Abstract
The pathogenicity of 52 isolates from several fungus species was studied for the false spider mite Brevipalpus phoenicis. In addition, the main stages during the course of infection by Hirsutella thompsonii, by far the most virulent pathogen, were studied by means of light and electron microscopy. Adult mites were confined to arenas prepared with citrus leaves in acrylic dishes containing agar–water. Conidial suspensions containing 108 conidia/ml were applied, except for H. thompsonii, where a concentration of 107 conidia/ml was used. The H. thompsonii isolates caused higher mortality, with indices higher than 90%. Observations under the scanning electron microscope (SEM) were performed at 0, 6, 12, 24, 48, 72, and 120 h after application of a H. thompsonii suspension containing 107 conidia/ml. Twenty-four hours after inoculation, H. thompsonii conidia were observed attached to the mite’s integument. The conidia germinated and penetrated through the base of the setae on the hysterosoma. Colonization occurred after 48 h, as evidenced by mortality. Conidiogenesis occurred after 120 h, with the development of mycelium and conidiophores emerging from the posterior and anterior parts of the mite.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus is one of the most important crops in Brazil, because of fruit and juice concentrate exports. The industry generates all sorts of services and other production-related activities (Neves 2000). One of the main obstacles limiting yield increase in citrus are pests, in particular Brevipalpus phoenicis (Geijskes, 1939) (Acari: Tenuipalpidae), known as the false spider mite and “flat mite.” Other crops that are susceptible to B. phoenicis injuries in Brazil are coffee (Coffea arabica) (Chagas et al. 2003) and passion fruit (Passiflora edulis) (Kitajima et al. 1997).
This species is a polyphagous mite and a total of 486 plants were identified as hosts (Childers et al. 2003a). The importance of B. phoenicis is directly associated with its capacity to transmit leprosis virus, a localized-action rhabdovirus (Gonzáles 1975; Oomen 1982; Childers et al. 2003b). The virus causes chlorotic spots and necrosis on leaves, branches, and fruits, pronounced fruit shedding, and decreases in fruit weight. Depending on the intensity of infestation by the pest, the plant may become unproductive or die (Guinado and Silvério 1992; Rodrigues et al. 1994).
The most important control strategy against the disease consists of reducing the mite population through application of chemical acaricides. In 1999, the estimated costs of the control of this pest were US$ 75 million, in the State of São Paulo (Brazil) only (FNP, 2000). Intensive pesticide applications aimed at reducing mite populations in citrus have caused the development of resistance to some products and increased residues in fruits (Omoto 1998; Alves et al. 2000). These problems, together with the resurgence of pests, have transformed brazilian citriculture into a low-sustainability activity.
Microbial control agents appear desirable alternatives within the IPM context. In addition to contributing to pest control, they limit the development of resistance and minimize impact on the environment. Fungi are the most important entomopathogens that occur in phytophagous mite populations; they can be introduced, increased, or protected at locations where they are already present (Alves 1998).
Among mite-attacking fungi the most relevant examples are the Entomophthorales species and the Deuteromycetes species Hirsutella thompsonii, an important pathogen of eriophyoid mites (Van der Geest et al. 2000). The Entomophthorales are the most important, since they cause epizootics under field conditions. However, their mass production is still incipient, making their practical use difficult to achieve. On the other hand, even though mitosporic fungi are not frequently observed causing epizootics in the field, they can be easily produced and could be used under the inundative strategy.
Therefore, as an initial stage in a microbial control program, virulent lines with suitable characteristics to be used as microbial products can be selected by laboratory bioassays (Alves 1998). The objectives of this research were to evaluate the susceptibility of B. phoenicis to isolates of entomopathogenic fungi and to observe the biological cycle of one isolate on the mite.
Materials and methods
Rearing and maintaining B. phoenicis in the laboratory
The mite was reared on ripe fruits of “Pêra Rio” and/or “Valence” varieties (Citrus sinensis) maintained in the laboratory. The mites were collected from an unsprayed citrus grove located in Piracicaba, State of São Paulo (Brazil), and have been mantained in the laboratory since 2000.
The fruits were collected in a pesticide-free citrus grove, washed in running water, and dried with paper towels in order to clean and disinfest the oranges. Each fruit was submitted to a melted paraffin bath covering 2/3 of its total area, leaving an arena of approximately 4 cm diameter on the surface. This arena was delimited with a sticky barrier (Tanglefoot®, Grand Rapids, MI, USA) to prevent mites from escaping. Most fruits selected showed citrus scab symptoms on the skin, since this condition favors mite maintenance because of their cryptical habit (Childers et al. 2003b).
The fruits were then infested with 50 adult mites and placed in plastic boxes (28 × 43 × 12 cm) with a perforated styrofoam bottom. The boxes were maintained in an air-conditioned room (25 ± 5°C; 12-h photophase, and 75 ± 10% RH) for a 20-day period. After this period, old fruits were replaced with new ones.
Selection of entomopathogenic fungal isolates
The isolates used in the selection bioassays came from the laboratory’s “Pathogen Bank” and were stored in a freezer ( − 12°C) in the form of pure conidia. Fifty-two isolates were tested: Beauveria bassiana (16), Beauveria brongniartii (1), Metarhizium anisopliae (18), Paecilomyces lillacinus (5), Paecilomyces fumosoroseus (2), Paecilomyces farinosus (1), Hirsutella thompsonii (4), Lecanicillium lecanii (1), Lecanicillium muscarum (1), and Sporothrix sp. (3). These isolates came from different localities in the Southeastern, Northeastern, Central-Western, and Southern regions of Brazil, and were isolated from several host species and from the soil (Table 1).
