Abstract
Essential oils of Ocimum basilicum (L.), Achillea fragrantissima (Forssk.) and Achillea santolina (L.) were obtained by hydrodistillation and analyzed using gas chromatography (GC) and GC/mass spectrometry (MS). Oil-in-water nanoemulsions (10% active ingredient) were prepared through a high-energy (ultrasonication) emulsification process. Nanoemulsions were characterized by viscosity, pH, thermodynamic stability, droplet size, polydispersity index (PDI) and scanning electron microscopy (SEM) measurements. The plant oils and their nanoemulsions showed considerable acaricidal activity against the mold mite, Tyrophagus putrescentiae (Schrank) (Sarcoptiformes: Acaridae). In a contact toxicity bioassay and 48 h post treatment, O. basilicum oil was the most toxic, followed by A. fragrantissima and A. santolina, where LC50 values were 8.4, 14.1 and 21.8 µl/cm2, respectively. LC50 for benzyl benzoate, a standard acaricide was 9.8 µl/cm2. Upon fumigation, responses also varied according to the test oil. Based on the 48-h LC50 values, the same manner of activity was also observed, where O. basilicum was the most toxic followed by A. fragrantissima and A. santolina. When prepared as nanoemulsions (particle size from 78.5 to 104.6) and tested as fumigants, toxicity of the oils was increased drastically with LC50 values of 2.2, 4.7, and 9.6 µl/l air for O. basilicum, A. fragrantissima and A. santolina, respectively. The oils showed a moderate to strong residual acaricidal activity, where O. basilicum oil was the most effective. The results suggest that appropriate nanoemulsions containing the tested oils can be developed to control T. putrescentiae after the required toxicological assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the storage ecosystem, mites play an important role in the deterioration process and are intimately associated with agricultural production. One of the various acarines associated with the stored grain ecosystem is the mold mite, Tyrophagus putrescentiae (Schrank) (Sarcoptiformes: Acaridae). It is a cosmopolitan post-harvest pest of durable stored foods with a high fat and protein content, such as dry cured ham, grains, aged cheese, spices, mushroom, dried fruit, dried eggs, nuts, and other stored foods (Hughes 1976; Brazis et al. 2008). In addition to destroying food, there is evidence that T. putrescentiae may be a source of allergens affecting farmers and workers handling heavily infested stored products, and causes acute enteritis, dermatitis (Mueller et al. 2005), and systemic anaphylaxis (Matsumoto et al. 1996) when contaminated food is ingested. T. putrescentiae also acts as a carrier of bacteria and toxigenic fungi such as Aspergillus spp. and Penicillium spp. in stored grain kept under warm and moist conditions (Franzolin et al. 1999). Mold mites, including T. putrescentiae, may be found at several points along the food storage, processing, and distribution system, including raw grain through to finished food products. Once a mite outbreak has been detected, it can be difficult to fully control the infestation (Gill et al. 2011).
Tyrophagus putrescentiae is one of the most difficult pests to control in the dry environment because of its morphological, ecological, physiological and behavioral characteristics (Boczek 1991). However, control of this mite depends heavily on the use of chemical methods such as fumigation, spraying with organophosphorus compounds, or treatment with benzyl benzoate because ecological control methods with high humidity and temperature cause alterations in food quality (Hagstrum et al. 2012). The repeated use of chemical acaricides, however, has resulted in the development of resistant strains of mites, showed undesirable effects on non-target organisms, and has fostered environmental and human health concerns (Park et al. 2014). These problems have highlighted the need for the development of new strategies for selective storage mite control using biorational tools, which do not affect human health as well as the organoleptic characters of food products. Natural plant products may provide ecofriendly alternatives to currently used pest control agents (Nenaah and Ibrahim 2011).
In the literature, much effort has been focused on the plant essential oils as potential sources of commercial storage mite control agents, especially against T. putrescentiae (Macchioni et al. 2002; Kim et al. 2003a, b, 2004; Lee et al. 2006; Sung et al. 2006; Park et al. 2014; Song et al. 2016). Plant-derived essential oils, in general, are considered minimum-risk pesticides and are exempt from Environmental Protection Agency registration under Sect. 25 (b) of the Federal Insecticide Fungicide and Rodenticide Act. However, the performance of most phytochemicals, including plant oils in pest control protocols, is inadequate for major practical use due to concerns associated with extraction, formulation and application (Nenaah 2014c; Nenaah et al. 2015). The urgent need now is the search for innovative strategies for developing and strengthening the use of plant-based products as alternative pest control agents (Almadiy et al. 2018).
In this context, nanotechnology has emerged as a promising area for developing and utilizing nano-sized particles with a wide range of uses in material science, medicine, pharmacology and pesticide applications (Khot et al. 2012). When transformed into nanoparticles, materials could acquire novel biological properties, as they offer large specific surface area and hence increased affinity to the target, penetrate rapidly and are selectively accumulated in biosystems enhancing various activities within the living cells (Weiss et al. 2006). The current study was designed to evaluate the chemical composition and the acaricidal activity of the essential oils of three plant species namely, Ocimum basilicum, Achillea fragrantissima, and A. santolina and their nanoemulsions against the mold mite, T. putrescentiae.
