. brunneum (adjusted p = 0.06). As expected, no differences were found between control treatments.
Discussion
The presence of entomopathogenic fungal conidia did not significantly affect the behavior of either predator species, either directly or indirectly, through changes in behavior of the prey. As described above, the conidia of the generalist fungus M. brunneum and the specialist fungus N. floridana both have the potential to influence predator behavior in different ways due to their very different biological characteristics.
The primary conidium of N. floridana germinates into an infective sticky capilliconidium on a long capillary that will rise 60–100 µm above the leaf surface (Keller 1997; Trandem et al. 2015). Capilliconidia easily break off and can attach to the body and legs of host and non-host arthropods (Delalibera et al. 2003). Specialist fungi cannot infect the predators and do therefore not pose a threat to them as such. This being said, we considered the physical presence of N. floridana conidia as likely to disturb the preying behavior of the predator, but this showed not to be the case. A longer observation time would perhaps reveal an interference between the specialist fungal conidia and the predators, as found by Wekesa et al. (2007).
Metarhizium brunneum produces smaller conidia (length 5.0–7.0 µm; Bischoff et al. 2009), and can induce avoidance responses, as other generalist entomopathogens do, by being perceived as a threat by predators that encounter them (Alma et al. 2010; Meyling and Pell 2006; Ormond et al. 2011). Previous studies with generalist entomopathogenic fungi have shown behavioral changes in predators (Pourian et al. 2011; Seiedy et al. 2012), but unlike in the present study, previous studies have been conducted with inoculated prey. Infected prey are likely producing a different volatile profile, ultimately increasing the likelihood of an altered predator response. Both situations are relevant and important for the understanding of the interactions between natural enemies, and both must be considered when developing strategies for pest control.
The lack of significant differences found in behavior in response to fungal conidia can also have been caused by low conidial concentrations. Because of distinct differences in life styles of the two fungal species, it was necessary to utilize two methods of applying fungal inoculum. The presence of fungal inoculum was established with agar imprints and visual observations with a microscope throughout the experiment, while the specific concentrations on the leaf discs were not known and not comparable between species.
There was a trend towards a significant effect of M. brunneum conidia on the behavior of O. majusculus. This predator spent more time feeding and had more prey encounters turning into feeding events on leaf discs without conidia than on leaf discs with M. brunneum, i.e. where there was no risk for the predator to engage in these behaviors. This trend needs confirmation with longer observation times. If confirmed, this would support the findings from other studies (Alma et al. 2010; Meyling and Pell 2006; Ormond et al. 2011).
Except for N. floridana, the organisms used in the present study are commonly used separately in augmentative biological control against various pests (Eilenberg et al. 2001; Gacheri et al. 2015; Gerson and Weintraub 2007; van Lenteren 2012). The outcome of such augmentative releases would be affected by the interaction between the released organisms. This study shows that the combined use of these natural enemies of taxonomically remote groups in augmentative releases will not interfere with each other, at least not initially. Thus, our initial expectation that the presence of entomopathogenic fungal conidia would alter the preying behavior of predators was not confirmed. Nevertheless, inoculated prey and risk of infection of predators should be investigated further as there may be long-term negative or positive effects on pest control.
References
Alma CR, Gillespie DR, Roitberg BD, Goettel MS (2010) Threat of infection and threat-avoidance behavior in the predator Dicyphus hesperus feeding on whitefly nymphs infected with an entomopathogen. J Insect Behav 23:90–99
Asalf B, Stensvand A, Trandem N, Klingen I (2011) Effect of powdery mildew on the interaction between two-spotted spider mite and a predatory mite in strawberry. Acta Hortic 70:101–105
Azevedo AGC, Steinwender BM, Eilenberg J, Sigsgaard L (2017) Interactions among the predatory midge Aphidoletes aphidimyza (Diptera: Cecidomyiidae), the fungal pathogen Metarhizium brunneum (Acomycota: Hypocreales), and maize-infesting aphids in greenhouse mesocosms. Insects 8:44
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Boomsma JJ, Jensen AB, Meyling NV, Eilenberg J (2014) Evolutionary interaction networks of insect pathogenic fungi. Annu Rev Entomol 59:467–485
Castro TR, Wekesa VW, Moral RA, Demétrio CGB, Delalibera I, Klingen I (2013) The effects of photoperiod and light intensity on the sporulation of Brazilian and Norwegian isolates of Neozygites floridana. J Invertebr Pathol 114:230–233
Delalibera I, Hajek AE, Humber RA (2003) Use of cell culture media for cultivation of the mite pathogenic fungi Neozygites tanajoae and Neozygites floridana. J Invertebr Pathol 84:119–127
Eilenberg J, Hajek A, Lomer C (2001) Suggestions for unifying the terminology in biological control. Biocontrol 46:387–400
