Abstract
Copulation in Ixodes scapularis involves physical contact between the male and female (on or off the host), male mounting of the female, insertion/maintenance of the male chelicerae in the female genital pore (initiates spermatophore production), and the transfer of the spermatophore by the male into the female genital pore. Bioassays determined that male mounting behavior/chelicerae insertion required direct contact with the female likely requiring non-volatile chemical cues with no evidence of a female volatile sex pheromone to attract males. Unfed virgin adult females and replete mated adult females elicited the highest rates of male chelicerae insertion with part fed virgin adult females exhibiting a much lower response. Whole body surface hexane extracts of unfed virgin adult females and males, separately analyzed by GC–MS, identified a number of novel tick surface associated compounds: fatty alcohols (1-hexadecanol and 1-heptanol), a fatty amide (erucylamid), aromatic hydrocarbons, a short chain alkene (1-heptene), and a carboxylic acid ester (5β-androstane). These compounds are discussed in terms of their potential role in female–male communication. The two most abundant fatty acid esters found were butyl palmitate and butyl stearate present in ratios that were sex specific. Only 6 n-saturated hydrocarbons were identified in I. scapularis ranging from 10 to 18 carbons.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks, like many other arthropods rely on chemical communication for courtship and reproduction. In some cases, pheromones help coordinate mating behaviors that bring adult males in contact with conspecific adult females, and guide male probing of the female genital pore. Transfer of the spermatophore follows soon after the successful completion of these mating behaviors (Sonenshine 2006). Despite similarities in courtship and reproduction among hard ticks in Ixodidae, there are distinct differences between metastriate and prostriate species. Metastriate ticks, such as Amblyomma, Dermacentor, and Rhipicephalus sp., engage in on-host aggregative mating strategies (Kiszewski et al. 2001). In adult females, consumption of blood triggers the production of a volatile sex pheromone that attracts fed or feeding adult males, and stimulates the males to achieve physical contact with emitting females. Following male–female contact, mounting pheromones induce male mounting behavior, which entails males crawling onto the ventral surface of females and locating the female genital pore. Males subsequently commence probing of the female genital pore with their chelicerae to detect genital pore sex pheromones. Perception of conspecific genital pore sex pheromones by sensilla on the male’s chelicerae triggers spermatophore synthesis, during which the male’s chelicerae are maintained in the female genital pore. Once the spermatophore is formed, the male’s chelicerae are removed from the female genital pore, and used to deposit the spermatophore into the female vulva, completing copulation. It is also important to note that metastriate ticks are not reproductively active until they commence feeding (Mulenga 2014).
The sex pheromones regulating mating in metastriate ticks have been well documented. The attractant sex pheromone is 2,6-dichlorophenol (2,6-DCP), and is produced by seven genera of Ixodidae ticks (Carr and Roe in press). Additionally, electrophysiological studies identified chemosensory sensilla in the Haller’s organ of adult male A. americanum (Haggart and Davis 1981), A. cajennense (Soares and Borges 2012), A. variegatum, D. variabilis, and R. appendiculatus (Mulenga 2014) that are responsible for detecting 2,6-DCP, although only fed or feeding males respond to it. Mixtures of cholesteryl esters present on the female dorsum have been identified as mounting pheromones in D. variabilis (Hamilton et al. 1989). Female genital pore sex pheromones have been identified as a combination of saturated fatty acids (C14–C22) and ecdysteroids in D. andersoni and D. variabilis (Allen et al. 1988; Allen et al. 2002). In comparison to our knowledge of metastriate reproductive pheromones, very little is known about the pheromones involved in prostriate tick courtship and mating.
Prostriate ticks, comprising the genus Ixodes, employ nest-based mating strategies, which do not require blood feeding in order to stimulate mating (Kiszewski et al. 2001). Adult female and male Ixodes engage in similar coordinated mounting, genital pore probing, and insemination behaviors as metastriate ticks, except that these behaviors can occur before attachment and feeding on the host, while the ticks are on the ground or in vegetation. Since unfed prostriate adult females can successfully retain endospermatophores without degradation for 4 months, mating can take place independent of blood feeding (Kiszewski and Spielman 2002). Ixodes virgin and previously mated adult females can also receive spermatophores from conspecific adult males on the host during blood feeding. The identity of compounds serving as pheromones in adult male–female attraction, mounting, genital pore probing, and sperm transfer of prostriate ticks are currently unknown. Evidence of a volatile attractant sex pheromone has been reported for I. persulcatus (Dobrotvorsky and Tkachev 1995) and I. ricinus (Dusbábek et al. 2001), however the identities of these volatile attractants have not been determined (Tkachev et al. 2000). Additionally, there has been no documentation of volatile sex pheromones, mounting pheromones, or genital pore sex pheromones in Ixodes scapularis, which is of special interest as the vector of the organism that causes Lyme disease. Investigations have identified the presence of aggregation pheromones (previously known as assembly pheromones) in I. holocylus (Treverrow et al. 1977), I. ricinus (Graf 1978), I. scapularis (Allen et al. 2002; Sonenshine et al. 2003), and I. uriae (Benoit et al. 2008), although it is still unclear if aggregation pheromones play a role in courtship and mating.
Due to the limited research available on prostriate reproductive pheromones, this study was conducted to further examine the mating behaviors of I. scapularis and determine, by bioassays, whether there is any evidence for pheromone involvement in male–female attraction, mounting and genital pore probing. In view of the widespread use of cuticular lipids in sexual communication in other arthropods, we also investigated potential sex differences in the cuticular lipids of adult I. scapularis. Only a few studies have been conducted on cuticular lipids of prostriate ticks, i.e., I. persulcatus and I. ricinus (Estrada-Peña et al. 1994; Tkachev et al. 2000; Dusbábek et al. 2001).
Materials and methods
Ticks
Adult I. scapularis were obtained from laboratory colonies of Dr. Daniel E. Sonenshine at Old Dominion University (Norfolk, VA, USA). Laboratory colonies were started with field collected I. scapularis from Armonk, New York and maintained by feeding larval, nymph and adult stages on New Zealand white rabbits, Oryctolagus cuniculus. When not feeding, I. scapularis larvae, nymphs, adult females and adult males were maintained in distinct containers at 21 ± 1 °C, 97 % humidity, and with a photoperiod of 16-h light:8-h dark (dusk and dawn periods of 1 h each at the beginning and ending of the scotophase).
