Abstract
Entomopathogenic fungi and predatory mites can independently contribute to suppressing the two-spotted spider mite, Tetranychus urticae Koch. It is important to assess the risk of possible fungal infections in predators when a combination of them are being considered as a tandem control strategy for suppressing T. urticae. The first part of this study tested 12 Beauveria bassiana isolates for virulence in T. urticae. Strains SCWJ-2, SDDZ-9, LNSZ-26, GZGY-1-3 and WLMQ-32 were found to be the most potent, causing 37.6–49.5% adult corrected mortality at a concentration of 1 × 107 m/L conidia 4 days post-treatment. The second part evaluated the pathogenicity of these five strains in five species of predatory phytoseiid mites. The bioassay results indicated that all adult predatory mite mortalities ranged from 7.5 to 9.1% 4 days post-treatment. No viable fungal hyphae were found on predator cadavers. Observations with scanning electron microscopy revealed that conidia were attached to the cuticle of predatory mites within 2–12 h after spraying with strain LNSZ-26, and had germinated within 24–36 h. After 48 h, conidia had gradually been shed from the mites, after none of the conidia had penetrated the cuticular surfaces. In contrast, the germinated conidia successfully penetrated the cuticle of T. urticae, and within 60 h the fungus colonized the mite’s body. Our study demonstrated that although several B. bassiana strains displayed a high virulence in T. urticae there was no evident pathogenicity to phytoseiid mites. These findings support the potential use of entomopathogenic fungus in combination with predatory mites in T. urticae control programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is a major economically important pest of agricultural and ornamental plants worldwide (Walter and Proctor 1999; Mondel and Ara 2006; Vergel et al. 2011). This mite species has become a serious problem due to the large scale use of chemical insecticides. One explanation for this increase is that widespread insecticide usage has eliminated many of the mites’ natural enemies, resulting in a reduction in predation pressure on the mite (Choi et al. 2004, Prischmann et al. 2005). Because of this effect on their natural enemies, coupled with the fact that increasing insecticide resistance in spider mites has evolved independently multiple times (Van Leeuwen et al. 2010), there has been an increasing demand for alternative approaches (Seiedy et al. 2010). Consequently, using biological control agents, such as entomopathogenic fungi (Maniania et al. 2008) and predatory mites (Zhang 2003) has become an increasingly popular option in suppressing spider mite populations because their use is considered to be more environmentally friendly.
Entomopathogenic fungi such as Beauveria bassiana (Balsamo) Vuillemin are widely distributed in nature (St. Leger et al. 1992). Beauveria bassiana has been tested in the laboratory and applied in the field to control numerous insect pest species (Legaspi et al. 2000), and has shown potential as an effective agent in controlling pest mite species (Maniania et al. 2008; Geroh et al. 2015). Although the potential for using some fungal agents for controlling T. urticae has been demonstrated under laboratory or greenhouse conditions (Bugeme et al. 2009; Gatarayiha et al. 2011; Seyed-Talebi et al. 2012), the control efficacy for T. urticae is somewhat limited because of the mites’ habits of feeding primarily on the ventral surface of the leaves. In addition, the mites produce silken webs (Morimoto et al. 2006), which tend to shield them from spray applications of the fungus (Gatarayiha et al. 2010). In order to increase efficiency in controlling T. urticae to minimize economic losses, it has been suggested that using predatory mites accompanied by applications of B. bassiana may be an alternative to traditional T. urticae management (Chandler et al. 2005). Considering the wide range of insect and mite species that are susceptible to B. bassiana, there is a risk that the fungus may be harmful to predatory mites (Jacobson et al. 2001). Therefore, evaluating the compatibility of B. bassiana and predatory mites may be critical to the success of potential IPM programs designed to control T. urticae.
