Abstract
From about 250 Cirsium spp., only two Aceria spp. (Acari: Eriophyoidea) have been described, Aceria anthocoptes (Nal.) and Aceria cirsii Pet. B. & Shi. Host specificity, which generally characterizes eriophyoid mites, potentially leads to speciation, so we may expect more than two Aceria spp. and/or other infraspecific taxa. Furthermore, studies on host-related variability in the morphology of Aceria mites are generally lacking. The purpose of this study was to investigate quantitative morphological traits of five Aceria populations inhabiting five Cirsium spp. in Serbia. MANOVA analysis revealed significant differences in 23 commonly used morphological traits as well as four additional traits related to the prodorsal shield design. In addition, the most important qualitative traits using scanning electron microphotographs were studied in order to clarify phenotypic differences among five Aceria spp. Discriminant analysis identified eight traits that significantly differentiate five populations. UPGMA cluster analysis of the squared Mahalanobis distances indicates that A. cirsii was morphologically the most divergent, while A. anthocoptes populations from Cirsium arvense and Aceria sp. from Cirsium heterophyllum were isolated from the branch clustering Aceria spp. populations from Cirsium eriophorum and Cirsium creticum. Analysis of qualitative traits using SEM confirmed results obtained from the analysis of morphometric features.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eriophyid mites have been considered to have a high potential for use as classical biological control agents of weeds (Smith et al. 2009). Plant species from the tribe Cardueae Cass. (Asteraceae), especially from Carduus L., Centaurea L., Cirsium Adans., Crupina Cass. and Onopordum L., are considered as invasive weeds which cause enormous environmental and economic damage in North America, Australia and New Zealand (Sobhian et al. 1989; Briese et al. 1990; Skinner et al. 2000; Winston et al. 2008). Most of the species nominated and investigated for biological control of weeds belong to the genus Aceria.
The genus Aceria includes over 900 species and is known to be a taxonomically difficult group (Amrine et al. 2003). According to the Catalogue of the Eriophyoidea of the World and Fauna Europaea (Amrine and Stasny 1994; de Lillo 2004), a total of 20 Aceria species have been recorded on Cardueae plants, 10 of which are known in Serbia (Petanović and Stanković 1999). Most of Aceria spp. inhabiting Cardueae plant taxa are poorly known. Majority of the species are known only from scattered localities and described in old-dated publications. The real status of congeneric Aceria spp., which are associated with Cardueae plant taxa, is difficult to assess because of the lack of recent detailed studies. Despite the descriptions of many species of Aceria, few papers have been published on morphological similarities or differences of congeneric taxa inhabiting closely related host plants.
From about 250 Cirsium spp. (Mabberley 1998), only two species of eriophyoid mites have been described, Aceria anthocoptes (Nalepa 1892) and Aceria cirsii Pet. B. & Shi (Petanović et al. 2000). Until recently, A. anthocoptes has been the only known Aceria species inhabiting Cirsium spp. It has so far been recorded on Cirsium arvense (L.) Scop., Cirsium heterophyllum (L.) Hill. and Cirsium vulgare (Savi) Tenore (Amrine and Stasny 1994), although its presence on C. vulgare was not confirmed (Ochoa et al. 2001; Smith et al. 2009). Another species, Aceria leontodontis (Lindroth 1904), was described from the host plants Leontodon autumnale (L.), C. arvense and C. heterophyllum in Finland and later it was recorded again in Finland and in Bulgaria (Roivainen 1951; Natcheff 1981). Recently, Petanović et al. (1997) proposed that these two species are synonymous, concluding that A. leontodontis is a deutogyne form of A. anthocoptes. A. anthocoptes has been noted in several European countries (Davis et al. 1982) and in 13 states in the USA. In Colorado, it has also been collected from four Cirsium spp., which are all native to North America (Smith et al. 2009). A. cirsii was described from Cirsium rivulare (Jacq.) All., and is known only from the type locality in Serbia (Petanović et al. 2000).