Bioassays
Bioassays were utilized to investigate the potential role of sex pheromones in I. scapularis mating behaviors including male attraction, mounting, and copulation. Copulation in I. scapularis is a two-step process: (1) insertion and maintenance of the chelicerae in the female genital pore (which initiates the production of the spermatophore) followed by (2) male placement of the spermatophore into the female genital pore. Of these two endpoints, bioassays were designed to evaluate male insertion and maintenance of the male chelicerae in the female genital pore only, and will be referenced in this paper as “male insertion/maintenance of chelicerae”. Two types of bioassays were conducted: a 4-port olfactometer assay (Fig. 1) and a Petri dish assay (Fig. 2). The 4-port olfactometer (ARS, Gainesville, FL, USA) was used to determine whether I. scapularis unfed virgin adult females emit volatile sex pheromones that are attractive to unfed virgin adult males. Tests were conducted in the dark for a 24 h period at 23 ± 1 °C with a relative humidity of 40 %. Adult ticks used in olfactometer assays were 3–4 months post-molt, with females and males stored separately after molting. All ticks were handled wearing gloves and using sterile soft-tipped (metal) forceps cleaned with absolute ethanol to prevent cross contamination between the sexes. Ticks were allowed 30 min to acclimate to experimental conditions prior to being placed into the olfactometer. A nylon mesh (440 squares/cm2) screen was used to isolate 50 I. scapularis unfed virgin adult females into one glass bulb of the 4-port olfactometer, and 20 I. scapularis unfed virgin adult males placed into the center of the apparatus (Fig. 1). Breathing quality air (AirGas, Radnor, PA, USA) was introduced into each glass bulb from a compressed air tank at a rate of 30 mL/min. A vacuum pump was used to remove air from the center of the 4-port olfactometer at a rate of 120 mL/min. Gases removed from the olfactometer were exhausted out of the test area. Airflow from the bulbs towards the central vacuum allowed males to detect putative volatile sex pheromones produced by the females without achieving physical contact. The position of males in the 4-port olfactometer was recorded 24 h after introduction into the central test arena. Ticks were identified as positive responders, and attracted to volatiles produced by unfed virgin adult females if they moved 1 cm past the choice point (Fig. 1), distal to the central arena. The experiment was replicated three times and the glass bulb containing the female ticks randomly rotated between the four positions to compensate for potential positional response bias. All equipment was washed with hot tap water and absolute ethanol, and allowed to air-dry between trials to eliminate contaminants. Results from the olfactometer assays were analyzed with the Student’s t test using Prism (GraphPad, La Jolla, CA, USA).
The 4-port olfactometer used to examine possible volatile secretions produced by Ixodes scapularis unfed virgin adult females that would be attractive to unfed virgin adult males. A vacuum hose attached to the bottom of the central arena of the olfactometer creates a unidirectional airflow from each glass bulb toward the center of the apparatus
a Petri dish assays used to examine Ixodes scapularis mounting behavior between free roaming unfed virgin adult males and unfed virgin adult females. This experiment was also used to examine the affects of detergent washing and blood feeding on female attraction, and impacts on chelicerae insertion/maintenance behavior in unfed virgin adult males. This Petri dish assay was used to examine chelicerae insertion/maintenance behavior of unfed virgin adult males with (1) detergent washed unfed virgin adult females, (2) unfed virgin adult females, (3) part-fed virgin adult females and (4) replete mated adult females. b Petri dish assays used to examine I. scapularis mounting behavior between free roaming unfed virgin adult males and unfed virgin adult females secluded inside a mesh to prevent direct contact between the sexes
Petri dish assays were conducted to determine whether I. scapularis unfed virgin adult female sex pheromones function through direct contact or spatially to induce mounting behavior in unfed virgin adult males. Petri dish assays were performed using black glass Petri dishes (148 mm diam × 20 mm) and lids (150 mm diam × 20 mm). No filter paper was employed during these experiments. Tests were conducted during the day between 1100 and 1500 hours at 23 ± 1 °C and a relative humidity of 40 %. The use of black Petri dishes eliminated light contamination from ambient (fluorescent) lighting and created a dark testing environment. Adult ticks used in Petri dish assays were 3–4 months post-molt, with females and males stored separately after molting. All ticks were handled wearing gloves and using sterile soft-tipped (metal) forceps cleaned with absolute ethanol to prevent cross contamination between the sexes. Ticks were allowed 30 min to acclimate to experimental conditions prior to being placed into Petri dishes. To observe normal mounting behavior of I. scapularis in the Petri dish assays, 20 unfed virgin adult males and 20 unfed virgin adult females were placed into a Petri dish, and the percentage of males mounting females documented at 5, 10, 15, 30, and 60 min (Fig. 2a). The experiment was replicated three times, and all equipment thoroughly washed with hot tap water and absolute ethanol, and allowed to air-dry between replicates. After documenting normal mounting behavior, Petri dish assays were performed under the same experimental conditions using 20 unfed virgin adult males (free to roam) and 20 unfed virgin adult females secluded in nylon mesh (440 squares/cm2) bags to prevent direct contact between the sexes (Fig. 2b). The percentage of males mounting females was documented at 5, 10, 15, 30, and 60 min after all ticks were added to the bioassay arena. The experiment was replicated three times and all equipment washed between replicates as previously described. Results were analyzed with an ANOVA and a Sidak’s multiple comparison test using Prism.
