Abstract
The salivary glands are vital to the biological success of ticks and they are a major route of pathogen transmission. Tick salivary glands undergo remarkable growth and differentiation during the blood-feeding period. MicroRNAs (miRNAs) are noncoding small RNA molecules found in diverse organisms that regulate gene expression at the post-transcriptional level. To explore transcriptional differences in the miRNAs of fed and unfed tick (Haemaphysalis longicornis) salivary glands, we investigated small RNA (sRNA) transcriptomes derived from the salivary glands and made a comparative analysis of miRNA profiles related to tick blood-feeding in the salivary glands. We generated two small RNA libraries from the salivary glands of unfed and fed H. longicornis, and obtained 14.8 and 10.3 million reads of 18–30 nt, respectively. The unfed-specific sRNAs were clearly richer than the fed-specific sRNAs in terms of the unique and total sRNAs. Overall, 769 conserved miRNA families were found in unfed samples, whereas 440 conserved miRNA families were found in fed samples. Six of the ten most abundant miRNA were found in both the unfed and fed tick salivary glands, i.e., miR-1, miR-375, bantam, miR-184, miR-739, and miR-263a. We found that known miRNA homologs displayed a wide variety of expression profiles in unfed and fed tick salivary glands. After blood-feeding, 162 known miRNAs were upregulated. The six main upregulated miRNAs were mir-1810, mir-2138, mir-2140, mir-425*, mir-429, and mir-516*. Likewise, 231 known miRNAs were downregulated after blood-feeding. The six main downregulated miRNAs were miR-2941-1*, miR-10-5p, miR-2973, miR-1183, miR-4006b-5p, and miR-881. We found that distinct microRNA profiles in the salivary glands of H. longicornis were relating to tick blood feeding. The differential expression of miRNAs in unfed and fed tick salivary glands supported their involvement at new levels in the regulation of tick blood-feeding. Our data provide an important resource for a more detailed functional analysis of miRNAs in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are ectoparasites with a global distribution that have veterinary and medical importance. They live in diversified habitats, making close contact with a wide range of vertebrate hosts and they transmit a variety of disease agents to human beings, domesticated animals, and wildlife (Sonenshine 1991; Magnarelli 2009). The salivary glands are vital to the biological success of ixodid ticks and the major route of pathogen transmission (Bowman et al. 1997). Their important functions include the absorption of water vapor from unsaturated air in free-living ticks, excretion of excess fluid to ensure blood meal concentration, and the secretion of bioactive protein and lipid compounds during tick feeding (Sauer et al. 2000). During the blood-feeding period, the tick salivary glands undergo remarkable growth and differentiation and a suite of qualitative and quantitative changes occur in the mRNA and protein content (Oaks et al. 1991; Valenzuela et al. 2002; Wang et al. 2007; Aljamali et al. 2009), although the factor(s) controlling this increased gene activity and salivary gland growth are poorly understood.
Recently discovered microRNA (miRNA)-directed posttranscriptional regulation may provide efficient fine-tuning of targeted gene expression in specific cell or tissue types, thereby coordinating their spatial and temporal control (Flynt and Lai 2008; Siomi and Siomi 2009). Furthermore, miRNA-guided suppression of the target genes may be rapidly released, so reactivation is faster than transcriptional activation of a genomic locus. Thus, miRNAs may act as reversible regulators (Flynt and Lai 2008). Because of their versatility, miRNAs have evolved as a major class of gene regulatory molecules that are critical for a diverse range of biological processes such as cell proliferation, differentiation, apoptosis, and stress responses (Banerjee and Slack 2002; Banerjee and Slack 2005; Bartel 2004, 2009). The discovery of miRNAs has provided new opportunities for understanding the biology of a number of species. Only a few miRNAs have been identified in the ticks Rhipicephalus microplus and Ixodes scapularis (Barrero et al. 2011; Griffiths-Jones et al. 2008), and there are no reports of specific miRNA profiles that are related to tick blood-feeding in the salivary glands.
The hard tick, Haemaphysalis longicornis, is distributed mainly in East Asia and Australia, where it transmits a wide range of pathogens including bovine theileriosis (Theileria spp), bovine babesiosis (Babesia ovata), canine babesiosis (Babesia gibsoni), and human rickettsiosis (Rickettsia japonica) (Fujisaki et al. 1994; Jongejan and Uilenberg 2004). The importance of this tick means that a greater understanding of their biology could lead to the development of effective control measures. To provide new insights into the biology of this tick and to expand our knowledge of tick miRNAs, we investigated small RNA transcriptomes derived from fed and unfed tick salivary glands of H. longicornis. We then performed a comparative analysis of miRNA profiles related to tick blood-feeding in the salivary glands.