Materials and methods
Test mites
A stock culture of T. putrescentiae was established from infested samples of dry stored grains obtained from a governmental drying store in Kafr Elsheikh Governorate, Egypt. Mites were identified according to the key described by Robertson (1959). Mites were maintained in the laboratory for 2 years without exposure to any pesticidal contamination. Mites were reared in plastic containers (15 × 10.5 × 6 cm) containing 30 g of sterilized diet (fry feed No. 1: dried yeast, 1: 1 (wt/wt); Korea Special Feed Meal, Inchon, Korea), each covered with a round plastic plate with a 3-cm-diameter hole in the center, which was sealed with a filter paper disk for ventilation. The rearing cages were kept at 25 ± 2 °C and 70 ± 5% r.h. in darkness. Adults were sexed by observing their secondary sexual characters (Hughes 1976).
Collection and preparation of the test plants
The aerial parts of A. fragrantissima and A. santolina were collected from Sinai Peninsula and Allamain desert, respectively, whereas O. basillicum were collected from local gardens at Gharbiya Governorate, Egypt, at the flowering period (May 2017). Plant samples were identified and authenticated by botanists of the Botany Department, Faculty of Science, Tanta University, Egypt, where voucher specimens were deposited for further reference (voucher numbers are Ob 01, Af 01 and As 01). The fresh plant samples were air-dried in the shade for 5 days at environmental temperature (28–32 °C daytime). The dried parts were powdered mechanically using an electric blender (Multiquick Immersion Hand Blender, B White Mixer MR 5550 CA, Braun, Germany), then sieved through a mesh size of 0.5 mm. The resulting fine powders were maintained in tightly closed dry bags until used for the extraction of the essential oils.
Extraction of essential oils
Powdered samples, 1 kg each, from the tested plants were hydrodistilled using a modified Clevenger-type apparatus to produce the plant oils. The extraction condition was as follows: 50 g powders, 500 ml distilled water, and 6 h distillation. Anhydrous sodium sulphate was utilized to remove water after extraction. The oil yield (% wt/wt) was calculated on a dry weight basis. The extracted oils were stored in a refrigerator at 4 °C until analyzed and tested.
Preparation of oil nanoemulsions
Oil-in-water nanoemulsion from the test plants was prepared by using the high-energy ultrasonic method (Badawy et al. 2017). The nanoemulsions were prepared in two phases. Firstly, EOs were emulsified using a high energy ultrasonic process, then mixed with surfactant (Tween 80) as a non-ionic surfactant and deionized water at a ratio of 1:2:7, respectively, with a final concentration of 10% yielding coarse emulsion formulations. Emulsions were prepared by dropping organic phase containing oil to water phase (water and surfactant) using a magnetic stirrer for 30 min at 4000 rpm. The emulsions formed were then subjected to ultrasonic emulsification for 15 min with sonication power of 10 kHz (9 cycle/sec) controlled by the software of the device (Ultrasonic Homogenizers HD 2070) with HF generator (GM 2070), ultrasonic converter UW2070, booster horn (SH 213 G) and probe microtip MS 73, Ø 3 mm). The difference of temperature from the initial coarse emulsion to the final emulsion was not more than 25 °C (Anjali et al. 2012).
Characterization of the oil nanoemulsions
Physicochemical characterization and stability of the prepared nanoemulsions were evaluated to different stress, such as thermodynamics, centrifugation, heating, cooling, and freeze cycles (Ghosh et al. 2013). Nanoemulsions were centrifuged at 5000 rpm for 30 min at 25 °C using Heraeus Labofuge 400R (Kendro Laboratory Products, Germany) and observed for phase separation, creaming, and cracking if any. This method has been widely used to determine the stability of nanoemulsions (Golemanov et al. 2006). Then, successful formulations that did not show any phase separation were subjected to the heating–cooling test, which contains six cycles between refrigerator temperatures at 4 °C for 48 h and 40 °C for 48 h. Then, formulations that did not show any phase separation were taken for the freeze–thaw stress test. For this purpose, nanoemulsions were kept alternatively at two temperatures (− 21 and 21 °C) for each temperature test of at least 24 h. Finally, the stable formulation nanoemulsions were stored for about 8 weeks at room temperature in closed tubes for additional observation like phase separation or creaming. The pH values of the stable nanoemulsions were measured at 25 ± 0.1 °C using an Adwa (AD 8000) pH meter. The dynamic (absolute) viscosity (μ) of the nanoemulsions was measured by a Rotary Myr VR 3000 digital viscometer with L4 spindle at 200 rpm at 25 °C without further dilution. Each reading was taken after the equilibrium of the sample for 2 min. All experiments are carried out in triplicates.
Droplet size and polydispersity index
The particle size distribution, mean particle diameter (Z-averages), and polydispersity index (PDI) of the samples were determined using dynamic light scattering (DLS) at 25 °C on a Zetasizer Nano-ZS90 (Malvern Instruments, Malvern, UK). The samples were diluted before measurement to 10% with deionized water to avoid multiple scattering effects. From the DLS data, the average particle size (z-diameter), which was determined by cumulate analysis of the intensity–intensity autocorrelation function (Stepanek 1993), and the PDI, which gives an indication of the width of the droplet size distribution, were determined. The analysis was performed at a scattering angle of 90°, and each recorded measurement was an average of three replicates.
Scanning electron microscopy (SEM)
Morphology of the oil nanoemulsions was investigated by SEM (JEOL, Model JFC-1600, Tokyo, Japan). A drop of each oil nanoemulsion (25 μL) was diluted with deionized water, transferred into a carbon-coated copper grid, then stained by phosphotungstic acid solution (2%, pH = 6.7) for 1 min. The replica was lifted to drying at room temperature (28 °C), and then, the image was visualized with SEM at 80-kV accelerating voltages.