Petri dish assays were also conducted to determine whether I. scapularis unfed virgin adult female sex pheromones affect male chelicerae insertion/maintenance behavior in unfed virgin adult males. Using the same Petri dish experimental procedure just described, male chelicerae insertion/maintenance behavior was observed between I. scapularis unfed virgin adult males and unfed virgin adult females after whole body washing of the latter with either distilled water or 1 % laboratory grade Triton X-100 detergent (Sigma-Aldrich, St. Louis, MO, USA) in distilled water. Using sterile soft-tipped (metal) forceps, 30 female ticks were placed into a 15 mL polypropylene centrifuge tube containing either 5 mL of distilled water or detergent (in distilled water) and the tubes repeatedly inverted for 5 min. The females were then removed from the tubes, checked for mortality, and rinsed with distilled water using the same procedure. Rinsed females were air-dried for 30 min, and subsequently placed into a Petri dish with 30 unfed virgin adult males (Fig. 2a). The percentage of males inserting/maintaining their chelicerae in the genital pore of females was documented at 0, 2, and 7 days. This behavior was differentiated from mounting by using a sterile probe to attempt to remove males from the ventral surface of females; males firmly affixed to the female genital pore were scored as having successfully inserted/maintained their chelicerae in the genital pore. Each treatment was replicated three times and the results analyzed with an ANOVA and a Sidak’s multiple comparison test using Prism.
Additional Petri dish assays were performed to determine whether blood feeding of adult female I. scapularis affects male chelicerae insertion/maintenance behavior in unfed virgin adult males. Using the same experimental procedure described just above, 20 I. scapularis unfed virgin adult males and 20 unfed virgin adult females were placed into a Petri dish, and the percentage of males inserting/maintaining their chelicerae in the genital pore of females was documented at 5, 10, 15, 30, and 60 min. The experiment was replicated three times. To compare the results from this bioassay with the impact of blood feeding, part-fed virgin, and replete mated adult females were paired with unfed virgin adult males in Petri dish assays. Due to the limited number of available I. scapularis, these experiments were conducted with only 4 females of each feeding stage. Part-fed virgin and replete mated adult females were paired with 20 unfed virgin adult males. The experiment was replicated three times for each treatment, and the percentage of males inserting/maintaining their chelicerae in the genital pore of females was documented at 5, 10, 15, 30, and 60 min after all ticks were added to the bioassay arena. Results were analyzed with an ANOVA and a Sidak’s multiple comparison test using Prism.
Cuticular extractions
A new bottle of high purity hexane (n-hexane) (≥98.5 %) was purchased for the purpose of these extractions (Thermo Fischer Scientific, Waltham, MA, USA). Hexane extractions were performed using live unfed virgin adult female and male I. scapularis. Ticks were 3–4 months post molt, with females and males stored separately since molting and extracted separately. To avoid cross contamination, extractions of opposite sexes were never conducted simultaneously, and each sex was assigned their own set of extraction tools. All extraction tools were autoclaved, washed five times with hexane, and allowed to air-dry prior to use. Extractions were conducted using new glass 2 mL Agilent GC–MS vials with polypropylene Teflon lined screw caps (Agilent, Santa Clara, CA, USA) also washed 5 times with hexane and air dried prior to use. Additionally, GC–MS vials and lids were never reused. Soft tipped (metal) forceps were used to transfer either 10 live unfed virgin adult female or male I. scapularis ticks into a GC–MS vial. Ticks were randomly selected for extraction. Once placed into a pre-weighed GC–MS vial, tick weight was recorded and a glass Hamilton syringe used to transfer 1 mL of hexane into the vial. Ticks were washed with hexane for 10 min at room temperature by inverting the GC–MS vial every few seconds. Soft-tipped forceps were used to remove the ticks from the vial. The hexane was then immediately concentrated just to dryness by warming the vial in a water bath (24 ± 1 °C) under a constant, slow stream of nitrogen gas (≥99.98 %; AirGas) using an 18 gauge stainless steel syringe needle inserted just into the top of the vial (Sigma-Aldrich). Then, an additional 10 μL of hexane was added to the vial, using a glass Hamilton syringe, to re-dissolve the extracted material. This procedure was replicated 5 times for females and 6 times for males. The number of replicates performed for each sex was based on the I. scapularis availability at the time. All extractions were analyzed using GC–MS (described in more detail later).
GLC analyses and operating conditions
A HP Mass Selective Detector 5973 (MSD) paired with a HP 6899 Gas Chromatograph (GC) System (Palo Alto, CA, USA) was used for all analyses. Instrumental control and program configuration was conducted using the MSD ChemStation software, Agilent G1701DA (Agilent). Manual injection of extractions (1 μL) was performed using the single electronic pressure controlled split-less injection port. Separations were performed on a TRACE TR-5MS coated fused silica capillary column (30 m length × 0.25 mm ID, 1 μM film thickness; Thermo Scientific). The column oven temperature was programmed to start at 50 °C, with no hold period, and a temperature ramp to 180 °C at 5°/min, and from 180° to 300 °C at 4°/min at the time of sample injection. Both the injector and detector temperature were set to 250 °C. The helium carrier gas (≥99.999 %; AirGas) was set to a constant flow of 1 mL/min, with an average velocity of 36 cm/s. A flame ionization detector was used to detect GC column eluants. The abundance of components identified in extraction samples was determined using GC peak areas without correcting for molar or weight response. Initial analyses focused on comparing retention times, peak areas and ion mass values (m/z) with the mass spectra database (Wiley 143,100). Secondary analysis consisted of manual comparison of m/z values and relative intensities using the MassBank spectrum search (MassBank Project, Tokyo, Japan) and the National Institute of Standards and Technology Chemistry Webbook (NIST, US Department of Commerce) to confirm compound identifications. Compound abundances were compared between females and males with the Student’s unpaired t-test with Welch’s correction using Prism.
Results
Bioassays
In the 4-port olfactometer assays (Fig. 1), the majority of I. scapularis unfed virgin adult males remained in the central arena and were unresponsive to any potential volatiles produced by unfed virgin adult females (Fig. 3). The percentage of unresponsive ticks was significantly higher than the percentage of ticks identified as attracted, i.e., moving towards the bulb containing the females (t = 40.93, df = 4, P ≤ 0.0001).