Recently, studies of morphological variations in populations of Aceria spp. collected from four Cirsium spp. showed that these Aceria spp. differed significantly in morphology, confirming differences between the previously recognized nominal species, A. anthocoptes and A. cirsii, but suggesting that Aceria from Cirsium eriophorum (L.) Scop., C. arvense and C. heterophyllum could represent three different species or biotypes (Vidović et al. 2008). However, it is not known if these populations are reproductively isolated or if they differ behaviourally in host plant preference. Populations of A. anthocoptes collected on C. arvense from two different locations in Serbia differ in morphology, which indicates significant phenotypic and, possibly, genetic and/or geographical heterogeneity within the species (Magud et al. 2007). In host range experiments, A. anthocoptes collected from C. arvense multiplied well only on C. arvense (Gassman et al. 2006). However, A. anthocoptes or a close relative has sometimes been collected on C. vulgare in the field, and some mites collected on C. arvense have developed on C. vulgare in laboratory experiments (Gassmann et al. 2005, 2006; Smith et al. 2009). During further surveys of Aceria spp. inhabiting Cirsium spp. in Serbia by the authors, either one more Aceria species or an infraspecific taxon has been collected from Cirsium creticum (Lam.) Urv.
The purpose of this study was to investigate quantitative morphological traits of five Aceria populations inhabiting five different Cirsium spp., bearing in mind the importance of the Aceria species as biological control agents and the necessity of their correct characterization and identification. Final aim of the long term study is to clarify systematics of Cirsium-associated Aceria spp. and their phyllogenetic relationships. This study presents results of phenotypic variability which could point to the subsequent research directions. As it was assumed by Skoracka et al. (2002), quantitative description of host related morphological variation can provide the basic information needed to improve the eriophyoid taxonomic system and enhance our understanding of mechanisms generating this variation. Besides the study of quantitative morphological traits and phenotypic differences among five Aceria spp., electron microphotographs were scanned in order to analyze the most important qualitative traits and to compare them among different populations. This would help to confirm the existence of variability in phenotypic traits between populations of Aceria mites living on different host plants and to characterize them as distinct morphological entities.
Materials and methods
During the surveys of the occurrence of eriophyoid mites on Cardueae plant taxa in Serbia, Aceria spp. have been registered on a few Cirsium spp. until now, although 22 species without infraspecific taxa and hybrids exist (Gajić 1975).
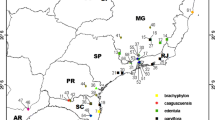
Samples of five Cirsium species were collected in Serbia at the following localities: C. arvense (Ca) (42°40′N, 22°18′E) and C. heterophyllum (Ch; 42°44′N, 22°19′E)—the Vlasina Mt. (altitude 1200 m); C. rivulare (Cr; 43°54′N, 19°25′E)—the Tara Mt. (altitude 1100 m), C. eriophorum (Ce; 44°06′N, 19°58′E)—the Maljen Mt. (altitude 1000 m), C. creticum (Cc; 43°33′N, 20°41′E)—the Goč Mt. (altitude 700 m).
Different host plants of the genus Cirsium and the previously confirmed presence of Aceria mites on them were the main criteria for the selection of samples. Geographical populations of plants were chosen mainly according to their distribution in Serbia. For instance, C. heterophyllum is distributed only on the Vlasina Mt. (Gajić 1975). C. rivulare was collected from the Tara Mt., because corresponding GPS coordinates represent the type locality of the mite A. cirsii. Although widely distributed, C. arvense was collected from the Vlasina Mt. because, according to literature (Roivainen 1951; Natcheff 1981), both C. arvense and C. heterophyllum are hosts of A. anthocoptes, etc.
Mites were collected using extracting methods described by de Lillo (2001). Twenty-five to 30 mites from each sample were mounted in a dorso-ventral position on slides in Kiefer’s F medium and identified (Amrine and Manson 1996). Protogyne females randomly selected from each population were examined by a phase-contrast microscope (LEICA DMLS). Twenty-seven traits were measured on each individual. Morphometry was performed using the software package M 1000 (Leica, Wetzlar, Germany; Fig. 1). Twenty-three of these morphometric traits (Fig. 1a) were commonly used, and the four others (Fig. 1b) were related by shield design, equally important for eriophyoid mite identification.