Gas chromatography–mass spectrometry
Gas chromatography–mass spectrometry (GC–MS) was carried out using an Agilent 6890 GC equipped with a 5973 N mass selective detector and an HP-5 (5% phenyl methylpolysiloxane) capillary column. The temperature of the oven was programmed from 50 to 280 °C at a rate of 4 °C/min and held at this temperature for 5 min. The temperatures of the inlet and interface were 250 and 280 °C, respectively. Helium, at a flow rate of 1.0 ml/min (constant flow), was used as the carrier gas. The sample (0.2 ml) was injected using a split of 20:1. Electron impact mass spectrometry was performed at 70 eV. The temperatures for the ion source and quadrupole were maintained at 230 and 150 °C, respectively. Compounds were identified by comparing their retention indices and mass spectra with those found in the NIST 98.1 and Mass Finder 3.1 commercial libraries. The integration area of the chromatographer was used to calculate the concentration of each component of the analyzed oils.
Contact acaricidal activity
An impregnated fabric disc bioassay was used for measuring the acaricidal activity of test plant oils and against T. putrescentiae (Kim et al. 2004). Test solutions of the plant oils were prepared by dissolving 3.2, 1.6, 0.8, 0.4, 0.2 and 0.1 ml of each material in 5 ml acetone. Each test concentration was applied uniformly to a black cotton fabric piece filter paper disc (6 cm diameter pieces, 28.3 cm2) using a Hamilton micro-applicator (Bonaduz, Switzerland) to obtain gradient concentrations of 113.2, 56.6, 28.3, 14.2, 7.1 and 3.5 µl/cm2. Control fabric discs received 5 ml acetone. After drying in a fume hood for 1 min, each disc was placed in the bottom of a Petri dish (6 cm diameter). Groups of 25 unsexed adults (7–10 days old) were placed separately in each Petri dish and covered with a lid. Treated and control mites were kept in the dark at 25 ± 2 °C and 70 ± 5% r.h. All treatments were set up in four replicates along with control. Benzyle benzoate, the conventional acaricide was used for comparison. Mortality was determined 48 h post treatment under a binocular microscope (20 ×), thereafter the end-point mortality was reached. The contact toxicity was expressed as μl/cm2. Adults were considered dead if their appendages did not move when they were prodded with a pin.
Fumigant acaricidal activity
The susceptibility of T. putrescentiae adults to the fumigant action of the plant oils and nanoemulsions was investigated according to Kwon and Ahn (2002). A fabric cotton piece (6.0 cm diameter) was impregnated with 25 µl of six graded concentrations of each oil or nanoemulsion to obtain equivalent fumigant concentrations of 45.85, 22.9, 11.5 5.7, 2.9 and 1.45 μl/l air or acetone only (control). After evaporating the solvent in a fume hood, the cotton piece was attached to the undersurface of the screw cap of a glass vial (54 ml volume), thus preventing direct contact of test adults with the test oils. Mites were transferred to the vials in groups of 25 unsexed (7–10 days old) adults. The vials were covered with fine steel gauze secured with adhesive tape. Treated and control mites were kept in the dark at 25 ± 2 °C and 70 ± 5% r.h. All treatments were set up in four replicates along with control. Mortality was determined 48 h post treatment under a binocular microscope (20 ×), thereafter the end-point mortality was reached. The fumigant toxicity was expressed as μl/l. Adults were considered dead if their appendages did not move when they were prodded with a pin.
Persistence activity assessment
This experiment was done in order to determine how long acaricidal activity of the plant oils was retained over the time. Each oil was diluted in acetone and admixed with sterilized crushed wheat grains in 1 l glass jars, at a concentration equal to the LC95 fraction of each oil in the contact toxicity bioassay. Jars containing the treated media were hand-shacked to ensure complete mixing. After evaporation of the solvent, the treated media were packed in tightly closed jute sacks (30 × 30 cm) and stored at darkness under the same laboratory conditions described before. Samples, each of 20 g of the stored food were withdrawn at various intervals (10, 20, 30, 40, 50, and 60 days) and offered 25 adults of T. putrescentiae (7–10 days old) in a Petri-dish (9 cm diameter). Control sets were made, where the same number of mites were offered food treated with acetone. Each experiment was replicated four times and mortality was recorded and corrected for mortality in control using Abbott’s formula (Abbott 1925).
Data analysis
Mortality data were recorded and corrected for that in the control using Abbott’s formula (Abbott’s 1925). The dose-mortality response was analyzed by probit analysis (SAS 1990) then the LC50 and LC95 values and their fiducial limits were estimated. Lethal concentrations at the 50% and slope levels were considered significantly different if their associated confidence intervals did not overlap. Significance of mean differences between treatments and control were compared using ANOVA (α = 0.05) followed by individual pairwise comparisons with Tukey’s honestly significant differences (HSD) test using SPSS v.15.0 software. LT50’s and their fiducial limits were estimated.