Mean percentage (+1 SEM) of Ixodes scapularis unfed virgin adult males attracted to odors produced by unfed virgin adult females in olfactometer assays (Fig. 1). Attraction is defined as males found between the females and point of choice (Fig. 1) 24 h after release in the center of the apparatus. Unresponsive males did not leave the central area of the apparatus. Treatments were compared using a paired Student’s t test (*P < 0.05)
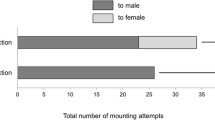
Petri dish assays (Fig. 2b) investigating mounting behavior of unfed virgin adult males with unfed virgin adult females secluded in mesh bags determined that I. scapularis does not engage in mounting without physical contact (Fig. 4). The percentage mounting observed between free roaming adults was significantly higher than that of the physically separated sexes for all time points; 5 min (t = 2.865, df = 20, P = 0.0096), 10 min (t = 3.123, df = 20, P = 0.0044), 15 min (t = 3.302, df = 20, P = 0.0036), 30 min (t = 4.109, df = 20, P = 0.0005), and 60 min (t = 4.471, df = 20, P = 0.0002). Additionally, there was no source of significant variation among time points (F = 0.4719, df = 20, P = 0.75).
Mean percentage mounting (+1 SEM) of Ixodes scapularis unfed virgin adult males with free roaming unfed virgin adult females, and with unfed virgin adult females enclosed in mesh bags in Petri dish bioassays (Fig. 2a, b). Attraction is defined as males mounting females and orienting towards the female genital pore. Arrows denote zero values. Treatments were compared using a 2-way ANOVA and a Sidak’s multiple comparisons test at each time point (*P < 0.05)
Petri dish assays (Fig. 2a) investigating possible sex pheromones that might elicit male chelicerae insertion/maintenance behavior determined that washing unfed virgin adult females with 1 % Triton-X 100 detergent resulted in a significant decrease in percent chelicerae insertion/maintenance of unfed virgin adult males when compared to distilled water washing, though only for day 0 (t = 4.907, df = 30 P ≤ 0.0001) (Fig. 5). There were no significant differences in percent chelicerae insertion/maintenance of unfed virgin adult males with unfed virgin adult females washed with distilled water or detergent for day 2 (t = 0.4147, df = 30, P = 0.68) and day 7 (t = 1.175, df = 30, P = 0.25). There was significant variation among time points, most notably at day 0 (F = 83.29, df = 30, P < 0.0001).
Mean percentage chelicerae insertion/maintenance (+1 SEM) of Ixodes scapularis unfed virgin adult males with unfed virgin adult females following female whole body washing with distilled water or with 1 % Triton-X 100 detergent in Petri dish bioassays (Fig. 2a). Treatments were compared using a 2-way ANOVA and a Sidak’s multiple comparison test at each time point (*P < 0.05)
Petri dish assays (Fig. 2a) investigating the impact of blood feeding on chelicerae insertion/maintenance behavior in I. scapularis identified significant decreases in percent chelicerae insertion/maintenance of unfed virgin adult males with part-fed virgin adult females at 15 min when compared to unfed virgin adult females (t = 2.903, df = 30, P = 0.0069) and replete mated adult females (t = 2.869, df = 30, P = 0.0075), at 30 min when compared to unfed virgin adult females (t = 3.213, df = 30, P = 0.0031) and replete mated adult females (t = 2.869, df = 30, P = 0.0075), and at 60 min when compared to unfed virgin adult females (t = 3.523, df = 30, P = 0.0014) and replete mated adult females (t = 2.869, df = 30, P = 0.0075) (Fig. 6). Additionally there was no significant difference observed in percent chelicerae insertion/maintenance of unfed virgin adults males with either unfed virgin adult females or replete mated adult females at 15 min (t = 0.03442, df = 30, P = 0.97), 30 min (t = 0.3442, df = 30, P = 0.73), and at 60 min (t = 0.6541, df = 30, P = 0.52). No significant difference was observed in percent chelicerae insertion/maintenance of unfed virgin adults males with adult females of all three feeding stages at 5 min and 10 min. There was significant variation among time points (F = 2.704, df = 30, P = 0.049) and between feeding stages (F = 20.06, df = 30, P < 0.0001).
Mean percentage chelicerae insertion/maintenance of Ixodes scapularis unfed virgin adult males with unfed virgin adult females, part-fed virgin adult females, and replete mated adult females in Petri dish assays (Fig. 2a). Arrows denote zero values. Treatments were compared using a 2-way ANOVA and a Tukey’s multiple comparison test for each time point. Treatments with the same letter at each time point are not statistically significant (P < 0.05)
GC–MS
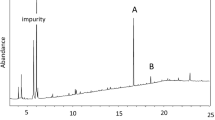
Analysis of replicate data for male and female I. scapularis whole body hexane extractions identified over 75 distinct compounds (Figs. 7, 8). n-Hexane was run individually as a control to ensure that peaks identified for analyses did not originate from the hexane or the process of extraction. Due to the variability in data analysis and repeatability, only compounds found in all replicates, of each sex respectively, and with a match probability ≥80 % were reported. These criteria identified 17 putative cuticle compounds including alkanes, an alkene, an aromatic hydrocarbon, carboxylic acid esters, fatty acid esters, fatty alcohols, a fatty aldehyde, a fatty amide, and terpenes. Despite having a low match probability (70 %) one steroid, repeatedly appearing in all replicates of both sexes, was also included in the analyses. These 18 compounds represent 70–75 % of the column eluants, and are listed in Table 1 and Fig. 9. Several additional compounds were identified in female and male whole body extracts, but in small amounts (<0.05 %), which impeded their confirmation of match identity. The majority of these unconfirmed, identified compounds were found in both sexes, though a few compounds were exclusive to females including androstanone, estronone, and valeric acid. But due to the difficulty in confirming peak identity, these compounds were excluded from further analyses. Compound abundances were converted to percentages using the sum of abundances for all 18 compounds as a divisor. Mean percent abundances are based on whole body extracts of 10 unfed virgin adult females or males. The two most abundant components identified in both female and male whole body extracts were the fatty acid esters butyl palmitate and butyl stearate (Fig. 9a). Butyl palmitate was the most abundant compound identified in male whole body extracts (35.30 ± 1.14) (Mean % ± SEM), and was identified in a significantly higher abundance than in female whole body extracts (18.57 ± 0.94; t = 11.32, df = 8.943, P < 0.001). Butyl stearate was the most abundant compound identified in female whole body extracts (27.53 ± 0.48) in a significantly higher abundance than in male whole body extracts (21.40 ± 0.51; t = 8.753, df = 8.977, P < 0.001) (Fig. 9a). There was also a substantial difference observed in the abundances of the aromatic hydrocarbons identified in male and female whole body extracts (Fig. 9b). 