Measurements of A. anthocoptes female morphology used in statistical analysis. Explanation of abbreviations: a A: length of body, B: length of prodorsal shield, C: width of prodorsal shield, D: length of scapular setae sc, E: scapular tubercles apart from sc, F: no. of dorsal annuli, G: no. of ventral annuli, H: length of lateral setae c2, I: length of I ventral setae d, J: length of II ventral setae e, K: length of III ventral setae f, L: length of genitalia, M: width of genitalia, N: length of setae 3a, O: spacing of tubercles 3a, P: spacing of tubercles 1b of coxa I, R: spacing of tubercles 1a of coxa I, S: spacing of tubercles 2a of coxa II, T: length of setae 2a, U: length of tibia I, W: length of tarsus I, V: length of tibia II, Z: length of tarsus II. b Md: length of median line, Ad: length of admedian line, Sd: length of submedian line, MA: distance between Md and Ad line
All variables that entered the analyses presented normal distribution, as well as homogeneity of variance. The data were tested for normality using Shapiro–Wilk tests. A multivariate analysis of variance (MANOVA) allows for the comparison of the population means of all variables of interest at the same time (multivariate response), rather than considering multiple responses as a suite of univariate responses (Zar 1999). The statistical significance of the MANOVA can be determined in a variety of ways. The most often used statistic test, Wilks’ Lambda, was applied (Zar 1999). A one-way MANOVA was used to examine the differences in morphological variation among Aceria spp. populations inhabiting different Cirsium species. To describe and interpret effects from MANOVA, a multivariate discriminant analysis (DA) was a useful post hoc method to employ following a MANOVA. Discriminant analysis was employed on 23 commonly used morphological traits and separately on four traits related to shield design in order to determine the relative importance of characters as discriminators between a priori groups and the relative positions of the centroids of those groups (Manly 1986). In addition, canonical variables were computed. All statistical analyses were conducted using the Statistica 6 software package (StatSoft 2001).
Finally, an UPGMA (Unweighted Pair Group Method with Arithmetic mean) dendrogram, based on squared Mahalanobis distance between species centroids, was generated using 23 commonly used quantitative traits. This was used to evaluate the phenetic relationships between species.
Qualitative morphological traits were investigated on live mites using scanning electron microscopy (SEM, JEOL-JSM 6390) at the Faculty of Agriculture, University of Belgrade. Live mites were collected individually with a fine entomological needle from fresh plant parts under a stereomicroscope and placed on the SEM holder.
Samples of plant species were stored as herbarium voucher specimens and deposited, along with the collection of slides, at the Department of Agricultural Entomology and Zoology, Faculty of Agriculture, University of Belgrade.
Results
Descriptive statistics of Aceria species quantitative traits are given in Table 1. The one-way MANOVA of five Aceria spp. populations revealed significant differences in the morphological variation of 23 commonly used morphological traits (Wilks’ Lambda = 0.00025 (92, 453) = 35.201; P < 0.001), and in four traits related to shield design (Wilks’ Lambda = 0.04561; F (16, 406) = 44.445; P < 0.001).
The results of the discriminant analysis of 23 traits and of four additional traits showed that the most important and distinct discrimination is between the population from Cirsium rivulare and the four others, i.e., C. eriophorum, C. heterophyllum, C. arvense and C. creticum, based on the first canonical axis (function; Figs. 2, 3). The total correct percent of classification matrix of all five groups was very high on 23 traits (99.291%) and high on four traits (74.468%).
Plots of scores of the first two canonical axes (CV 1 and CV 2) of five populations of Aceria spp. from 23 commonly used morphological traits. Abbreviations of Aceria populations associated with different host plants are described in the “Materials and Methods” section
Plots of scores of the first two canonical axes (CV 1 and CV 2) of five populations of Aceria spp. populations from four traits related to the shield design. Abbreviations of Aceria populations associated with different host plants are described in the “Materials and Methods” section
From the standardized canonical discriminant function coefficients (Table 2), it is evident that the first canonical function describes 68.19% of the total variability; the first and second 92.80%; first, second and third together 98.82%; and all four roots 100% of the total variability. It should be emphasized that the first and the second canonical functions describe most of the variability. The length of the second ventral (e) setae, the length of the first ventral (d) setae and the distance between the first tubercles (1b) have the most distinct discriminative power based on the first canonical function. These functions clearly separate A. cirsii from the other four Aceria populations.