Results
Chemical composition of the plant oils
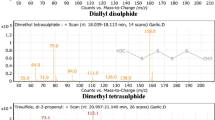
Data in Table 1 show the amount yielded and the chemical composition of each oil. A total of 30 compounds constituting 98.7% were identified in O. basilicum oil. The major components were methyl eugenol (71.3%), α-cubebene (6.4%), and linalool (4.1%). It is indicated that cis-thujone (24.9%), 3,3,6-trimethyl-1,5-heptadien-4-one (Artemisia ketone) (19.8%), 2,5-dimethyl-3-vinyl-4-hexen-2-ol (Santolina alcohol) (14.3%), and trans-thujone (13.5%) were the major constituents of A. fragrantissima oil. For A. santolina, fragranyl acetate (26.1%), 1,6-dimethyl-1,5 cyclooctadiene (12.6%), 1,8 cineole (11.8%), and cis-thujone (9.4%) were the major components.
Formation and physicochemical characteristics of the oil nanoemulsions
The experimental design and the optimum conditions for the preparation of nanoemulsions containing EOs from O. basillicum, A. fragrantissima and A. santolina are shown in Table 2. Oil in water nanoemulsions were prepared in the proportions as follows: the active ingredient (10%), water phase (70%), surfactant (20%, lipophilic emulsifier), sonication time (15 min), sonication cycle (five cycles per second), and sonication power (10 kHz). The results of nanoemulsions preparation also include the viscosity (mPa.s) and the pH values. Nanoemulsions were exposed to extreme storage conditions to evaluate their stability during storage. The results of the thermodynamic characterization studies (centrifugation at 5000 rpm, temperature stability at 25 °C, heating–cooling cycle at 4–40 °C, and freezing cycle at − 4 °C of nanoemulsions are also presented in Table 2. All of the prepared oil nanoemulsions exhibited thermodynamic stability during the extreme storage conditions.
Visual appearance and particle size
The appearance of nanoemulsions was visualized before and after the sonication process by the naked eye. The nanoemulsion formulations were transparent after sonication. The particle size and PDI of the prepared nanoemulsions are presented in Table 2 and illustrated in Fig. 1. The data indicated that the droplet size varied from 78.5 to 104.6 nm with PDI values ranged between 0.18 and 0.26. Measurements showed that the polydispersity indices obtained from O. basilicum and A. fragrantissima nanoemulsions were lower than that obtained from A. santolina. This result proved that all these liquid formulations were successful in their preparation in the nanometric size range. SEM study showed that the obtained nanoemulsions consisted of dispersed spherical shape nanoparticles with different particle sizes (Fig. 2). On average, the transparent nanoemulsions obtained from O. basilicum and A. fragrantissima samples had smaller particles (78.5 and 91.3 nm, respectively) than the translucent nanoemulsion obtained from A. santolina (104.6 nm).
Acaricidal activity
The contact toxicity bioassay of the test oils against adults of T. putrescentiae is recorded in Table 3. The widely used benzyl benzoate served as a standard control for comparison. Based on 48 h LC50 values, O. basilicum was the most toxic, followed by A. fragrantissima and A. santolina, where LC50’s were 8.4, 14.1, and 21.8 µl/cm2, respectively. LC50 for benzyl benzoate was 9.8 µl/cm2. In this case, the oil of O. basilicum was more toxic than the standard acaricide, benzyl benzoate. Upon fumigation, response of T. putrescentiae adults to the tested oils also varied according to the test oil and the formulation used (Table 4). Based on the 48-h LC50 values, the same manner of activity was also observed, where O. basilicum was the most toxic with LC50 = 6.7 µl/l air, followed by A. fragrantissima and A. santolina. When prepared as nanoemulsions and tested as fumigants, toxicity of the plant oils was increased drastically with LC50 values of 2.2, 4.7, and 9.6 µl/l air for O. basilicum, A. fragrantissima and A. santolina, respectively.
Persistence activity of essential oils
Results in Tables 5 and 6 show the residual acaricidal effect of the test oils against adults of T. putrescentiae, where the oil of O. basilicum showed the potent residual protecting activity with LT50 value of 63.2 days (adult mortality reached 54.6 after 60 days of storage). The essential oil of A. fragrantissima showed a moderate residual activity with LT50 values of 46.6 days, whereas A. santolina oil showed a weak activity. As shown in Fig. 3, all of the tested oils undergo degradation over time, whereas those of O. basilicum and A. fragrantissima showed moderate stability, with a promising residual activity against T. putrescentiae during storage.
Discussion
Composition of the plant oils was similar to previous reports concerning the same plant species (Özcan and Chalchat 2002; Bader et al. 2003; Nenaah 2014a, b, c; Almadiy et al. 2016). Still, differences were observed both in the composition and abundance of the major components of A. santolina (Mohamed and Abdelgaleil 2008) and O. basilicum (Sartoratto et al. 2004). Sources of compositional variability include the plant part extracted, phenological state of the plant, environmental conditions (climatic, seasonal and geographical), genetic and chemotype differences, soil variations and nutritional status of plants (Nenaah 2014b, c; Nenaah et al. 2015; Isman 2016).