Several studies have evaluated the effects of pathogens on predators by investigating the predator mortality after exposure to spray residues (Flexner et al. 1986). These studies, however, are commonly laboratory-based with bioassay experiments being conducted in confined environments (Chang 1996), for example, in Petri dishes covered with parafilm (Sun et al. 2002) or polyvinyl chloride (PVC) film (Wu et al. 2015). Based on our observations, these enclosed environments often result in a high humidity situation, with a layer of condensate on the inside surface of the film during incubation, often leading to a higher mortality in small arthropods, including predatory mites. The current lack of information on susceptibility of predatory mites to B. bassiana has restricted their combined utilization for controlling pest mite species. In order to determine the effect of the fungus on predator mites, we first conducted a bioassay of one of the highly virulent strains of B. bassiana on T. urticae, and then observed and documented the fungal infection process in five species of predatory mites, Neoseiulus [=Amblyseius] cucumeris (Oudemans) (Easterbrook et al. 2001), Neoseiulus californicus (McGregor) (Greco et al. 2005), Neoseiulus womersleyi (Schicha) (=Amblyseius pseudolongispinosus Xin, Liang and Ke) (Xin et al. 1984), Phytoseiulus persimilis Athias-Henriot (Opit et al. 2004), Amblyseius swirskii Athias-Henriot (van Houten et al. 2007), using scanning electron microscopy (SEM). These five predatory mite species are the subject of ongoing research in China (Xu et al. 2013, 2015) and have shown potential for use in biological control of T. urticae.
Materials and methods
Entomopathogenic fungi
The origin and source of the 12 fungal isolates are shown in Table 1. All isolates were stored and conidia were produced on Sabouraud Dextrose Agar (SDA) at 26 ± 1 °C and incubated for 7 days. Conidia powder used for the experiments was harvested using an inoculation loop, and then suspended in sterile 54 g/L Tween-80 and diluted to 1 × 107 m/L conidia, according to the method described by Goettel and Inglis (1997). Viability of the conidia was confirmed on SDA medium (Wen et al. 2003), and the percentage germination of the conidia was determined to be >90% for all strains.
Mite colonies tested
The five species of predatory mite, N. cucumeris, N. californicus, N. womersleyi, P. persimilis, and A. swirskii, were obtained from colonies maintained in the laboratory at the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing, China. The spider mite T. urticae colony, originally obtained from Nanjing Agriculture University (Nanjing, China), was lab-reared on kidney beans (Phaseolus vulgaris) in a walk-in growth chamber at 20–30 °C, 60–70% RH and L12:D12 h. To maintain similar age adult T. urticae mites for the bioassays, deutonymphs taken from plants were carefully transferred onto detached bean leaves using a fine paintbrush. The leaves ware placed on petri dishes (7 cm diameter) of 15 g/L agar containing rhizocaline (100 mg/mL) and maintained at 26 ± 1 °C and L14:D10 photoperiod until development of the adults (Shi and Feng 2009). The similar age adult predatory mites were obtained as being consistent with T. urticae mites, except that deutonymphs were separately cultured in plastic boxes (15 × 15 × 10 cm) with lids and supplied with T. urticae as prey. Circular moistened sponges (3 cm thick, 10 cm diameter) were placed on the bottom of the boxes to prevent the mites from escaping. A hole (12 cm diameter) was cut in the lid and covered with fine mesh for ventilation. Culture boxes were kept in climatic chambers and maintained at 26 ± 1 °C, 60–70% RH and L14:D10 photoperiod. Newly emerged adult female predatory mites and T. urticae mites (no more than 2 days after final ecdysis) were used for experimental treatments.
Screening of 12 fungal isolates
Twenty T. urticae adults were transferred to a Petri dish (7 cm diameter) lined with a freshly excised bean leaf, and placed on a moist filter paper. The leaf stalk was wrapped with moistened cotton to slow leaf desiccation. Fungal conidia were prepared at a concentration of 1 × 107 m/L conidia (the middle concentration recommended for laboratory bioassays by Shi et al. 2008). Deposits of sprayed conidia on the surface of T. urticae (no. conidia/mm2) were counted using an optical microscope at 400× magnification in five random views under a glass coverslip (2 × 2 cm), that had been placed on the dish to collect the conidia from the spray. Counts of conidia were then averaged as the number of conidia per mm2.
The effect of the fungal isolates on T. urticae survival was evaluated by spraying mites with B. bassiana using a hand-held pressure sprayer (2 mL) producing a wet deposit of 195 ± 13 (mean ± SE) conidia/mm2. Untreated control mites were handled in a similar manner, except sterile 54 g/L Tween-80 was used in place of the conidia spray. The Petri dishes were sealed with polyvinyl chloride (PVC) film, which was pricked with minute pins for ventilation (Wu et al. 2014), and then incubated in a climate chamber maintained at 26 ± 1 °C, 60–70% RH and L14:D10 photoperiod. The number of dead mites was recorded daily by means of a 10× hand magnifier after the PVC cover was temporarily removed to enable observation. Cadavers were transferred onto a moistened filter paper in a Petri dish and left undisturbed for 5–6 days. The presence of fungal outgrowths on the mites visible under an optical microscope (Nikon, SMZ1500, Japan) at 50× magnification was used as an indication of death due to fungal infection. The bioassays of all fungal isolates and controls were replicated six times and 20 adult mites were used in each replicate. Mortality of mites was assessed 4 days after treatment. Every replicate in a control treatment was <15%.