The width of the prodorsal shield, the length of the lateral (c2) setae, the length of the second (e) ventral setae, the length of the scapular (sc) setae, the distance between (3a) tubercles and the distance between (sc) tubercles have the most discriminative power based on the second canonical function. Bearing in mind that the second canonical function describes 24.61% of the total variability, it could be inferred that its discriminative power is significantly lower in comparison with the first canonical function. It should be stressed that this function evidently separates Aceria spp. populations from C. eriophorum and C. creticum on one hand and Aceria anthocoptes from C. arvense and Aceria sp. from C. heterophyllum on the other (Fig. 2).
From the standardized canonical discriminant function coefficients for the four shield characters (Table 2), it is evident that the first canonical function describes 90.27% of the total variability; the first and second 98.29%; first, second and third together 99.60%; and all four roots 100% of the total variability. It should be emphasized that the first and the second canonical functions describe most of the variability. The distance between median and admedian line (MA) and length of submedian (Sd) line have the most distinct discriminative power based on the first and second canonical functions. These functions clearly separate A. cirsii from the other four Aceria species. Bearing in mind that the third canonical function describes only 1.3% of the total variability, it could be inferred that its discriminative power is significantly lower in comparison with the first and second canonical functions (Fig. 3).
All pairwise squared Mahalanobis distances between populations were significant at the 99% level. UPGMA cluster analysis of the squared Mahalanobis distances (Fig. 4) clustered all Aceria populations, except for C. rivulare, in the same branch, indicating that A. cirsii from C. rivulare was the most divergent population. Within this main branch, the populations from C. arvense and C. heterophyllum were isolated from the branch clustering Aceria populations from C. eriophorum and C. creticum.
UPGMA tree diagram (dendrogram) of five Aceria spp. populations from different Cirsium species based on squared Mahalanobis distances (scale showed) obtained from commonly used 23 morphological traits. Abbreviations of populations are described in the “Materials and Methods” section
Aceria cirsii mites from the host plants C. rivulare are characterized by the shorter length of their first and second ventral setae, and by the smaller distance between the first coxal setae, compared with four other populations. Aceria spp. mites from the host plants C. arvense and C. heterophyllum are characterized by a narrower prodorsal shield, smaller distance between scapular setae, longer length of lateral setae and longer length of the second ventral setae, compared with populations of Aceria mites from C. eriophorum and C. creticum.
Morphological differences exist among Aceria populations from C. arvense and C. heterophyllum. Aceria sp. from C. heterophyllum is characterized by the shorter length of its first ventral seta, shorter length of the second ventral seta and smaller distance between first coxal setae, compared with A. anthocoptes population from C. arvense. Also, morphological differences exist among Aceria sp. mites from C. eriophorum and C. creticum. Aceria sp. mites from C. eriophorum are characterized by a narrower prodorsal shield, longer length of scapular setae, shorter length of lateral setae, and longer length of the first and second ventral setae, compared with Aceria sp. population from C. creticum.
Regarding the quantitative traits which characterize shield design, A. cirsii mites in comparison with the other four species have a smaller submedian line and a shorter distance between median and admedian lines. Results obtained in this study indicate that there are no differences between the length of shield lines and the distance among median and admedian lines regarding Aceria populations from C. arvense, C. heterophyllum, C. eriophorum and C. creticum.
However, from the SEM photographs of the dorsal shield of five Aceria populations, it is obvious that the appearance of the dorsal shield of A. cirsii is the most distinct. Median and admedian lines are complete, but both submedains are partial. Moreover, numerous short dashes are visible on the whole surface, except in the main field between the shield lines (Fig. 5).