Fathipour Y, Maleknia B (2016) Mite predators. In: Omkar (ed) Ecofriendly pest management for food security. Academic Press, Elsevier, Cambridge, pp 329–366
Fischhoff IR, Keesing F, Ostfeld RS (2017) The tick biocontrol agent Metarhizium brunneum (= M. anisopliae) (strain F52) does not reduce non-target arthropods. PLoS ONE 12:11
Gacheri C, Kigen T, Sigsgaard L (2015) Hot-spot application of biocontrol agents to replace pesticides in large scale commercial rose farms in Kenya. Biocontrol 60:795–803
Gerson U, Weintraub PG (2007) Mites for the control of pests in protected cultivation. Pest Manage Sci 63:658–676
Hajek AE, Eilenberg J (2018) Natural enemies. An introduction to biological control, 2nd edn. Cambridge University Press, Cambridge, p 439
Inglis GD, Enkerli J, Goettel MS (2012) Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA (ed) Manual of techniques in invertebrate pathology. Academic Press, New York, pp 189–253
Keller S (1997) The genus Neozygites (Zygomycetes: Entomophthorales) with special reference to species found in tropical regions. Sydowia 49:118–146
Kennedy GG, Smitley DR (1988) Method of controlling plant feeding mites with the fungus Neozygites floridana. U.S. Patent No 4,752,468, 21 June 1988
Leite LG, Smith L, Moraes GJ, Roberts DW (2000) In vitro production of hyphal bodies of the mite pathogenic fungus Neozygites floridana. Mycologia 92:201–207
McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42:291–321
Meyling NV, Pell JK (2006) Detection and avoidance of an entomopathogenic fungus by a generalist insect predator. Ecol Entomol 31:162–171
Oduor GI, Moraes GJ, Yaninek JS, van der Geest LPS (1995) Effect of temperature, humidity and photoperiod on mortality of Mononychellus tanajoa (Acari: Tetranychidae) infected by Neozygites cf. floridana (Zygomycetes: Entomophthorales). Exp Appl Acarol 19:571–579
Ormond EL, Thomas APM, Pell JK, Freeman SN, Roy HE (2011) Avoidance of a generalist entomopathogenic fungus by the ladybird, Coccinella septempunctata. FEMS Microbiol Ecol 77:229–237
Pourian H-R, Talaei-Hassanloui R, Kosari AA, Ashouri A (2011) Effects of Metarhizium anisopliae on searching, feeding and predation by Orius albidipennis (Hem., Anthocoridae) on Thrips tabaci (Thy., Thripidae) larvae. Biocontrol Sci Technol 21:15–21
R Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 2 Oct 2019
Rauch H, Steinwender BM, Mayhofer J, Sigsgaard L, Eilenberg J, Enkerli J, Zelger R, Strasser H (2017) Field efficacy of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae), Metarhizium brunneum (Hypocrales: Clavicipitaceae), and chemical insecticide combinations for Diabrotica virgifera larval management. Biol Control 107:1–7
Roy HE, Pell JK (2000) Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci Technol 10:737–752
Roy HE, Pell JK, Clark SJ, Alderson PG (1998) Implications of predator foraging on aphid pathogen dynamics. J Invertebr Pathol 71:236–247
Seiedy M, Saboori A, Allahyari H (2012) Interactions of two natural enemies of Tetranychus urticae, the fungal entomopathogen Beauveria bassiana and the predatory mite, Phytoseiulus persimilis. Biocontrol Sci Technol 22:873–882
Trandem T, Bhattarai UR, Westrum K, Knudsen GK, Klingen I (2015) Fatal attraction: male spider mites prefer females killed by the mite-pathogenic fungus Neozygites floridana. J Invertebr Pathol 128:6–13
Trandem N, Berdinesen R, Pell JK, Klingen I (2016) Interactions between natural enemies: effect of a predatory mite on transmission of the fungus Neozygites floridana in two-spotted spider mite populations. J Invertebr Pathol 134:35–37
van Lenteren JC (2012) The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 57:1–20
Wekesa VW, Moraes GJ, Knapp M, Delalibera I Jr (2007) Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulus longipes (Acari: Phytoseiidae). Biol Control 41:408–414
Wekesa VW, Moraes GJ, Ortega EMM, Delaibera I Jr (2010) Effect of temperature on sporulation of Neozygites floridana isolates from different climates and their virulence against the tomato red spider mite, Tetranychus evansi. J Invertebr Pathol 103:36–42
Wu S, Gao Y, Smagghe G, Xu X, Lei Z (2016) Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol Control 98:43–51
Acknowledgements
We thank Karin Westrum (NIBIO) and Natasha Iwanicki (University of São Paulo, ESALQ) for assistance throughout the experiment. Professor Italo Delalibera Jr. and his group at ESALQ-USP are acknowledged for providing the N. floridana isolate ESALQ 1420 used in this study. We also thank the anonymous reviewers and associate editor of the journal for devoting their time into improving the manuscript. This study is part of the research project IMBICONT (Improved Biological Control for IPM in Fruits and Berries) (Project Number 1024151001) funded by the Innovation Fund, Denmark, and also supported by the project BERRYSYS (project number 190407/110) funded by the Norwegian Foundation for Research Levy on Agricultural Products (FFL) and the Agricultural Agreement Research Funds (JA) and the project SMARTCROP (Project Number 244626) funded by The Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacobsen, S.K., Klingen, I., Eilenberg, J. et al. Entomopathogenic fungal conidia marginally affect the behavior of the predators Orius majusculus (Hemiptera: Anthocoridae) and Phytoseiulus persimilis (Acari: Phytoseiidae) foraging for healthy Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 79, 299–307 (2019). https://doi.org/10.1007/s10493-019-00441-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-019-00441-w