Effects of five highly virulent Beauveria bassiana strains on predatory mites
Based on previous screening of the 12 fungal strains as reported above, strains SCWJ-2, SDDZ-9, LNSZ-26, GZGY-1-3 and WLMQ-32 were further tested against T. urticae and the five species of predatory mites using a spray concentration of 1 × 107 m/L conidia. To limit the searching behavior of the predatory mites to the leaves and to prevent them from escaping from the Petri dishes, the experimental design consisted of two uniform pieces of Plexiglas (6 × 5 × 0.4 cm). A water-saturated filter paper was placed on the bottom piece, and a freshly excised kidney bean leaf placed ventral side up on the filter paper surface. A 2.5-cm-diameter hole was cut in the second piece of Plexiglas and was pressed on the leaf. The circular chamber that was formed between the two outer pieces of Plexiglas served as the experimental arena (Wu et al. 2016). Each predatory mite species that had been added to the leaf disc inside the chamber were sprayed and their mortality was assessed as described above. Ample T. urticae immature prey was supplied daily to the chambers containing predatory mites. The chamber of the experimental unit was then sealed with a coverslip. All dead individuals were removed, placed on a moist filter paper in a Petri dish, and examined under an optical microscope for the presence of fungal mycelia. The bioassays of five fungal isolates and controls were replicated six times using 20 adults of mites in each replicate for each of the five mite species. The number of B. bassiana conidia deposited onto each species of mite was determined as above, and estimated as 206 ± 16 (mean ± SE) conidia/mm2.
Scanning electron microscope (SEM) observations
A SEM was used to observe and document the micromorphological processes that occurred due to fungal conidial inoculation in each of the predatory mite species. Mites were first sprayed with one of highly virulent B. bassiana strains LNSZ-26 (1 × 107 conidia/mL) as described above. Then 10 individuals of the predatory mite species being observed were placed into one of the chambers and supplied with ample T. urticae immatures as prey. After 2, 12, 24, 36, 48 and 60 h, the B. bassiana-treated mites were removed, fixed in 5% glutaraldehyde in cacodylate buffer for 24 h, and then dehydrated in an ascending series of ethyl alcohol solutions (70, 75, 80, 90, 95 and 100%, 6 min each). After dehydration, the samples were dried using the critical-point method, mounted on SEM stubs, and sputter coated with gold. The samples were viewed using a Quanta 200 FEG SEM under a high-vacuum mode at 5–10 kV. To compare the fungal infection process in the predatory mites and T. urticae, the same procedure for SEM of T. urticae as described above was carried out.
Statistical analysis
Mortality data were control mortality corrected using Abbott’s formula (Abbott 1925), and then normalized using square root transformation. Differences in mortality among different fungal isolates were analyzed by one-way analysis of variance (ANOVA) using SPSS 13.0. Means were separated using the Tukey–Kramer honestly significant difference (HSD) test (α = 0.05).
Results
Screening fungal isolates
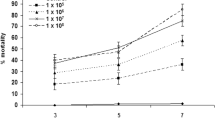
All test fungal isolates were shown infective to adult T. urticae (Fig. 1). Correct mortalities of T. urticae showed significant differences among the different isolates (F 11,60 = 9.09, P < 0.0001) 4 days after spray application. However, there were no significant differences among the isolates of SCWJ-2, SDDZ-9, LNSZ-26, GZGY-1-3 and WLMQ-32 (F 4,25 = 2.60, P = 0.06), resulting in mite corrected mortalities of 37.6–49.5% 4 days post-treatment. Fungal outgrowths were noticeable on T. urticae cadavers on day five or six (Fig. 2a). These five fungal strains were determined to be the most virulent strains and were chosen for further evaluation on the predatory mite species.