The main field of the prodorsal shield in four other populations is characterized by clearly distinctive, complete median line, one admedian line (on each side of the median line), as well as one submedian line on each side. Submedian lines are curved at their end and complete. Aceria sp. from C. creticum differs very clearly from three other populations, having a smooth prodorsal shield without additional ornamentation between the main shield lines.
A common characteristic of the ornamentation of the prodorsal shield of Aceria spp. from C. arvense, C. heterophyllum and C. eriophorum is the presence of additional broken lines and dashes between admedian and submedian lines (although less numerous and short, like in A. cirsii), as well as in the lateral fields of the prodorsal shield. On the other hand, a common characteristic of the ornamentation of the prodorsal shield of Aceria spp. from C. heterophyllum, C. eriophorum, and C. creticum are two short lines at the base of the median line, which are almost parallel in Aceria spp. from C. eriophorum and C. creticum, but oblique in Aceria sp. from C. heterophyllum. Besides, SEM photographs clearly show the shield lobe above the gnathosoma, which is usually not visible from the slides prepared for the phase contrast microscope.
Discussion
The results of our study show that A. cirsii is clearly separate from the four other Aceria populations from C. arvense, C. heterophyllum, C. eriophorum and C. creticum host plants. Our investigations confirm the results presented in the original description by Petanović et al. (2000) that A. cirsii mites are characterized by a narrower prodorsal shield, shorter length of first (d) and second ventral setae (e) and smaller distance between first coxal (1b) setae. Besides that, by comparing the shield design, it is obvious that A. cirsii mites have a shorter median line, a significantly shorter submedian line, and a twice shorter distance between median and admedian lines. Sukhareva (2001), analyzing 15 morphological features of 35 Aceria species living on Asteraceae, stated that the principal differences between species are the length of the median line and the length and form of submedian lines. Moreover, from the SEM photographs it is obvious that shield design is made of complete median and admedian lines, partial submedians and numerous short dashes on the whole surface except the main field between shield lines.
The original description of A. anthocoptes (Nalepa 1892) and other old publications (Roivainen 1950; Farkas 1965) included a relatively low number of quantitative characteristics, without a set of comparative data from other specimens. Petanović et al. (1997) supplemented the description of this species from the C. arvense host plant adding more quantitative traits. Magud et al. (2007) compared morphological variation in different populations of A. anthocoptes inhabiting two infraspecific host plant taxa of C. arvense. Recently, investigations of morphological variations in populations of Aceria spp. collected from four Cirsium spp. showed that these Aceria spp. differed significantly in morphology, confirming differences between previously recognized species, A. anthocoptes and A. cirsii, but suggesting that Aceria spp. from C. eriophorum, C. arvense and C. heterophyllum could represent three different species or biotypes (Vidović et al. 2008).
Results of present studies of phenotypic variability indicate the separation between A. anthocoptes from C. arvense and Aceria sp. from C. heterophyllum on one hand, and Aceria spp. from C. eriophorum and C. creticum on the other. Characteristics which separate these two groups are the width of the prodorsal shield, length of scapular (sc) setae, distance between scapular setae, length of lateral (c2) setae, length of second ventral (e) setae, and distance between genital (3a) setae. On the other hand, the shield design is quite similar regarding the length of lines and the distance between them, so these characters cannot be considered as adequate for species characterization. However, the shield ornamentation differs in details.
Although A. anthocoptes mites from C. arvense and Aceria sp. from C. heterophyllum are very similar, there are still characters in which they differ. Aceria sp. from C. heterophyllum is characterized by the shorter length of the first ventral seta, shorter length of the second ventral seta, and smaller distance between the first coxal setae. The shield design of these two populations is similar. The only difference is in the length of the submedian line, which is longer in Aceria sp. inhabiting C. heterophyllum. From the SEM photographs, it is obvious that the shield design of these two species differs a little bit in the presence of two short oblique lines at the base of the median line in Aceria sp. from C. heterophyllum, and the absence of such a line in A. anthocoptes. Bearing in mind that samples were collected on the same day and from the same locality, the explanation regarding the seasonal variability (seasonal dimorphism) or geographical races is excluded. We may presume the hypothesis of host plant impact on intraspecific and/or interspecific phenotypic differences.