1,2,3-Trimethylbenzene was the third most abundant component identified in male whole body extracts (6.30 ± 0.18), and occurred in a significantly higher abundance than in female whole body extracts (2.13 ± 0.19; t = 15.93, df = 8.970, P < 0.001). The third most abundant component identified in female whole body extracts was not an aromatic hydrocarbon, but the fatty acid amide erucylamide, and occurred in a significantly higher abundance in female whole body extracts (6.53 ± 0.26) than in male whole body extracts (4.90 ± 0.46; t = 3.085, df = 7.720, P = 0.016) (Fig. 9b). Alkanes identified in female and male whole body extracts include compounds with 10, 14, 15, 16, 17, and 18 carbons (Fig. 9c). The abundances in male whole body extracts of C-10 (1.97 ± 0.15), C-15 (1.44 ± 0.031), C-16 (0.70 ± 0.06), and C-18 (1.87 ± 0.09) were all significantly higher than those identified in female whole body extracts; C-10 (1.23 ± 0.14; t = 3.488, df = 8.889, P = 0.007), C-15 (0.97 ± 0.04; t = 10.18, df = 8.578, P < 0.001), C-16 (0.00 ± 0.00; t = 11.67, df = 5.000, P < 0.001), and C-18 (1.43 ± 0.8; t = 3.654, df = 9.000, P = 0.0053). There was no significant difference between the abundances of C-17 identified in female (0.767 ± 0.88) and male (0.767 ± 0.12) whole body extracts. The final alkane identified, C-14, was present only in female whole body extracts (1.69 ± 0.1; t = 16.90, df = 4.00, P < 0.001). In addition to the alkanes there was one alkene C-7, also identified only in female whole body extracts (0.85 ± 0.03; t = 28.33, df = 4.000, P < 0.001) (Fig. 9c). The carboxylic acid esters, butyl benzoate and ethyl-4-ethoxybenzoate, were identified in the whole body extracts of both sexes (Fig. 9a). The abundance of ethyl-4-ethoxybenzoate identified in female whole body extracts (5.30 ± 1.0) was significantly higher than the abundance identified in male whole body extracts (2.56 ± 0.35; t = 2.586, df = 4.980, P = 0.049). There was no significant difference between the abundances of butyl benzoate identified in female (1.26 ± 0.03) and male (1.10 ± 0.10) whole body extracts. Two fatty alcohols were identified in female and male whole body extracts, 1-heptanol and 1-hexadecanol (Fig. 9b). 1-Hexadecanol was identified in both female (1.5 ± 0.11) and male (1.67 ± 0.03) whole body extracts with no significant difference in abundance between the sexes. 1-Heptanol was identified in only female whole body extracts (0.65 ± 0.03; t = 21.67, df = 4.000, P < 0.001). The remaining compounds identified in female and male whole body extracts were the steroid 5β-androstane, and the terpenes phytol and thymol (Fig. 9b). Significant differences in abundances between the sexes were found for both 5β-androstane and thymol. 5β-androstane was identified in female whole body extracts (2.87 ± 0.30) in a significantly higher abundance than in male whole body extracts (0.76 ± 0.12; t = 6.530, df = 5.274, P = 0.0010). Thymol was identified only in male whole body extracts (0.70 ± 0.06; t = 11.67, df = 5.000, P < 0.001). There was no significant difference between the abundances of phytol identified in female (1.43 ± 0.1) and male (1.90 ± 0.40) whole body extracts.
Example of a GC–MS chromatogram of a whole body surface hexane extraction of 10 unfed virgin adult female Ixodes scapularis ticks. The compounds identified with ≥80 % match probability are numbered and further described in Table 1 and Fig. 9. Missing numbers represent compounds not identified in females. Abundances were determined using flame ionization detection. Time = min
Example of a GC–MS chromatogram of a whole body surface hexane extraction of 10 unfed virgin adult male Ixodes scapularis ticks. The compounds identified with ≥80 % match probability are numbered and further described in Table 1 and Fig. 9. Missing numbers represent compounds not identified in males. Abundances were determined using flame ionization detection. Time = min
Mean percent abundance (+1 SEM) of compounds identified (Table 1) in whole body hexane extractions of Ixodes scapularis unfed virgin adult females versus unfed virgin adult males. Mean abundance for a compound was calculated by averaging the peak areas identified in all 5 female and 6 male replicate GC–MS runs. Percentages were calculated out of the total abundance obtained for all 18 compounds identified. Arrows denote zero values. Treatments were compared using a paired student’s t test (*P < 0.05). Numbers in parenthesis correlate to assigned numbers in Table 1 and peak numbers in Figs. 7 and 8
Discussion
Laboratory bioassays demonstrated that I. scapularis adults rely on direct contact for reproduction. If unfed, mate seeking males can respond to sex attractant volatiles from unfed, virgin females, we would have expected the males to aggregate around and/or on top of the mesh bags in which the females were confined. As shown in Fig. 4, this did not happen. Our findings do not support the hypothesis that volatile pheromones are required for male mating behavior. In our studies, unfed, virgin adult males did not engage in mating behavior unless direct contact with females was possible. Additionally, washing of unfed virgin adult females decreased chelicerae insertion/maintenance behavior of unfed virgin adult males, removing surface compounds that possibly function as sex pheromones in I. scapularis. It is interesting to note that washed females apparently were capable of regenerating these compounds after a 48 h period, restoring normal mating behavior. Since I. scapularis are natural ambushers when it comes to host seeking, it is plausible that their mating strategies employ a similar behavior of waiting for transient male–female contact. This eliminates the need for volatile attractant sex pheromones to engage adult male I. scapularis to actively search for emitting adult females. Additionally, prostriate tick reproduction is not dependent on blood feeding, and would not require effective attractant sex pheromones like those in metastriate ticks to markedly influence male behavior, i.e., cessation of blood feeding and initiation of mating. Instead, it is probable that I. scapularis couple aggregation behavior with mating activity. These two behaviors act synergistically to congregate conspecific adults that can subsequently mate. As I. scapularis encounter each other through aggregation, adult males come in direct contact with adult females, and with putative sex pheromones located on the cuticle providing the necessary chemical cues for successful mounting and insemination. The large size discrepancies between adult and immature I. scapularis, as well as the lack of reproductive orifices, makes adults easily distinguishable from other life stages through physical contact. This coupling of aggregation and mating behavior is also evident in blood feeding I. scapularis, congregating on the host in protected locations behind ears and in axilla. Pairing aggregation and mating behaviors ensures the viability of I. scapularis populations by increasing the probability of contact between reproductives and subsequent mating.