The plant oils showed a marked acaricidal activity against the mold mite, T. putrescentiae. Superior activity was achieved by the essential oils of O. basilicum and A. fragrantissima. In the literature, these plants showed insecticidal and acaricidal activity against insects and mites of stored food products. In a related study, A. fragrantissima growing in Saudi Arabia exhibited acaricidal activity against Hyalomma dromederi, a prevalent tick species of camels (Al-Harbi et al. 2015). Essential oil of Achillea millefolium was 2.62 times more potent than that of benzyl benzoate as a contact acaricide against T. putrescentiae (Song et al. 2016). Ebadollahi (2017) obtained similar results with Achillea filipendulina oil against the spider mite Tetranychus urticae. In the current study, O. basilicum (contatining a high percentage of eugenol) showed the strongest acaricidal activity against T. putrescentiae. These findings are in accordance with those of Hüe et al. (2015) who studied the acaricidal activity of essential oils from three Ocimum species on 14- to 21-day-old larvae of the cattle tick Rhipicephalus microplus. The essential oils of O. urticaefolium and O. gratissimum were the most effective with matching LC50 values. Both oils contain high amounts of eugenol (33.0 and 22.3%, respectively). Assis et al. (2011) reported a direct relationship between high concentration of eugenol and high mortality values when testing the acaricidal activity of essential oils of Cinnamomum zeylanicum and Suidasia pontifica against T. putrescentiae. According to many studies, O. basilicum showed a marked acaricidal activity against a wide range of species such as Rhipicephalus sanguineus (Manzoor et al. 2013) and R. (Boophilus) microplus (Veeramani et al. 2014). Many authors such as Macchioni et al. (2002) and Kim et al. (2003b) studied the control of T. putrescentiae using plant oils. Chemical profile of the plant oils tested herein revealed their high content of monoterpenoids. It is well known that the acaricidal activity of many plant oils are mainly due to their monoterpenoid constituents (Sànchez-Ramos and Castanera 2001; Kim et al. 2003a, b, 2004; Lee et al. 2006; Jeong et al. 2008; Jeon et al. 2009). Kim et al. (2004) suggest that hydrophobicity appears to play a crucial role in T. putrescentiae toxicity and that the presence of a hydroxyl moiety in the ortho position may have a considerable influence on toxicity.
Nanoemulsions (particle size 78.5 to 104.6 nm) were prepared from the plant oils through a green procedure without using toxic chemical solvents. Optimum conditions for the preparation of nanoemulsions depend on the active ingredient (10%), water phase (70%) and the lipophilic emulsifier (surfactant) (20%). Nanoemulsions were exposed to extreme storage conditions to judge their stability during storage. Stabilization of nanodroplets in the emulsion with a 1:3 ratio of oil and surfactant would be due to the surfactant, which reduces interfacial free energy and provides a mechanical barrier to coalescence (Reiss 1975). The sonication time (15 min), sonication cycle (five cycles per second), and sonication power (10 kHz) are also determinant factors for nanoemulsion stability (Anjali et al. 2012; Badawy et al. 2018). Centrifugation can accelerate the rate of cremation or sedimentation, demonstrating that the rupture of an emulsion may be related to the action of the gravity force. The results showed that all nanoemulsions passed from the centrifugation test at 5000 rpm. Stability at 25 to 4 °C indicated that all samples were stable without phase separation up to 8 weeks. These findings are consistent with other studies that reported an increase in surfactant concentration and the emulsification time had a direct relationship to the stability of the emulsion (Ghosh et al. 2013; Badawy et al. 2017). In non-equilibrium systems, emulsions tend to reduce their interfacial areas and free energy through several breakdown processes, such as creaming, sedimentation, flocculation, Ostwald ripening, and coalescence (Taylor 2003; Tadros et al. 2004). Compared to conventional emulsions, nanoemulsions have a good stability against creaming, sedimentation, flocculation, and coalescence due to the small size of the droplets (Reddy and Fogler 1981). The stability of emulsions can also improve considerably with increasing the surface charge due to the repulsive forces produced between droplets against flocculation and coalescence (Stachurski and Michalek 1996). In our study, the overall pH value of the various formulations is around 6.0. The pH can exert a vital effect on the stability of nanoemulsions. Variations at different levels of pH cause a change in the surface charge of the globules and thus their stability during storage. According to Badawy et al. (2017), an increase in the surface charge of the globules promotes electrostatic repulsion and reduces the flocculation and dissolution of the nanoemulsions. The polydispersity index (PDI) is a measure of the uniformity and stability of the droplet size in the formulation. Low polydispersity results in high uniformity of droplet size. According to the current study, PDI of the oil nanoemulsions ranged between 0.18 and 0.26. It has been reported that PDI values lower than 0.25 indicate a narrow particle size distribution, proving good physical stability of the nanoemulsion, due to the reduced Ostwald ripening (Hoeller et al. 2009). Shakeel et al. (2007) stated that PDI value remaining below 0.2 reflects the relative homogeneity of the nanoemulsion, and PDI > 0.3 indicates system heterogeneity. On the other hand, an increase in viscosity of the continuous phase reduces oil droplet mobility, which delays instability phenomena, resulting in oil droplets with a more homogeneous particle size (Arancibia et al. 2016). Similar results have been reported by other studies on nanoemulsions of plant oils such as cinnamon oil (Ghosh et al. 2013), neem oil (Anjali et al. 2012), and basil (Ghosh et al. 2014). The spherical form of the nanoemulsions reported herein and their size range (78.5–104.6 nm) are in agreement with the findings of many authors. Li and Chiang (2012) stated that nanoemulsions containing D-limonene by ultrasonic emulsification had a droplet size of less than 100 nm. It is reported that small nanoemulsion droplets can be found when the hydrophilic–lipophilic balance (HLB) value of the surfactant pair coincides with the required HLB value of the oil (Fernandes et al. 2014).