Furthermore, there are differences between Aceria mites from C. eriophorum and C. creticum, which refer to the width of the prodorsal shield, length of scapular setae, length of lateral setae, and length of the first and second ventral setae. According to statistical analyses, there are no differences in the shield design, yet SEM photographs have shown that clear differences do exist. The prodorsal shield of Aceria sp. mites from C. creticum is smooth compared to the prodorsal shield of Aceria sp. from C. eriophorum, which have short lines on posterior and lateral parts of the shield.
The phenogram supplied in this study confirms that A. cirsii is the most divergent in comparison with the other four species. On the other hand, populations of A. anthocoptes from C. arvense and Aceria sp. from C. heterophyllum were isolated from Aceria spp. from C. eriophorum and C. creticum. Results suggest that there could be five morphologically different taxa on the Cirsium spp. investigated in this study. One of them, i.e., A. cirsii, is most clearly excluded from the group of conspecific (congeneric?) taxa, which are, to some extent phenotypically different. According to Skoracka et al. (2002) variability in phenotypic traits between populations of mites living on different host plants may originate from several causes: total separation of gene pools—different mite species, partial differentiation of gene pools, host races, and no separation of gene pool—phenotypic plasticity.
For the time being, it is impossible to understand the origin of the phenetic variability among the investigated taxa. In addition to classical taxonomy, molecular techniques should be applied in clarifying the taxonomic status of Aceria spp. inhabiting Cirsium spp.
References
Amrine JW, Manson DCM (1996) Preparation, mounting and descriptive study of Eriophyoid Mites. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid Mites. Their biology, natural enemies and control. Elsevier Science BV, Amsterdam, pp 383–396
Amrine JW Jr, Stasny TAH (1994) Catalog of the Eriophyoidea (Acarina, Prostigmata) of the world. Indira Publ. House, West Bloomfield, p 531
Amrine JW Jr, Stasny TAH, Flechtmann CHW (2003) Revised keys to world genera of Eriophyoidea (Acari: Prostigmata). Indira Publishing House, Michigan, p 244
Briese DT, Lane D, Hyde-Wyatt BH, Crocker J, Driver RG (1990) Distribution of thistles of the genus Onopordum in Australia. Plant Prot Q 5:23–27
Davis R, Flechtmann CHW, Boczek JH, Barke HF (1982) Catalogue of eriophyid mites (Acari: Eriophyoidea). Warsaw Agricultural University Press, Warsaw
de Lillo E (2001) A modified method for eriophyoid mite extraction (Acari: Eriophyoidea). Int J Acarol 27:67–70
de Lillo E (2004) Fauna Europaea: Eriophyoidea. In: W. Magowski (ed) Fauna Europea: Acariformes. Fauna Europea version 1.1, http://www.faunaeur.org
Farkas H (1965) Spinnentiere, Eriophyidae (Gallmilben). Die Tierwelt Mittleuropas 3:1–155
Gajić M (1975) Asteraceae In: Josifović M (ed) Flora of Serbia 7:1–465
Gassman A, Tosevski I, Petanovic R, Magud B, Haefliger P, Chevillat V, Rheinhold T (2006) Biological control of Canada Thistle Cirsium arvense. In: CABI Bioscience Switzerland Centre Annual Report 2005, CABI Bioscience, Delemont, Switzerland, p 27
Gassmann A, Gerber E, Toševski I, Petanović R, Magud B, Cortat G (2005) Biological control of Canada Thistle Cirsium arvense. In: CABI Bioscience Switzerland Centre Annual Report 2004, CABI Bioscience, Delemont, Switzerland, p 27
Lindroth JI (1904) Nyz salsynta finska Eriophyde. Acta Soc Fauna Flora Fenn 26:1–18
Mabberley DJM (1998) The plant book, a portable dictionary of the vascular plants. Cambridge University Press, Cambridge