Ixodes scapularis unfed virgin adult males demonstrated the highest percent chelicerae genital pore insertion/maintenance when paired with unfed virgin adult females and replete mated adult females in laboratory bioassays. Zemek et al. (2002) observed similar results with I. ricinus. Unfed virgin adult females had the highest percentage of male chelicerae insertion/maintenance and spermatophore transfer. However, replete mated adult females were deemed more attractive to unfed virgin adult males having the fastest rate of mounting and female genital pore penetration. Further analysis revealed that despite males remaining in copula with replete females, the majority of males did not transfer spermatophores. Zemek et al. (2002) theorized that this decrease in successful insemination of replete mated adult females was due to a “copulation inhibiting system” that is turned on after the females become fully engorged. This may not be necessarily true. It has been shown that unknown factors, likely associated with spermatophores and male saliva, cause males to abort insemination with females that have been previously mated. This re-mating inhibition slowly decreases after females fully engorge, becoming less apparent 5 days post-repletion, when they are capable of being re-inseminated (Kiszewski and Spielman 2002). This decrease in re-mating inhibition may be explained by the degradation of spermatophores and the recapicitation of sperm that have become sessile again during storage, providing an opportunity for sperm displacement (Kiszewski et al. 2001). It is also possible that re-mating inhibiting factors are sequestered from the female genital pore upon completion of blood feeding and initiation of oocyte maturation.
Zemek et al. (2002) concluded that the increase in attractiveness of replete mated adult females was due to an increase in production of attractive pheromones. This seems counter-intuitive for replete mated adult females to focus on re-mating rather than oviposition. It is known that male I. rubicundus and I. holocylus regard replete females as sources of nourishment, gaining nutrients through copula or by inserting their mouthparts into the body wall of females (Moorhouse and Heath 1975; Fourie et al. 1988). An alternative hypothesis is that prolonged feeding and host association naturally changes the cuticular chemistry of replete females, becoming attractive to males that primarily seek nourishment, engaging in mating after nutritional needs have been met, and if there are no re-mating inhibition factors. This theory may also explain the distinct decrease in mating activity with part-fed females. Changes in the cuticular chemistries of I. scapularis adult females from host interactions and feeding may be gradual, and require a prolonged period of time before becoming apparent to males. Since pheromone recognition in ixodid ticks is dependent on the ratio of signals from generalized chemosensory sensilla, I. scapularis males may only identify cuticular chemistries of unfed and replete females (Osterkamp et al. 1999; Waladde and Rice 1982). Subtle changes occurring in part-fed females may be distinct enough to distinguish them from unfed females, preventing mating, but not be substantial enough to induce male attraction like replete females. Since it is highly unlikely that adult males would encounter part-fed, virgin adult females in the wild, there may be other factors that deter male mating. The increase rate of mounting between unfed virgin adult males and replete mated adult females also may be explained by the cessation of locomotor activity in replete females. It would be easier for males to contact females when they are not moving. With both sexes wandering, waiting to achieve contact may be more difficult and take longer, decreasing the speed of mating. This also further illustrates the importance of coupling aggregation and mating behavior to increase the efficacy of mating in I. scapularis. More research is needed in general to understand the different male mating preferences in Ixodes sp.
Whole body cuticular extractions of male and female I. scapularis have identified a few compounds that may be components of reproductive pheromones regulating courtship and mating. Fatty acids function as aggregation pheromones in Amblyomma ticks (Carr and Roe, in press) and are also components of genital pore pheromones in Dermacentor ticks (Allen et al. 1988). The two most abundant fatty acids in I. scapularis whole body extracts, identified as the derivatized esters butyl palmitate and butyl stearate, are also present in R. annulatus, R. bursa, and R. sanguineus body extracts. Both fatty acid esters were present in ratios that were distinct for each Rhipicephalus species (Shimshoni et al. 2013). In I. scapularis, there are clear distinctions in the ratios of butyl palmitate and butyl stearate between males and females. The fatty acid palmitic acid, which co-elutes with butyl palmitate, is also present in body extracts of I. persulcatus, though in combination with additional fatty acids not identified in I. scapularis (Tkachev et al. 2000). It is apparent that fatty acids create unique cuticular chemistries that are species and sex specific, and may be important during male–female interactions and mating. Perhaps fatty acids allow for sex differentiation among reproductives searching for conspecifics for mating, though this is still unclear.
In insects, fatty alcohols act as both pheromones and precursors for fatty aldehydes. 1-Hexadecanol and 1-heptanol are the first fatty alcohols to be identified in ixodid tick whole body extracts. 1-Hexadecanol is an important component of female sex pheromone in the cotton bollworm Helicoverpa armigera (Zhang et al. 2012). 1-Heptanol is a primary pheromone component of the female pine beetle Dendroctonus jeffreyi, and attractive to both adult females and males (Paine et al. 1999). Since fatty alcohols function as both reproductive and aggregation pheromones in insects, further investigations of their role in I. scapularis reproduction is warranted.
There is currently no evidence that cuticular hydrocarbons function as pheromones in ticks (Estrada-Peña et al. 1994). The cuticular hydrocarbons identified in I. scapularis whole body extracts are less diverse and shorter overall than those reported in metastriate ticks. Estrada-Peña et al. (1992, 1993) documented over 100 distinct hydrocarbons in varying lengths of 11–39 carbons, and degrees of saturation and branching in whole body extracts of several Rhipicephalus and Amblyomma species. Only 6 linear, saturated hydrocarbons were identified in I. scapularis whole body extracts ranging from lengths of 10–18 carbons. The short chain alkene 1-heptene, previously undocumented in Ixodes species, was also identified (Estrada-Peña et al. 1994; Tkachev et al. 2000). Since metastriate ticks actively hunt for or ambush hosts, they are more susceptible to environmental conditions than prostriate ticks, which quest and usually remain on or near plants that offer shelter and protection. Perhaps metastriate ticks have a thicker epicuticular layer consisting of a greater number of more diverse hydrocarbons to provide additional protection when host seeking, though this requires further investigation.