Results of the current study revealed that the oil nanoemulsions showed increased fumigant acaricidal activity against T. putrescentiae. When transformed into nanoparticles, materials could acquire novel biological properties, as they offer large specific surface area and hence increased affinity to the target, penetrate rapidly and are selectively accumulated in biosystems enhancing various activities within living cells (Weiss et al. 2006; Nenaah 2014c; Nenaah et al. 2015; Almadiy et al. 2016, 2018). It is possible that a reduction in droplet size, and hence an increase in surface area of the droplets, increases the rate of accumulation by the mites of the acaricidal component of the oil nanoemulsions (Margulis-Goshen and Magdassi 2013). The stability of the prepared nanoemulsions, the high acaricidal activity, and the absence of organic toxic solvents make these formulations suitable as biorational acaricidal products. In a related study, Sarapothong et al. (2017) studied the contact acaricidal activity of nano essential oil of black pepper, Piper nigrum, against the African red mite, Eutetranychus africanus. After 24 h, and a test concentration of 1%, 96% mortality was recorded (LC50 was 0.34%). Nanoemulsions from Callistemon viminalis and Origanum vulgare and two monoterpenes (R-limonene and pulegone) exhibited high acaricidal activity against T. urticae with 100% reduction at 5 mg/l after 2–3 days of application (Badawy et al. 2018). Garlic oil nanoemulsion with droplet size 93.4 nm showed a high acaricidal activity against injurious eriophyid mites Aceria oleae and Tegolophus hassani with LC50 298.3 and 309.63 μg/ml, respectively (Mossa et al. 2018). As many of the pesticides known today are organic compounds with poor water solubility, development of nanomaterials, including nanoemulsions appear to solve this problem by enhancing water solubility of poorly water-soluble substances. Their bioavailability results in stable formulations without utilization of organic toxic solvents (Nenaah et al. 2015). These formulations should also degrade rapidly with residue levels below the regulatory criteria in foodstuffs and environment (Khot et al. 2012).
The molecular targets associated with the mechanism of action of essential oils include inhibition of acetylcholinasterase (AChE), antagonism with the receptors of tyramine and/or octopamine neurotransmitter that modulate vital functions ranging from metabolism to behavior. Closure of the chloride channels by GABA is also confirmed (Blenau et al. 2012; Isman 2016). Based on our results, the test plant oils and their nanoemulsions could be a very promising control strategy to protect stored food from mite infestation. However, challenges still have to be overcome for the effective use of plant oils as natural acaricides. These include scarcity of the natural resource, the need for chemical standardization and quality control, in addition to some challenges associated with formulation, application, stability, and storage of these compounds. It is also necessary to extend the residual life of oil-based pesticides in open areas. Innovative technologies in the formulation of acaricides, such as microencapsulation and nanoformulation, provide clues to solving problems with essential oil-based products. However, many of the plant essential oils studied herein are used as pharmaceuticals, therefore considered less harmful to humans than most conventional insecticides and they can be used as relatively safe fumigants and/or contact acaricides after experiments required to validate various limitations about their mammalian and environmental safety.
References
Abbott WS (1925) A method for computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Chicago
Al-Harbi KB, El-Ashmawy IM, Omar HM, Al-Wabel NA (2015) Anti-tick activity of some methanol-extracted plants indigenous in Saudi Arabia. J Anim Vet Adv 14(13):07–412
Almadiy A, Nenaah G, Al Assiuty B, Moussa E, Mira N (2016) Chemical composition and antibacterial activity of essential oils and major fractions of four Achillea species and their nanoemulsions against foodborne bacteria. LWT-Food Sci Technol 69:529–537
Almadiy A, Nenaah G, Shawer D (2018) Facile synthesis of silver nanoparticles using harmala alkaloids and their insecticidal and growth inhibitory activities against the khapra beetle. J Pest Sci 91(2):27–737
Anjali C, Sharma Y, Mukherjee A, Chandrasekaran N (2012) Neem oil (Azadirachta indica) nanoemulsion-a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci 68:158–163
Arancibia C, Navarro-Lisboa R, Zúniga RN, Matiacevich S (2016) Application of CMC as thickener on nanoemulsions based on olive oil: physical properties and stability. Int J Polym Sci 2016:10
Assis CPO, Gondim MG, Siqueira HA, Câmara CA (2011) Toxicity of essential oils from plants towards Tyrophagus putrescentiae (Schrank) and Suidasia pontifica Oudemans (Acari: Astigmata). J Stored Prod Res 47(4):311–315
Badawy MEI, Saad A-FS, Tayeb E-SH, Mohammed SA, Abd-Elnabi AD (2017) Optimization and characterization of the formation of oil-in-water diazinon nanoemulsions: modeling and influence of the oil phase, surfactant and sonication. J Environ Sci Health, Part B 12:896–911