Magud B, Stanisavljević Lj, Petanović R (2007) Morphological variation in different populations of Aceria anthocoptes (Acari: Eriophyoidea) associated with the Canada thistle, Cirsium arvense, in Serbia. Exp Appl Acarol 42:173–183
Manly FJB (1986) Multivariate statistical methods—a primer. Chapman and Hall, New York
Nalepa A (1892) Les acarocecides de Lorraine (Suite). Feuiele (3) 22 (258): 120 (no38)
Natcheff P (1981) Eriofidni akari v Bulgaria. Habilitacionen trud, Katedra entomologija, Visc Selskostopanski institut “V.Kolarov”, Plovdiv, p 310
Ochoa R, Erbe EF, Wergin WP, Frye C, Lydon J (2001) The presence of Aceria anthocoptes (Nalepa) (Acari: Eriophyidae) on Cirsium species in the United States. Int J Acarol 27:179–187
Petanović R, Stanković S (1999) Catalogue of Eriophyoidea (Acari: Prostigmata) of Serbia and Montenegro. Acta Ent Serbica, Special Issue, p 143
Petanović R, Boczek JH, Stojnić B (1997) Taxonomy and bioecology (Acari: Eriophyoidea) associated with Canada thistle, Cirsium arvense (L.) Scop. Acarologia 38:181–191
Petanović R, Boczek J, Shi A (2000) Four new Aceria species (Acari: Eriophyoidea) from Serbia. Acta Ent Serbica 5:119–129
Roivainen H (1950) Eriophyid news from Sweden. Acta Ent Fenn 7:1–51
Roivainen H (1951) Contribution to the knowledge of the eriophyids of Finland. Acta Ent Fenn 8:1–70
Skinner K, Smith L, Rice P (2000) Using noxiois weed lists to prioritize targets for developing weed managements strategies. Weed Sci 48:640–644
Skoracka A, Kucynski L, Magowski W (2002) Morphological variation in different host populations of Abacarus hystrix (Acari: Prostigmata: Eriophyoidea). Exp Appl Acarol 26:187–193
Smith L, de Lillo E, Amrine JW Jr (2009) Effectiveness of Eriophyid Mites for biological control of weedy plants and challenges for future research. Exp Appl Acarol. doi:10.1007/s10493-009-9299-2
Sobhian R, Katsoyannos BI, Kashefi J (1989) Host specificity of Aceria centaureae (Nalepa), a candidate for biological control of Centaurea diffusa De Lamarck. Entomologica Hellenica 7:27–30
StatSoft, Inc. (2001) STATISTICA (data analysis software system), version 6. http://www.statsoft.com
Sukhareva SI (2001) Four-legged mites (Acari: Tetrapodili) of the genus Aceria from plants of the family Asteraceae with the description of a new species. Acarina 9:131–141
Vidović B, Petanović R, Stanisavljević Lj (2008) Morphological variation of Aceria spp. (Acari: Eriophyoidea) inhabiting Cirsium species (Asteraceae) in Serbia. In: Bertrand S, McCoy KD, Migeon A, Navajas M, Tixer M-S, Vial L (eds) Integrative Acarology proceedings of the 6th european congress, European Association of Acarologist, Montpellier, France, 21–25 July, 2008, pp 331–339
Winston R, Hansen R, Schwarzländer M, Coombs E, Randall CB, Lym R (2008) Biology and biological control of exotic true thistles. Forest Health Technology Enterprise Team Technology Transfer, USDA Forest Service, University of Idaho, Moscow
Zar J (1999) Biostatistical analysis, 4th edn. Prentice-Hall, New Jersey
Acknowledgments
The authors are grateful to Dr. Vladimir Stevanović, Faculty of Biology, University of Belgrade, and to Dr. Vladimir Randjelović, Department of Biology and Ecology, University of Niš, for the identification of plant species. The study was supported by the Serbian Ministry of Science and Environmental Protection (Grant # 143006B).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vidović, B., Stanisavljević, L. & Petanović, R. Phenotypic variability in five Aceria spp. (Acari: Prostigmata: Eriophyoidea) inhabiting Cirsium species (Asteraceae) in Serbia. Exp Appl Acarol 52, 169–181 (2010). https://doi.org/10.1007/s10493-010-9354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-010-9354-z