This is the first documentation of fatty amides, specifically erucylamide in ticks. Fatty amides are the major component of cuticle extracts of psocopterans (Howard and Lord 2003), and in particular erucylamide a component of whole body extracts of the malaria mosquito, Anopheles gambiae (Caputo et al. 2005). Fatty amides are also present on the cuticle and in the Dufour gland of the paper wasps Polistes dominulus and P. sulcifer (Dani 2006). Unfortunately, fatty amides are not well studied because they were previously hypothesized to be plastic contaminants. The majority of plastic contaminants identified in our I. scapularis whole body extracts were phthalates, with no fatty amides identified in our controls. Additionally, GC–MS analyses of ants (Lenoir et al. 2012) and spiders (Xiao et al. 2009) housed in similar plastic containers to ours identified the same phthalate contaminants. Thus, we can assume that the identified fatty amide is not a plastic contaminant, and may play a role in prostriate tick chemical communication or cuticular function.
This study is the first documentation of aromatic hydrocarbons in I. scapularis whole body extracts. Aromatic hydrocarbons are functional components of insect assembly pheromones rather than sex pheromones (Torto et al. 1994). Aromatic hydrocarbons have also been identified in mite exocrine glands that secrete complex species-specific oils used as alarm pheromones (Heethoff 2012). In some mite species, components of alarm and aggregation pheromones also functions as components of reproductive pheromones. The secretion of these chemical components in distinct concentrations allows mites to discriminate between the pheromones and respond accordingly (Carr and Roe in press). Since ticks are closely related to mites, aromatic hydrocarbons should be further evaluated as functional components of alarm, aggregation, and reproductive pheromones and the concentrations that distinguish one pheromone from another.
Carboxylic acids and acid derivatives are functional components of many insect pheromones. This is the first documentation of carboxylic acid esters in tick whole body extracts. In lepidopterans, carboxylic acids are commonly biosynthesis intermediates for pheromones (Bjostad et al. 1987). Unfortunately, the role of carboxylic acids in ticks is currently unknown. 5β-Androstane is a steroid hormone and the precursor for all estrogens and a variety of axillary steroids that function as pheromones in mammals. Since tick steroids have mammalian counterparts, for example tick ecdysteroid and vertebrate testosterone, it is possible that the component currently identified as 5β-androstane in I. scapularis body extracts is a closely related steroid regulating mating, which has not yet been discovered (De Loof 2006). Terpenes are typically associated with plants and disregarded as environmental contaminants. With the repeated identification of terpenes in tick whole body extracts, these compounds also should be further studied to determine their exact role in the Ixodidae.
In summary, our bioassays provided no evidence of a volatile female sex pheromone to attract males. It appears that direct male contact with the female and chemical cues from the female are needed to elicit male mounting of the female and the insertion of his chelicerae into the female genital track. This occurs for both virgin unfed and mated replete females but not virgin part-fed females. A number of unique compounds were identified by GC–MS from hexane surface extracts of unfed males and females, not previously described in other ticks, with potential roles in chemical communication. Sex specific differences were found in some compounds extracted from the cuticle, and a preference for shorter and less diverse cuticular hydrocarbons in I. scapularis was found compared to metastriate ticks.
References
Allen SA, Phillips JD, Taylor D, Sonenshine DE (1988) Genital sex pheromones of ixodid ticks: evidence for the role of fatty acids from the anterior reproductive tract in mating of Dermacentor variabilis and Dermacentor andersoni. J Insect Physiol 34:315–323
Allen SA, Sonenshine DE, Burridge MJ (2002) Tick pheromones and uses thereof. United States Patent Office, patent no. 6331297
Benoit JB, Lopez-Martinez G, Philips SA, Elnitsky MA, Yoder JA, Lee RE Jr, Denlinger DL (2008) The seabird tick, Ixodes uriae, uses uric acid in penguin guano as a kairomone and guanine in tick feces as an assembly pheromone on the Antarctic Peninsula. Polar Biol 31:1445–1451
Bjostad LB, Wold WA, Roelofs WL (1987) Pheromone biosynthesis in lepidotpterans: desaturation and chain shortening. In: Prestwich GD, Blomquist GJ (eds) Pheromone biochemistry. Academic Press, Orlando, pp 77–120
Caputo B, Dani FR, Horne GL, Petrarca V, Turillazzi S, Coluzzi M, Priestmand A, della Torre A (2005) Identification and composition of cuticular hydrocarbons of the major Afrotropical malaria vector Anopheles gambiae s.s. (Diptera: Culicidae): analysis of sexual dimorphism and age related changes. J Mass Spectrom 40:1595–1604
Dani FR (2006) Cuticular lipids as semiochemicals in paper wasps and other social insects. Ann Zool Fennici 43:500–514
De Loof A (2006) Ecdysteroids: the overlooked sex steroid of insects? Males: the black box. Insect Sci 13:325–338
Dobrotvorsky AK, Tkachev AV (1995) Evidence of a volatile sex pheromone in the unfed adult taiga tick Ixodes persulcatus Schulze. In: 2nd international conference on tick-borne pathogens at the host-vector interface: an agenda for research, proceedings and abstract, vol 1, pp 372–375
Dusbábek FP, Simek EAP, Bouman R, Zahradnikova H, Zemek R (2001) Composition and effect of several semiochemicals in Ixodes ricinus L. (Acari: Ixodidae). In: Blaszak C, Bucsek A (eds) Stawonogi Pasozyty i Nosiciele. Lublin, Wydawnictwo KGM, pp 33–43