Effects of five highly virulent Beauveria bassiana strains on predatory mites
The mortalities of five species of predatory mites in the untreated control treatments ranged from 7.5 to 9.1% 4 days post-treatment (Fig. 3). There were no significant differences of mortalities between five B. bassiana strains (SCWJ-2, SDDZ-9, LNSZ-26, GZGY-1-3 and WLMQ-32) and untreated control for each of predatory mites (P > 0.5) 4 days after spray application. No fungal hyphae were observable under an optical microscope on the predatory mite cadavers (Fig. 2b).
Comparison of mean (+SEM) mortality of five Beauveria bassiana isolates (SCWJ-2, SDDZ-9, LNSZ-26, GZGY-1-3 and WLMQ-32) and untreated control in each of five species of phytoseiid mites at 4 days post-treatment in the laboratory. a Neoseiulus cucumeris; b N. californicus; c Phytoseiulus persimilis; d Amblyseius swirskii; e N. womersleyi
Scanning electron microscopic (SEM) observations
Conidia adhered to the cuticle of predatory mites within 2–12 h (Figs. 4a, 5a, 6a, 7a, 8a). Some secretions were detected on the interface of the conidia (Figs. 7b, 8b). Although conidia germinated within 24–36 h, they apparently did not penetrate the cuticle (Figs. 4c, 5c, 6c, 7c, 8c). Most conidia were shed from the cuticle, and a number of them had begun to shrivel within 48 h (Figs. 4d, 5d, 8d). Few conidia were detected on the cuticular surface after 48 h. Similarly, conidia adhered to the cuticle of T. urticae within 2–12 h (Fig. 9a) and germinated within 24–36 h (Fig. 9b). However, Germ tubes of conidia successfully penetrated the mite cuticle within 48 h (Fig. 9c), with fungal mycelia prolifically growing from its bodies within 60 h (Fig. 9d).
Discussion
The entomopathogenic fungus B. bassiana has been reported as being capable of infecting over 100 species of insects from a wide range of insect orders, although many fungal isolates vary in host range and some isolates have displayed high host specificity (Maurer et al. 1997; McCoy et al. 1988). The possible effects of pathogens on natural enemies are particularly relevant to biological control of agricultural pests. If natural enemies are susceptible to infection by pathogens, applying combinations of these two controls for suppression of pests would not be compatible. In contrast, applying multiple species may act synergistically in reducing a pest population if interference between species is minimal or nonexistent (Roy and Pell 2000). Since many of these evaluation studies, however, have been conducted in simplified laboratory rearing containers, conclusions from such studies tend to be limited by the context in which the insects were incubated. Ludwig and Oetting (2001) indicated that natural enemies were highly susceptible to infection by B. bassiana under laboratory conditions, while lower infection rates occurred in the greenhouse. Shi and Feng (2004) reported that they had failed in many of their attempts to conduct bioassays on the carmine spider mite, Tetranychus cinnabarinus (Boisduval), using a leaf-dish system for evaluating the lethal effects of fungus on the immobile eggs because high natural mortalities had occurred. In the present study, although the bean leaf stems were wrapped with moistened cotton in the Petri dishes, the leaves gradually began to wilt 4 days after inoculation, which necessitated assessing mite mortalities on day four. In the sealed chambers, the high humidity would increase the natural mortality of mites, with more than 20% mortality in the untreated control after 5 days.
The bioassays for predatory mites showed that roughly identical mortalities were caused by B. bassiana and the Tween-80 control. However, no formation of hyphae was observed on cadavers after being sprayed with B. bassiana, indicating that the predatory mites had died naturally during the treatment. Our SEM observations further verified that strain LNSZ-26 was not pathogenic to predatory mites, since the conidial germ tube was not able to penetrate the cuticle. These results were consistent with our previous study, demonstrating that the same fungal species displayed no virulence to the predatory mite Neoseiulus barkeri (Hughes) (Wu et al. 2014). In contrast, B. bassiana strain LNSZ-26 conidia penetrated T. urticae cuticle soon after germination. These results do raise questions why B. bassiana is so virulent to pest mite species, but shows no apparent infectivity in predatory mite species. Furtado et al. (1996) did report that a strain of the fungal pathogen, Neozygites acaricida (Petch) S. Keller and Milner was pathogenic to the predatory mite Euseius citrifolius Denmark and Muma, while many other studies have indicated that other fungal pathogens displayed no detrimental effects on predatory mites under both laboratory conditions and field investigations (Jacobson et al. 2001; Wekesa et al. 2007; Wang et al. 2011). It remains unknown how entomopathogenic fungi can selectively identify and infect their host species.