Materials and methods
Tick sampling and total RNA preparation
A colony of parthenogenetic H. longicornis ticks was collected from deer in the Shanghai Wildlife Park, China. A single engorged female was used to establish the tick colony. Tick rearing was performed in the laboratory in a dark incubator at 25 °C with 92 % relative humidity, with feeding on a New Zealand white rabbit. After three generations had been maintained under laboratory conditions, the tick colony was established in the Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences, Shanghai, China (Zhou et al. 2004). To collect the fed tick salivary glands, adult ticks were recovered from rabbit ears after 4 days of feeding and the salivary glands were immediately dissected under the microscope. The same batches of unfed adult ticks were used to dissect the unfed tick salivary glands. Dissection of ticks was conducted as described elsewhere (Gao et al. 2011). Briefly, unfed or partially fed ticks were submerged in autoclaved ice-cold phosphate-buffered saline (PBS; pH 7.4) under a dissection light microscope and held down with a pair of soft-tissue forceps. The dorsal cuticle was cut and the salivary glands were separated from the remaining organs using 18-gauge needles. After dissection, the salivary glands were pipetted into microcentrifuge tubes, washed once with PBS, and stored at −80 °C until use. The total RNA was extracted using Trizol reagent (Invitrogen), according to the manufacturer’s protocol. The purity and integrity of the total RNA was examined by standard agarose gel electrophoresis, while the concentration was determined using a BioPhotometer (Eppendorf). The purified total RNA was stored at −80 °C until use.
Small RNA isolation and high-throughput sequencing
RNA fragments measuring 20–30 bases in length were isolated and purified from 10 μg of total RNA using a Novex 15 % TBE-Urea gel. We added 5′ and 3′ adaptors (Illumina) to the ends of fragments. Reverse transcription PCR (RT-PCR) was performed using an RT-PCR kit (Invitrogen). The fragments were purified using a 6 % TBE PAGE gel and used for high-throughput sequencing with a Solexa sequencer at Huada Genomics Institute Co. Ltd, China. All the gels and kits used for small RNA purification and amplification were bought from Invitrogen Co. Ltd.
Sequence analysis and identification of homologs of conserved miRNA
After masking the adaptor sequences and removing redundant reads smaller than 18 nt, the clean reads were screened against GenBank and the Rfam database (version 9.0; http://www.sanger.ac.uk/software/Rfam/mirna) to remove noncoding RNAs, such as rRNAs, tRNAs, snRNAs, snoRNAs, and other ncRNAs. The sequences of candidate precursors were analyzed using RepeatMasker (http://www.repeatmasker.org) to eliminate any repetitive sequences, before the remaining reads were searched against the Sanger miRBase (version 17.0) to identify conserved miRNAs. Based on the nomenclature of miRNAs, reads with high similarity to known miRNAs from other organisms (mismatches ≤2) were classified into the same miRNA family (Wei et al. 2009; Chen et al. 2009). The family distribution of conserved miRNA and the nucleotide bias were analyzed statistically to determine the expression and coding characters of miRNAs. Reads that did not match any database hits were marked as unannotated. Clean reads were then mapped onto the genome of Drosophila melanogaster (http://www.fruitfly.org/sequence/release5genomic.shtmlt) using the program Short Oligonucleotide Analysis Package (SOAP) (Li et al. 2008).
Differential expression analysis of miRNAs
We compared the expression of known miRNAs in the two samples to identify any differentially expressed miRNAs. The expression of miRNAs in the two samples was plotted as the log2 ratio and a scatter plot. The procedures were as follows:
-
1.
Normalize the miRNA expression in the two samples (control and treatment) as transcripts per million (TPM). Normalization formula:
$$ {\text{Normalized}}\;{\text{miRNA}}\;{\text{expression}} = ({\text{actual}}\;{\text{miRNA}}\;{\text{count/total}}\;{\text{count}}\;{\text{of}}\;{\text{clean}}\;{\text{reads}}) \times 1000000 $$ -
2.