Badawy MEI, Abdelgaleil SAM, Mahmoud NF, Marei AM (2018) Preparation and characterizations of essential oil and monoterpene nanoemulsions and acaricidal activity against two-spotted spider mite (Tetranychus urticae Koch). Int J Acarol. https://doi.org/10.1080/01647954.2018.1523225
Bader A, Flamini G, Cioni PL, Morelli I (2003) Essential oil composition of Achillea santolina L. and Achillea biebersteinii Afan. collected in Jordon. Flav Frag J 18:36–38
Blenau W, Rademacher E, Baumann A (2012) Plant essential oils and formamidines as insecticides/acaricides: what are the molecular targets? Apidologie 43(3):334–347
Boczek J (1991) Mite pests in stored food. In: Gorham JR (ed) Ecology and Management of Food Industry Pest. FDA Technical Bulletin 4. Association of Official Analytical Chemists, Arlington, pp 57–79
Brazis P, Serra M, Selles A, Dethioux F, Biourge V, Puigdemont A (2008) Evaluation of storage mite contamination of commercial dry dog food. Vet Dermatol 4:209–214
Ebadollahi A (2017) Chemical composition, acaricidal and insecticidal effects of essential oil from Achillea filipendulina against two arthropod pests; Oryzaephilus surinamensis and Tetranychus urticae. Toxin Rev 36(2):132–137
Fernandes CP, de Almeida FB, Silveira AN, Gonzalez MS, Mello CB, Feder D, Apolinário R, Santos MG, Carvalho JCT, Tietbohl LAC (2014) Development of an insecticidal nanoemulsion with Manilkara subsericea (Sapotaceae) extract. J Nanobiotechnol 12:22–30
Franzolin MR, Gambale W, Cuero RG, Correa B (1999) Interaction between toxigenic Aspergillus flavus Link and mites (Tyrophagus putrescentiae Schrank) on maize grains: effects on fungal growth and aflatoxin production. J Stored Prod Res 35:215–224
Ghosh V, Mukherjee A, Chandrasekaran N (2013) Formulation and characterization of plant essential oil based nanoemulsion: evaluation of its larvicidal activity against Aedes aegypti. Asian J Chem 25(Supplementary Issue):S321–S323
Ghosh V, Mukherjee A, Chandrasekaran N (2014) Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloid Surf B 114:392–397
Gill C, McEwan N, McGarry J, Nuttal T (2011) House dust and storage mite contamination of dry dog food stored in open bags and sealed boxes in 10 domestic households. Vet Dermatol 22(2):162–172
Golemanov K, Tcholakova S, Denkov ND, Gurkov T (2006) Selection of surfactants for stable paraffin-in-water dispersions, undergoing solid-liquid transition of the dispersed particles. Langmuir 22(8):3560–3569
Hagstrum DW, Phillips TW, Cuperus G (2012) Stored product protection. Kansas State University Printing Services, Manhattan
Hoeller S, Sperger A, Valenta C (2009) Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm 370:181–186
Hüe T, Cauquil L, Fokou JB, Dongmo PM, Bakarnga-Via I, Menut C (2015) Acaricidal activity of five essential oils of Ocimum species on Rhipicephalus (Boophilus) microplus larvae. Parasitol Res 114(1):91–99
Hughes AM (1976) The mites of stored food and houses, 2nd edn. Ministry of Agriculture, Fisheries and Food, Technical Bulletin 9, London, p 400
Isman MB (2016) Pesticides based on plant essential oils: phytochemical and practical considerations. In: Jeliazkov VD, Cantrell CL (eds) Medicinal and aromatic crops: production, phytochemistry, and utilization. American Chemical Society, Washington, pp 13–26
Jeon JH, Lee CH, Lee HS (2009) Food protective effect of geraniol and its congeners against stored food mites. J Food Protect 72:1468–1471
Jeong EY, Lim JH, Kim HG, Lee HS (2008) Acaricidal activity of Thymus vulgaris oil and its main components against Tyrophagus putrescentiae, a stored food mite. J Food Prot 71(2):351–355
Khot L, Sankaran S, Maja J, Ehsani R, Schuster E (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70
Kim E-H, Kim H-K, Choi D-H, Ahn Y-J (2003a) Acaricidal activity of clove bud oil compounds against Tyrophagus putrescentiae (Acari: Acaridae). Appl Entomol Zool 38(2):261–266
Kim E-H, Kim H-K, Ahn Y-J (2003b) Acaricidal activity of plant essential oils against Tyrophagus putrescentiae (Acari: Acaridae). J Asia-Pac Entomol 6:77–82
Kim H-K, Kim J-R, Ahn Y-J (2004) Acaricidal activity of cinnamaldehyde and its congeners against Tyrophagus putrescentiae (Acari: Acaridae). J Stored Prod Res 40:55–63
Kwon JH, Ahn YJ (2002) Acaricidal activity of butylidenephthalide identified in Cnidium officinale rhizome against Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae). J Agric Food Chem 50:4479–4483
Lee C-H, Sung B-K, Lee H-S (2006) Acaricidal activity of fennel seed oils and their main components against Tyrophagus putrescentiae, a stored-food mite. J Stored Prod Res 42:8–14
Li PH, Chiang BH (2012) Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface methodology. Ultrason Sonochem 19:192–197
Macchioni F, Cioni PL, Flamini G, Morelli I, Perrucci S, Franceschi A, Macchioni G, Ceccarini L (2002) Acaricidal activity of pine essential oils and their main components against Tyrophagus putrescentiae, a stored food mite. J Agric Food Chem 50(16):4586–4588
Manzoor F, Fazal S, Munir N, Naz S, Khalid A (2013) Acaricidal Activity of Essential Oils from Tulsi (Ocimum basilicum), Bach (Acorus calamus) and Mint (Mentha arvensis) Against Rhipicephalus sanguineus (Latreille). Asian J Chem 25:6787–6790