The ability of fungal conidia to attach to the insect cuticle is strongly correlated with virulence (Altre et al. 1999). Insect infection occurs following germination of conidia on the cuticle, with subsequent penetration of the cuticle by specialized infection structures (Butt 1990). Insect cuticle constitutes a defensive barrier to fungal penetration (Samuels and Paterson 1995). In our studies, although conidia were able to germinate, they apparently were not able to penetrate the cuticle of any of the predatory mite species. We speculated that their proteinaceous outer integument forms an effective barrier against fungi. Furthermore, the self-grooming behavior of fungal conidia (Wu et al. 2016) probably reduced the probability of fungal infection in predatory mites. In addition, several shriveled conidia that remained on the cuticle 48 h after spraying with B. bassiana had likely lost their viability due to the lack of an appropriate nutrient source from the host mite. The defense mechanism present in the cuticle of predator mites that is responsible for this apparent immunity to fungal penetration deserves further study.
Evaluating the compatibility of entomopathogenic fungi and predatory mites is a critical issue for the successful implementation of IPM programs to control pest mite species (Vergel et al. 2011). Chandler et al. (2005) reported that a strain of the commercial fungal pathogen Naturalis-L caused a reduction in the numbers of P. persimilis on greenhouse tomatoes, but did not know if this was caused directly by the fungal infection. Our study tested the B. bassiana strains that were virulent to T. urticae, but were found to be non-infective to five species of predatory mites under laboratory bioassay and SEM observation. The findings here support the potential use of B. bassiana in combination with predatory mites to control T. urticae.
References
Abbott WS (1925) A method of computing the effectiveness of and insecticide. J Econ Entomol 18:265–267
Altre JA, Van Denberg JD, Cantone FA (1999) Pathogenicity of Paecilomyces fumosoroseus isolates to diamondback moth, Plutella xylostella: correlation with spore size, germination speed, and attachment to cuticle. J Invertebr Pathol 73:332–338
Bugeme DM, Knapp M, Boga HI, Wanjoya AK, Maniania NK (2009) Influence of temperature on virulence of fungal isolates of Metarhizium anisopliae and Beauveria bassiana to the two-spotted spider mite Tetranychus urticae. Mycopathologia 167:221–227
Butt TM (1990) Fungal infection process—a mini-review. In: Proceedings and abstracts, Vth international colloquium on invertebrate pathology and microbial control. Adelaide, Australia, pp 121–124
Chandler D, Davidson G, Jacobson RJ (2005) Laboratory and glasshouse evaluation of entomopathogenic fungi against the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), on tomato, Lycopersicon esculentum. Biocontrol Sci Technol 15:37–54
Chang GC (1996) Comparison of single versus multiple species of generalist predators for biological control. Environ Entomol 25:207–212
Choi WI, Lee SG, Park HM, Ahn YJ (2004) Toxicity of plant essential oils to Tetranychus urticae (Acari: Tetranychidae) and Phytoseiulus persimilis (Acari: Phytoseiidae). J Econ Entomol 97:553–558
Easterbrook MA, Fitzgerald JD, Solomon MG (2001) Biological control of strawberry tarsonemid mite Phytonemus pallidus and two-spotted spider mite Tetranychus urticae on strawberry in the UK using species of Neoseiulus (Amblyseius) (Acari: Phytoseiidae). Exp Appl Acarol 25:25–36
Flexner JL, Lighthart B, Croft BA (1986) The effects of microbial pesticides on non-target, beneficial arthropods. Agric Ecosyst Environ 16:203–254
Furtado IP, Moraes GJ, Keller S (1996) Infection of Euseius citrifolius (Acari: Phytoseiidae) by an entomophthoralean fungus in Brazil. Rev Ecossistema 21:85–86
Gatarayiha MC, Laing MD, Miller RM (2010) Effects of adjuvant and conidial concentration on the efficacy of Beauveria bassiana for the control of the two spotted spider mite, Tetranychus urticae. Exp Appl Acarol 50:217–229