Calculate the fold-change and P value from the normalized expression. Generate the log2 ratio plot and scatter plot. Fold-change formula:
$$ {\text{Fold-change}} = \log _{2} ({\text{treatment/control}}) $$
P value formula:
Results
Profile characteristics of small RNA pools in libraries
We obtained raw data by sequencing two small RNA (sRNA) pools from the salivary glands of unfed and fed-day-fed adults of the tick H. longicornis, using the latest Solexa sequencing technology (Glazov et al. 2008; Hafner et al. 2008). We filtered the low quality reads according to the base quality value, trimmed the adaptor sequence at the 3′ primer terminus, cleaned up 5′ adaptor contaminants formed by ligation, and finally collected the small RNAs and analyzed their size distribution. All identical sequence reads in each sRNA library were then grouped and converted into unique sequences with their associated counts for individual reads (Additional File 1).
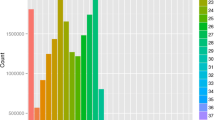
Deep sequencing yielded about 15.0 and 10.9 million reads with high quality from the unfed and fed tick salivary glands, respectively. Based on the size distribution of the total reads, we found a distinct bimodal distribution in both libraries, with one peak around 21–23 nt representing miRNAs and another around 27–29 nt that mainly represented longer piRNA-like sRNAs (Fig. 1). The peak at the 27–29 nt size class was larger in the unfed salivary glands, suggesting that these sRNAs were more diverse in unfed ticks. Overall, the peak in the 27–29 nt size class was larger than that in the 21–22 nt size class, which suggested that larger-sized sRNAs were more abundant in both unfed and fed tick salivary glands.
In the next step, we removed reads smaller than 18 nt and a total of 14.8 million clean reads remained with 3.6 million (24.32 %) unique sequences in the unfed tick salivary glands. Similarly, a total of 10.3 million clean reads remained with 1.3 million (12.62 %) unique sequences in the fed tick salivary glands. Common and specific sRNAs from the fed and unfed tick salivary glands are shown in Table 1, which indicates that the unfed sRNAs were more abundant than the fed sRNAs in terms of both unique sRNAs and total sRNAs. Among the clean reads of unfed and fed samples, only 5.59 and 7.97 % were perfectly mapped to the Drosophila melanogaster genome, including 0.26 and 0.49 % unique sequences, respectively.
Among the clean reads, 4.41 and 4.42 % were ncRNAs in the unfed and fed samples, respectively, including rRNAs, tRNAs, snRNAs, and snoRNAs. Repeat-associated small RNAs derived from high-repeat regions of genomes or transposon-regions were found to belong to two types of repeat, i.e., rRNA:1 and ambi. The proportion of known miRNAs in unfed tick samples was 12.42 % from 1,847,554 reads, which included 37,853 unique sequences. The sRNAs matching known miRNAs from unfed tick salivary glands are shown in Additional File 2. Similarly, the proportion of known miRNAs in fed tick samples was 14.63 % from 1,513,624 reads, which included 14,144 unique sequences. The sRNAs matching known miRNAs from fed tick salivary glands are shown in Additional File 3. Apart from the miRNA, rRNA, and repeats mentioned above, 83.15 % (unfed) and 80.94 % (fed) sequences had no matches and they were marked as unannotated (Table 2).
Expression profiles of miRNA in unfed and fed tick salivary glands
Overall, we found 769 conserved miRNAs families in the unfed tick salivary glands, where 588 types of miRNA had copy numbers <100, while 130 types had only one copy. We found that 97 types of miRNA had copy numbers between 100 and 1,000, while only 47 types of miRNA had copy numbers between 1,000 and 10,000. Furthermore, only 37 types of miRNA had copy numbers >10,000 (Additional File 4).
Overall, 440 conserved miRNA families were found in the fed tick salivary glands. We found 322 types of miRNA had copy numbers <100, while 39 types had only one copy. There were 67 types of miRNAs with copy numbers between 100 and 1,000, while only 32 types of miRNA had copy numbers between 1,000 and 10,000. Moreover, only 19 types of miRNA had copy numbers >10,000 (Additional File 5).