Estrada-Peña A (1993) Climate and cuticular hydrocarbon variations in Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol Res 79:512–516
Estrada-Peña A, Estrada-Peña R, Peiro JM (1992) Differentiation of Rhipicephalus ticks (Acari, Ixodidae) by gas-chromatography of cuticular hydrocarbons. J Parasitol 78:982–993
Estrada-Peña A, Castellá J, Siuda K (1994) Cuticular hydrocarbon composition and pheromone variability in sympatric populations of Ixodes ricinus ticks from Poland. Exp Appl Acarol 18:247–263
Fourie LJ, de Jager T, Petney TN (1988) Prolonger or repeated copulation and male longevity in the tick Ixodes rubicundus. J Parasitol 74:609–612
Graf JF (1978) Ecology and ethology of Ixodes ricinus (Ixodidae). B Swiss Entomol Soc 50:241–253
Haggart DA, Davis EE (1981) Neurons sensitive to 2,6-dichlorophenol on the tarsi of the tick Amblyomma americanum (Acari: Ixodidae). J Med Entomol 3:187–193
Hamilton JGC, Sonenshine DE, Lushby WR (1989) Cholesteryl oleate; mounting sex pheromone of the hard tick, Dermacentor variabilis. (Say) (Acari: Ixodidae). J Insect Physiol 35:873–879
Heethoff M (2012) Regeneration of complex oil-gland secretions and its importance for chemical defense in an oribatid mite. J Chem Ecol 38:1116–1123
Howard RW, Lord JC (2003) Cuticular lipids of the booklouse, Liposcelis bostrychophila: hydrocarbons, aldehydes, fatty acids, and fatty acid amides. J Chem Ecol 29:615–627
Kiszewski AE, Spielman A (2002) Preprandial inhibition of re-mating in Ixodes ticks (Acari: Ixodidae). J Med Entomol 39:847–853
Kiszewski AE, Matuschka F, Spielman A (2001) Mating strategies and spermiogenesis in ixodid ticks. Annu Rev Entomol 46:167–182
Lenoir A, Cuvillier-Hot V, Devers A, Christidès J, Montigny F (2012) Ant cuticles: a trap for atmospheric phthalate contaminants. Sci Total Environ 441:209–212
Moorhouse DE, Heath CG (1975) Parasitism of female ticks by males of the genus Ixodes. J Med Entomol 12:571–572
Mulenga A (2014) Molecular biology and physiology of chemical communication. In: Sonenshine DE, Roe RM (eds) Biology of ticks, vol I. Oxford University Press, New York, pp 368–397
Osterkamp J, Wahl U, Schmalfuss G, Haas W (1999) Host odor recognition in two tick species coded in a blend of vertebrate volatiles. J Comp Physiol 185:59–67
Paine TD, Millar JG, Hanlon CC, Hwang JS (1999) Identification of semiochemicals associated with Jeffrey pine beetle, Dendroctonus jeffreyi. J Chem Ecol 25:433–453
Shimshoni JA, Erster O, Cuneah O, Soback S, Shkap V (2013) Cuticular fatty acid profile analysis of three Rhipicephalus tick species (Acari: Ixodidae). Exp Appl Acarol 61:481–489
Soares SF, Borges MLF (2012) Electrophysiological responses of the olfactory receptors of the tick Amblyomma cajennense (Acari: Ixodidae) to host-related and tick pheromone-related synthetic compounds. Acta Trop 124:192–198
Sonenshine DE (2006) Tick pheromones and their use in tick control. Annu Rev Entomol 51:557–580
Sonenshine DE, Adams T, Allan SA, McLaughlin J, Webster FX (2003) Chemical composition of some components of the arrestment pheromones of the black-legged tick, Ixodes scapularis (Acari: Ixodidae) and their use in tick control. Med Entomol 40:849–859
Tkachev AV, Dobrotvorsky AK, Vjalkov AI, Morozov SV (2000) Chemical composition of lipophylic compounds from the body surface of unfed adult Ixodes persulcatus ticks (Acari: Ixodidae). Exp Appl Acarol 24:145–158
Torto B, Obeng Ofori D, Njagi PGN, Hassanali A, Amiani A (1994) Aggregation pheromones systems of adult gregarious desert locust Schistocerca gregaria. J Chem Ecol 20:1749–1762
Treverrow NL, Stone BF, Cowie M (1977) Aggregation pheromone in two Australian hard ticks, Ixodes holocylus and Aponomma concolor. Experientia 33:680–682
Waladde SM, Rice MJ (1982) The sensory basis of tick feeding behavior. In: Obenchain FD, Galun R (eds) Physiology of ticks. Pergamon Press, Oxford, pp 71–118
Xiao YH, Zhang JX, Li SQ (2009) A two-component female produced pheromone of the spider Pholcus beijingensis. J Chem Ecol 35:769–778
Zemek R, Bouman EAP, Socha R, Dusbábek F (2002) The effect of feeding status on sexual attractiveness of Ixodes ricinus (Acari: Ixodidae) females. Exp Appl Acarol 27:137–149
Zhang J, Salcedo C, Fang Y, Zhang R, Zhang Z (2012) An overlooked component: (z)-9-tetradecenal as a sex pheromone in Helicoverpa armigera. J Insect Physiol 58:1209–1216
Acknowledgments
This work was funded by grants to RMR and DES from NIH (1R21AI096268) and NSF (IOS-0949194) and from support to RMR from the NCSU Ag. Research Station. ALC was also supported in part by a Graduate Student Teaching Assistantship from the Department of Entomology at North Carolina State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest in the publication of the results reported in this paper.
Ethical standard
Students conducting research on this project have received training in a Graduate School approved ethics course at NC State University, which complies to the NSF standards.
Human and animal rights
No human subjects were used in the research reported in this paper. All use of animals in this study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols were approved by the Old Dominion University Institutional Animal Care and Use Committee (#10-018 and #10-032) and are on file at the Office of Research, Old Dominion University, Norfolk, Virginia. Tranquilizers (Acepromazine) were administered to the animals prior to handling to minimize anxiety and/or discomfort. No animal work was conducted at NC State University.
Rights and permissions
About this article
Cite this article
Carr, A.L., Sonenshine, D.E., Strider, J.B. et al. Evidence of female sex pheromones and characterization of the cuticular lipids of unfed, adult male versus female blacklegged ticks, Ixodes scapularis . Exp Appl Acarol 68, 519–538 (2016). https://doi.org/10.1007/s10493-015-0009-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-0009-y