Margulis-Goshen K, Magdassi S (2013) Nanotechnology: an advanced approach to the development of potent insecticides (A book chapter). In: Ishaaya I, Palli SR, Horowitz R (eds) Advanced technologies for managing insect pests. Springer, Dordrecht, pp 295–314
Matsumoto T, Hisano T, Hamaguchi M, Miike T (1996) Systemic anaphylaxis after eating storage-mite contaminated food. Int Arch Allergy Imm 109:197–200
Mohamed MI, Abdelgaleil SA (2008) Chemical composition and insecticidal potential of essential oils from Egyptian plants against Sitophilus oryzae (L.) (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Appl Entomol Zool 43(4):599–607
Mossa A, Afia S, Mohafrash S, Abou-Awad B (2018) Formulation and characterization of garlic (Allium sativum L) essential oil nanoemulsion and its acaricidal activity on eriophyid olive mites (Acari: Eriophyidae). Environ Sci Pollut 25(11):10526–10537
Mueller RS, Fieseler KV, Rosychuk RA, Greenwalt T (2005) Intradermal testing with the storage mite Tyrophagus putrescentiae in normal dogs and dogs with atopic dermatitis in Colorado. Vet Dermatol 16:27–31
Nenaah G (2014a) Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J Asia-Pac Entomol 17:701–709
Nenaah G (2014b) Chemical composition, insecticidal and repellence activities of essential oils of three Achillea species against the Khapra beetle (Coleoptera: Dermestidae). J Pest Sci 87(2):273–283
Nenaah G (2014c) Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind Crop Prod 53:252–260
Nenaah G, Ibrahim S (2011) Chemical composition and the insecticidal activity of certain plants applied as powders and essential oils against two stored-products coleopteran beetles. J Pest Sci 84:393–402
Nenaah G, Ibrahim S, Al-Assiuty B (2015) Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J Stored Prod Res 61:9–16
Özcan M, Chalchat J-C (2002) Essential oil composition of Ocimum basilicum L. and Ocimum minimum L. in Turkey. Czech J Food Sci 20(6):223–228
Park J-H, Yang J-Y, Lee H-S (2014) Acaricidal activity of constituents derived from peppermint oil against Tyrophagus putrescentiae. J Food Prot 77(10):1819–1823
Reddy SR, Fogler HS (1981) Emulsion stability: determination from turbidity. J Colloid Interface Sci 79:101–104
Reiss H (1975) Entropy-induced dispersion of bulk liquids. J Colloid Interface Sci 53:61–70
Robertson EL (1959) A revision of the genus Tyrophagus with a discussion on its taxonomic position in the Acarina. Aust Zool 7:146–181
Sànchez-Ramos I, Castanera P (2001) Acaricidal activity of natural monoterpenes on Tyrophagus putrescentiae (Schrank), a mite of stored food. J Stored Prod Res 37:93
Sarapothong K, Pumnuan J, Insung A (2017) Acaricidal toxicity of nano essential oil of black pepper against African red mite (Eutetranychus africanus (Tucker). Int J Agric Technol 13(73):2267–2274
Sartoratto A, Lúcia A, Machado M, Delarmelina C, Figueira GM, Duarte MC, Rehder VL (2004) Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol 35:275–280
SAS (Statistical Analysis System) Institute (1990) SAS/STAT User’s Guide, Version 6. SAS Institute, Cary
Shakeel F, Baboota S, Ahuja A, Ali J, Aqil M, Shafiq S (2007) Nanoemulsions as vehicles for transdermal delivery of aceclofenac. AAPS PharmSciTech 8(4):E104. https://doi.org/10.1208/pt0804104
Song JE, Kim JM, Lee NH, Yang JY, Lee HS (2016) Acaricidal and insecticidal activities of essential oils against a stored-food mite and stored-grain insects. J Food Prot 79(1):174–178
Stachurski J, Michalek M (1996) The effect of the z potential on the stability of a non polar oil-in-water emulsion. J Colloid Interface Sci 184:433–436
Stepanek P (1993) Data analysis in dynamic light scattering. In: Brown W (ed) Dynamic light scattering: the method and some applications. Clarendon Press, Oxford, pp 177–241
Sung B, Lim JH, Lee H (2006) Food protective and color alteration effects of acaricidal aldehydes on Tyrophagus putrescentiae (Schrank). J Food Prot 69(7):1728
Tadros T, Izquierdo P, Esquena J, Solans C (2004) Formation and stability of nano-emulsions. Adv Colloid Interface Sci 108–109:303–318
Taylor P (2003) Ostwald ripening in emulsions: estimation of solution thermodynamics of the disperse phase. Adv Colloid Interface Sci 106(1–3):261–285
Veeramani V, Sakthivelkumar S, Tamilarasan K, Aisha SO, Janarthanan S (2014) Acaricidal activity of Ocimum basilicum and Spilanthes acmella against the ectoparasitic tick, Rhipicephalus (Boophilus) microplus (Arachinida: Ixodidae). Trop Biomed 31(3):414–421
Weiss J, Takhistov P, McClements JD (2006) Functional materials in food nanotechnology. J Food Sci 71:107–116
Acknowledgements
The authors thank Prof. Dr. Abdel Naieem Al-Assuity for the identification and authentication of the mite species.
Author information
Authors and Affiliations
Contributions
GN and BA conceived and designed the experiments. All authors conducted bioassays. GN achieved GC–Ms. All authors collected and analyzed data. GN and BA wrote the manuscript. All read and approved manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Al-Assiuty, B.A., Nenaah, G.E. & Ageba, M.E. Chemical profile, characterization and acaricidal activity of essential oils of three plant species and their nanoemulsions against Tyrophagus putrescentiae, a stored-food mite. Exp Appl Acarol 79, 359–376 (2019). https://doi.org/10.1007/s10493-019-00432-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-019-00432-x