Previous reports have indicated that evolutionarily conserved miRNAs are often highly expressed (Zhang et al. 2007; Glazov et al. 2008; Reddy et al. 2009). In the tick H. longicornis, the ten most abundant miRNAs from the unfed and fed salivary glands are shown in Table 3. Six of the ten most abundant miRNAs were found in both the unfed and fed tick salivary glands, i.e., miR-1, miR-375, bantam, miR-184, miR-739, and miR-263a. Thus, these highly expressed miRNAs may be evolutionarily conserved miRNAs in the tick H. longicornis. However, some of the most abundant miRNAs in salivary glands also changed significantly with blood-feeding in terms of miRNA families and the order of their abundance. These conserved families are known to be presented in a large range of organisms. This indicates that H. longicornis miRNAs are distributed widely.
Differentially expression of miRNAs in unfed and fed samples
We compared the global miRNA expression levels in samples by normalizing all of the known miRNA transcripts in each sample as reads per million. We calculated the fold-change and P value from the normalized expression data. We then generated a log2 ratio plot and a scatter plot (Fig. 2 and Additional File 6). We found that 78 miRNAs were expressed equally (within a two-fold change), 42 miRNAs were expressed within a one-fold change in unfed and fed samples (Table 4), which included six of the ten most abundant miRNAs, i.e., mir-1, mir-375, mir-184, mir-263a, mir-739, and bantam. These conserved miRNAs must have essential roles in tick salivary glands.
We found that 162 known miRNAs were upregulated after tick blood-feeding (>two-fold), while 42 known miRNAs showed more than tenfold upregulation (Table 5). The six most upregulated miRNAs were mir-1810, mir-2138, mir-2140, mir-425*, mir-429, and mir-516*, which increased more than 15-fold compared with the unfed samples. Three of the ten most abundant miRNAs in the fed tick salivary glands, i.e., let-7, miR-183, and miR-275, were upregulated three- to ten-fold, of which miR-275 was recently found to be involved in blood digestion and egg development in a mosquito vector (Bryant and Macdonald 2010).
We found that 231 known miRNAs were downregulated after tick blood-feeding (>two-fold), while 81 known miRNAs showed greater than ten-fold downregulation (Table 6). The 23 most downregulated miRNAs had more than 15-fold lower levels than that found in the fed samples. Three of the ten most abundant miRNAs in the unfed tick salivary glands (miR-1268, miR-276, and miR-184b) were upregulated six-fold to 13-fold.
Discussion
Next generation sequencing technologies have been used for the genome-wide profiling and discovery of miRNAs. This process normally requires a reference genome sequence. Of the tick, only the Ixodes scapularis genome draft is currently available, wherein 37 miRNAs have been identified (Griffiths-Jones et al. 2008). However, a recent report on miRNAs in cattle ticks found that evolutionary conserved microRNAs were ubiquitously expressed compared with tick-specific miRNA (Barrero et al. 2011), which indicates the possibility of detecting dynamic changes in miRNA profiles by comparing known miRNA homologs in ticks. Therefore, this study presented distinct microRNA profiles relating to tick blood-feeding in the salivary glands of H. longicornis, although no reference genome sequence was available for H. longicornis.
Significant differences in the sRNA expression levels of tick salivary glands during the unfed and fed phases indicated their potential functions in tick blood-feeding. The two phases of the tick share the same genome but they exhibit different gene expression profiles and phenotypes, suggesting differential regulation of gene expression (Kang et al. 2004).
The salivary glands of tick undergo remarkable growth and differentiation during tick feeding, where the mass and protein content of the salivary glands increases about 25-fold, although the number of cells does not increase (Sauer et al. 2000). During a blood meal, new mRNAs are induced in the salivary glands, leading to the synthesis of a wide range of new proteins (Leboulle et al. 2002). Correspondingly, we found that some specific unfed sRNAs were more abundant than specific fed sRNAs in terms of unique and total sRNAs. Almost double the number of conserved miRNA families were found in unfed samples compared with fed samples. More different types of miRNAs were downregulated rather than upregulated after blood-feeding. These phase changes might be explained by the miRNA-guided suppression of targeted genes in unfed tick salivary glands. Similarly, larvae exposed to a host for 6 h showed a significant reduction in the accumulation of miRNA transcripts compared with unexposed larvae in the tick R. microplus (Barrero et al. 2011). Changes in the miRNA expression profile might indicate the fine-tuning or tight regulation of their targets in a spatial- and temporal-specific manner.
Two recent studies have reported the secretion of miRNAs into saliva (Park et al. 2009). Interestingly, of the most abundantly expressed H. longicornis miRNAs in unfed and fed tick salivary glands, only miR-375 is known to be expressed in human salivary glands (Park et al. 2009; Zhang et al. 2009). The expression of miR-375 in the salivary glands of ticks and vertebrates suggests a conserved functional role for this miRNA throughout the evolution of this organ.