Gatarayiha MC, Laing MD, Miller RM (2011) Field evaluation of Beauveria bassiana efficacy for the control of Tetranychus urticae Koch (Acari: Tetranychidae). J Appl Entomol 135:582–592
Geroh M, Gulati R, Tehri K (2015) Determination of lethal concentration and lethal time of entomopathogen Beauveria bassiana (Balsamo) Vuillemin against Tetranychus urticae Koch. Int J Agric Sci 7:523–528
Goettel MS, Inglis DG (1997) Fungi: hyphomycetes. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, London, pp 213–249
Greco NM, Sánchez NE, Liljesthröm GG (2005) Neoseiulus californicus (Acari: Phytoseiidae) as a potential control agent of Tetranychus urticae (Acari: Tetranychidae): effect of pest/predator ratio on pest abundance on strawberry. Exp Appl Acarol 37:57–66
Jacobson RJ, Chandler D, Fenlon J, Russell KM (2001) Compatibility of Beauveria bassiana (Balsamo) Vuillemin with Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae) to control Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) on cucumber plants. Biocontrol Sci Technol 11:391–400
Legaspi JC, Poprawski TJ, Legaspi BC (2000) Laboratory and field evaluation of Beauveria bassiana against sugarcane stalkborers (Lepidoptera: Pyralidae) in the Lower Rio Grande Valley of Texas. J Econ Entomol 93:54–59
Ludwig SW, Oetting RD (2001) Susceptibility of natural enemies to infection by Beauveria bassiana and impact of insecticides on Ipheseius degenerans (Acari: Phytoseiidae). J Agric Urban Entomol 18:169–178
Maniania NK, Bugeme DM, Wekesa VW, Delalibera I Jr, Knapp M (2008) Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae), pests of horticultural crops. Exp Appl Acarol 46:259–274
Maurer P, Couteaudier Y, Girard PA, Bridge PD, Riba G (1997) Genetic diversity of Beauveria bassiana and relatedness to host insect range. Mycol Res 101:159–164
McCoy CW, Samson RA, Boucias DG (1988) Entomogenous fungi, in CRC handbook of natural pesticides. In: Ignoffo CM (ed) Microbial insecticides, part A; entomogenous protozoa and fungi, vol V. CRC Press, Florida, pp 151–236
Mondel M, Ara N (2006) Biology and fecundity of the two spotted spider mite, Tetranychus urticae Koch. (Acari: Tetranychidae) under laboratory conditions. J Life Earth Sci 1:43–47
Morimoto K, Furuichi H, Yano S, Osakaba MH (2006) Web-mediated interspecific competition among spider mites. J Econ Entomol 99:678–684
Opit GP, Nechols JR, Margolies DC (2004) Biological control of two spotted spider mites, Tetranychus urticae Koch (Acari: Tetranychidae), using Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseidae) on ivy geranium: assessment of predator release ratios. Biol Control 29:445–452
Prischmann DA, James DG, Wright LC, Teneyck RD, Snyder WE (2005) Effects of chlorpyrifos and sulfur on spider mites (Acari: Tetranychidae) and their natural enemies. Biol Control 33:324–334
Roy HE, Pell JK (2000) Interactions between entomopathogenic fungi and other natural enemies: implications for biological control. Biocontrol Sci Technol 10:737–752
Samuels RI, Paterson IC (1995) Cuticle degrading proteases from insect moulting fluid and culture filtrates of entomopathogenic fungi. Comp Biochem Physiol B Biochem Mol Biol 110:661–669
Seiedy M, Saboori A, Allahyari H, Talaei-Hassanloui R, Tork M (2010) Laboratory investigation on the virulence of two isolates of the entomopathogenic fungus Beauveria bassiana against the two spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 36:527–532
Seyed-Talebi FS, Kheradmand K, Talaei-Hassanloui R, Talebi-Jahromi K (2012) Sublethal effects of Beauveria bassiana on life table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Biocontrol Sci Technol 22:293–303
Shi WB, Feng MG (2004) Lethal effect of Beauveria bassiana, Metarhizium anisopliae, and Paecilomyces fumosoroseus on the eggs of Tetranychus cinnabarinus (Acari: Tetranychidae) with a description of a mite egg bioassay system. Biol Control 30:165–173
Shi WB, Feng MG (2009) Effect of fungal infection on reproductive potential and survival time of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 48:229–237