The differential expression of the ten most abundant miRNAs in unfed and fed tick salivary glands might be important molecules, because their changes may lead to more than a simple alteration in their miRNA levels. Interestingly, miR-275 was recently found to be involved in blood digestion and egg development in the mosquito (Bryant and Macdonald 2010). Given that both are blood-feeding vectors, the function of miR-275 in the tick may reward further investigation.
Conclusions
We generated two small RNA libraries from the unfed and fed tick salivary glands of H. longicornis. We found a greater abundance of specific unfed sRNAs compared with specific fed sRNAs in terms of both unique sRNAs and total sRNAs. Our results showed that known miRNA homologs have a wide variety of expression profiles in unfed and fed tick salivary glands. The differential expression of miRNAs in unfed and fed tick salivary glands supports their involvement at new levels in the regulation of tick blood-feeding. Our data provide an important resource for more detailed functional analysis of miRNAs in this species.
References
Aljamali MN, Ramakrishnan VG, Weng H, Tucker JS, Sauer JR, Essenberg RC (2009) Microarray analysis of gene expression changes in feeding female and male lone star ticks, Amblyomma americanum. Arch Insect Biochem Physiol 71:236–253
Banerjee D, Slack F (2002) Control of developmental timing by small temporal RNAs: a paradigm for RNA-mediated regulation of gene expression. BioEssays 24:119–129
Banerjee D, Slack FJ (2005) Temporal and spatial patterning of an organ by a single transcription factor. Genome Biol 6:205
Barrero RA, Keeble-Gagnère G, Zhang B, Moolhuijzen P, Ikeo K, Tateno Y, Gojobori T, Guerrero FD, Lew-Tabor A, Bellgard M (2011) Evolutionary conserved microRNAs are ubiquitously expressed compared to tick-specific miRNAs in the cattle tick Rhipicephalus (Boophilus) microplus. BMC Genomics 12:328
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Bowman AS, Coons LB, Needham GR, Sauer JR (1997) Tick saliva: recent advances and implications for vector competence. Med Vet Entomol 11:277–285
Bryant B, Macdonald W, Raikhel AS (2010) microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. PNAS 107(52):22391–22398
Chen X, Li Q, Wang J, Guo X, Jiang X, Ren Z, Weng C, Sun G, Wang X, Liu Y, Ma L, Chen JY, Wang J, Zen K, Zhang J, Zhang CY (2009) Identification and characterization of novel amphioxus microRNAs by Solexa sequencing. Genome Biol 10:R78
Flynt AS, Lai EC (2008) Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet 9:831–842
Fujisaki K, Kawazu S, Kamio T (1994) The taxonomy of the bovine Theileria spp. Parasitol Today 10:31–33
Gao X, Shi L, Zhou Y, Cao J, Zhang H, Zhou J (2011) Characterization of the anticoagulant protein Rhipilin-1 from the Rhipicephalus haemaphysaloides tick. J Insect Physiol 57:339–343
Glazov EA, Cottee PA, Barris WC, Moore RJ, Dalrymple BP, Tizard ML (2008) A microRNA catalog of the developing chicken embryo identified by a deep sequencing approach. Genome Res 18(6):957–964
Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res 36(Database issue):D154–D158
Hafner M, Landgraf P, Ludwig J, Rice A, Ojo T, Lin C, Holoch D, Lim C, Tuschl T (2008) Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods 44(1):3–12
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3–S14
Kang L, Chen X, Zhou Y, Liu B, Zheng W, Li R, Wang J, Yu J (2004) The analysis of large-scale gene expression correlated to the phase changes of the migratory locust. Proc Natl Acad Sci USA 101:17611–17615
Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E (2002) Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J Biol Chem 277:10083–10089
Li R, Li Y, Kristiansen K, Wang J (2008) SOAP: short oligonucleotide alignment program. Bioinformatics (Oxford, England) 24(5):713–714
Magnarelli LA (2009) Global importance of ticks and associated infectious disease agents. Clin Microbiol Newsl 31:33–37
Oaks JF, McSwain JL, Bantle JA, Essenberg RC, Sauer JR (1991) Putative new expression of genes in ixodid tick salivary gland development during feeding. J Parasitol 77:378–383
Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT (2009) Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res 15(17):5473–5477
Reddy AM, Zheng Y, Jagadeeswaran G, Macmil SL, Graham WB, Roe BA, Desilva U, Zhang W, Sunkar R (2009) Cloning, characterization and expression analysis of porcine microRNAs. BMC Genomics 10:65
Sauer JR, Essenberg RC, Bowman AS (2000) Salivary glands in ixodid ticks: control and mechanism of secretion. J Insect Physiol 46(7):1069–1078
Siomi H, Siomi MC (2009) On the road to reading the RNA-interference code. Nature 457:396–404
Sonenshine D (1991) Biology of ticks. Oxford University Press, Oxford
Valenzuela JG, Francischetti IMB, Pham VM, Garfield MK, Mather TN, Ribeiro JMC (2002) Exploring the sialome of the tick vector of Lyme disease. J Exp Biol 205:2843–2864
Wang M, Guerrero FD, Pertea G, Nene V (2007) Global comparative analysis of ESTs from the southern cattle tick, Rhipicephalus (Boophilus) microplus. BMC Genomics 8:368
Wei Y, Chen S, Yang P, Ma Z, Kang L (2009) Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol 10:R6
Zhang Y, Zhou X, Ge X, Jiang J, Li M, Jia S, Yang X, Kan Y, Miao X, Zhao G, Li F, Huang Y (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129:1401–1414
Zhang X, Cairns M, Rose B, O’Brien C, Shannon K, Clark J, Gamble J, Tran N (2009) Alterations in miRNA processing and expression in pleomorphic adenomas of the salivary gland. Int J Cancer 124(12):2855–2863
Zhou J, Zhou Y, Gong H, Chen L, Cao J (2004) Discovery of parthenogenesis population of Haemaphysalis longicornis in China and its biological characteristic. Chin J Vector Bio Control 15(3):173–174
Acknowledgments
This work was supported by grants from the the Special Fund for Public Welfare Industry of Chinese Ministry of Health (201202019) and grants from the Basic Research Foundation for National Commonweal Institute of China (2012JB12).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10493_2012_9604_MOESM2_ESM.xls
Additional file 2: small RNAs of matching known miRNAs from unfed tick salivary glands. This file contains small RNAs whose sequences are similar with miRNAs deposited at miRBase, the format include: 1.small RNA sequnece Id, 2.length of small RNA sequence, 3.count of small RNA sequence, 4.small RNA sequence, 5.homolog (best one), 6.homolog length, 7.homolog sequence, 8.family, 9.match number, 10.mismatch number, 11.gap number, 12.score. Supplementary material 2 (XLS 75 kb)
10493_2012_9604_MOESM3_ESM.xls
Additional file 3: small RNAs of matching known miRNAs from fed tick salivary glands. This file contains small RNAs whose sequences are similar with miRNAs deposited at miRBase, the format include: 1.small RNA sequnece Id, 2.length of small RNA sequence, 3.count of small RNA sequence, 4.small RNA sequence, 5.homolog (best one), 6.homolog length, 7.homolog sequence, 8.family, 9.match number, 10.mismatch number, 11.gap number, 12.score. Supplementary material 3 (XLS 1991 kb)
10493_2012_9604_MOESM4_ESM.xls
Additional file 4: Summary of known miRNA in fed tick salivary glands This file contains known miRNA name, total count (sum of the counts of all variants), reference sequence (sequence of a miRNA who has the highest count in the whole family). Supplementary material 4 (XLS 1429 kb)
10493_2012_9604_MOESM5_ESM.xls
Additional file 5: Summary of known miRNA in fed tick salivary glands This file contains known miRNA name, total count (sum of the counts of all variants), reference sequence (sequence of a miRNA who has the highest count in the whole family). Supplementary material 5 (XLS 91 kb)
10493_2012_9604_MOESM6_ESM.xls
Additional file 6: Comparison of changes in salivary glands miRNA between fed and unfed ticks This file contains known miRNA name, normalized expression level, fold change of miRNAs in the pair of samples, P value and Significance label. Supplementary material 6 (XLS 58 kb)
Rights and permissions
About this article
Cite this article
Zhou, J., Zhou, Y., Cao, J. et al. Distinctive microRNA profiles in the salivary glands of Haemaphysalis longicornis related to tick blood-feeding. Exp Appl Acarol 59, 339–349 (2013). https://doi.org/10.1007/s10493-012-9604-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-012-9604-3