Shi WB, Zhang L, Feng MG (2008) Time-concentration-mortality responses of carmine spider mite (Acari: Tetranychidae) females to three hypocrealean fungi as biocontrol agents. Biol Control 46:495–501
St. Leger RJ, Allee LL, Mai B, Staples RC, Roberts DW (1992) Worldwide distribution of genetic variation among isolates of Beauveria spp. Mycol Res 96:1007–1115
Sun J, Fuxa JR, Henderson G (2002) Sporulation of Metarhizium anisopliae and Beauveria bassiana on Coptotermes formosanus and in vitro. J Invertebr Pathol 81:78–85
van Houten YM, Hoogerbrugge H, Bolckmans KJF (2007) The influence of Amblyseius swirskii on biological control of two-spotted spider mites with the specialist predator Phytoseiulus persimilis (Acari: Phytoseiidae). IOBC-WPRS Bull 30:129
Van Leeuwen T, Vontas J, Tsagkarakou A, Dermauw W, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Vergel SJN, Bustos RA, Rodríguez CD, Cantor RF (2011) Laboratory and greenhouse evaluation of the entomopathogenic fungi and garlic–pepper extract on the predatory mites, Phytoseiulus persimilis and Neoseiulus californicus and their effect on the spider mite Tetranychus urticae. Biol Control 57:143–149
Walter DE, Proctor HC (1999) Mites: ecology, evolution and behaviour. CABI Publishing, Wallingford
Wang J, Lei ZR, Xu HF, Gao YL, Wang HH (2011) Virulence of Beauveria bassiana isolates against the first instar nymphs of Frankliniella occidentalis and effects on natural enemy Amblyseius barkeri. Chin J Biol Control 27:479–484
Wen JZ, Lei ZR, Tan ZH, Wang Y, Fu W, Huang H (2003) Pathogenicity of five Beauveria bassiana strains against Locusta migratoria. China Plant Prot 29:50–52
Wekesa VW, Moraes GJ, Knapp M, Jr Delalibera (2007) Interactions of two natural enemies of Tetranychus evansi, the fungal pathogen Neozygites floridana (Zygomycetes: Entomophthorales) and the predatory mite, Phytoseiulue longipes (Acari: Phytoseiidae). Biol Control 41:408–414
Wu SY, Gao YL, Zhang YP, Wang ED, Xu XN, Lei ZR (2014) An entomopathogenic strain of Beauveria bassiana against Frankliniella occidentalis with no detrimental effect on the predatory mite Neoseiulus barkeri: evidence from laboratory bioassay and scanning electron microscopic observation. PLoS ONE 9:e84732
Wu SY, Gao YL, Xu XN, Goettel MS, Lei ZR (2015) Compatibility of Beauveria bassiana with Neoseiulus barkeri for control of Frankliniella occidentalis. J Integr Agric 14:98–105
Wu SY, Gao YL, Smagghe G, Xu XN, Lei ZR (2016) Interactions between the entomopathogenic fungus Beauveria bassiana and the predatory mite Neoseiulus barkeri and biological control of their shared prey/host Frankliniella occidentalis. Biol Control 98:43–51
Xin JL, Liang LR, Ke LS (1984) Biology and utilization of Amblyseius pseudolongispinosus (Acarina: Phytoseiidae) in China. In: Griffiths DA, Bowman CE (eds) Acarology VI, vol 2. Ellis Horwood, Chichester, pp 693–698
Xu XN, Lv JL, Wang ED (2013) Research and applications of predatory mites in China. China Plant Prot 33:26–34
Xu XN, Lv JL, Wang ED (2015) Predatory mite research in mass rearing and field applications. Chin J Biol Control 31:647–656
Zhang ZQ (2003) Phytoseiid mites. Mites of greenhouses: identification, biology and control. CABI Publishing, Oxon, pp 171–202
Acknowledgements
We thank Dr. Cecil L. Smith for helping with the language editing. We also thank Prof. Dr. Xiaoyue Hong for providing the spider mite T. urticae. This research was supported by National Natural Science Foundation of China (31501704) and China Agriculture Research System (CARS-25).
Author contributions
Conceived and designed the experiments: SY. W, XN. X and ZR. L. Performed the experiments: SY. W, HC. X, MY. L Analyzed the data: SY. W. Wrote the paper: SY. W and ZR. L.
Author information
Authors and Affiliations
Corresponding authors
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10493-016-0097-3.
Rights and permissions
About this article
Cite this article
Wu, S., Xie, H., Li, M. et al. Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp Appl Acarol 70, 421–435 (2016). https://doi.org/10.1007/s10493-016-0090-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0090-x