A sample from each isolate tested was initially transferred to Petri dishes containing complete culture medium (0.36 g KH2PO4, 1.05 g NaHPO4·7H2O, 0.60 g MgSO4·7H2O, 1.0 g KCl, 10.0 g glucose, 5 g yeast extract, 20 g agar, 1,000 ml sterile water), previously autoclaved at 120°C for 20 min. After inoculation, the dishes were maintained for 10 days under controlled condition (26 ± 0.5°C; 12 h photophase and 70 ± 10% RH) for pathogen growth and sporulation.
The bioassays were performed using leaves of variety “Pêra-Rio” collected from a pesticide-free citrus grove. After cleaning and disinfesting, 2.6 cm diameter arenas were cut out with a metal hole punch. Each arena was placed on an acrylic plate (3.5 cm diameter) containing 5 ml of a solidified agar–water mixture (2%). In order to fix the leaf onto the agar and allow it to last longer, a border was made on the leaf using 2 ml of the agar–water mixture (2%), thus delimiting a confinement arena. Twenty B. phoenicis teneral females showing high mobility were transferred to each arena from the stock colony.
After preparing the arenas and transferring the mites, the plates were sprayed with a suspension containing 108 conidia/ml for all isolates tested, with the exception of H. thompsonii, whose isolates were tested at a concentration of 107 conidia/ml. Five plates containing 20 mites were sprayed for each isolate, totaling 100 mites per treatment. The spray was performed in a Potter Spray Tower (Burkard Manufacturing, Rickmansworth, Hertfordshire, United Kingdom), calibrated at 1.034 bar and 2 ml of the conidial suspensions were used (0.2 μl suspension/cm2).
In the control treatment sterile water with spreader-sticker at 0.01% (Tween 40®) was applied. In addition, a standard isolate of B. bassiana (ESALQ-447—ARSEF/USDA-APHIS 2722) was used in all bioassays, sprayed at a concentration of 108 conidia/ml.
After the spray, the plates were maintained for a few minutes in a room to allow the suspension to dry and were then transferred to closed plastic boxes (11 × 33 × 20.5 cm) with a moistened paper towel sheet on the bottom and maintained under controlled condition (26 ± 0.5°C; 12-h photophase and 70 ± 10% RH) during the entire evaluation period. Daily evaluations were made until the sixth day after application, and the number of dead mites on each leaf disc was recorded.
The dead mites were sorted, transferred to acrylic containers holding the agar–water mixture (2%) and maintained in an incubator (26 ± 0.5°C; 12-h photophase; 70 ± 10% RH) for 5 days to confirm death by the fungus. After sporulation, each individual was transferred to a slide containing Hoyer’s medium + lactophenol-cotton blue (2:1) and was observed under the optical microscope for pathogen structure visualization.
The mortality values were corrected in relation to control treatment using the formula proposed by Schneider-Orelli (1947).
Hirsutella thompsonii biological cycle stages on B. phoenicis
Some H. thompsonii (ESALQ-1269) developmental stages on B. phoenicis adults were studied in the laboratory by scanning electron microscopy (Zeiss Leo 435 VP). In this experiment arenas consisting of variety “Pêra Rio” citrus leaves were used as substrate for the mites, following procedures as described above.
Adults from the stock colony were transferred to the leaves (15 adults/leaf arena) and sprayed with a suspension containing 107 conidia/ml, using a Potter Spray Tower. After applying the pathogen, the plates were placed into plastic boxes with a moistened paper towel sheet on the bottom and stored under controlled condition (26 ± 0.5°C, 70 ± 10% relative humidity, and 12-h photophase).
After 0, 6, 12, 24, 48, 72, and 120 h from inoculation, each group of three plates was removed from the incubator and stored at − 40°C to cause mite death and preserve the material. A corresponding stub was prepared for each plate, containing a piece of the leaf with a variable number of mites.
After this preparation, the samples were fixated in osmium tetroxide vapor (OsO4) for 48 h and left for 72 h in a glass dehumidifier with silica-gel to maintain relative humidity near 0%. Later, the material was sputtered with gold in a Balzers Evaporator (MED 010) for 120 s and observed under the electron microscope.
Results and discussion
Selection of entomopathogenic fungal isolates
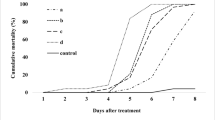
Based on B. phoenicis mortality values the isolates were separated into two groups: the first combined B. bassiana, B. brongniartii, M. anisopliae, Sporothrix sp., L. lecanii, L. muscarum, and Paecilomyces spp. strains; the second group combined the H. thompsonii strains (see Fig. 1). Isolates from the second group caused 90–100% mite mortality, 6 days after inoculation. Isolates from the first group caused mite mortality of less than 30%, ranging from 0% to 29.3% after the sixth day from application. Despite the great genetic variability of these isolates, the pathogenicity and virulence of an isolate are not related to its origin. The isolate may cause high mortality to a wide array of hosts (Moino Junior et al. 1998; Almeida et al. 1997; Tamai et al. 1999; Alves et al. 2005). This variation in mortality is frequently related to factors such as low virulence, specificity, and host tolerance, among others (Alves 1998).
Intraspecific variation was observed among the B. bassiana and M. anisopliae isolates, because the mean mortality values observed on days 3 and 6 after application were 2% and 10.4%, and 3.9% and 15.1%, respectively. There are few reports in the literature on the natural occurrence of these fungi in phytophagous mites, but they are found in ticks (Chandler et al. 2000). Still, some mite species are very susceptible to these fungi.
The ESALQ-447 B. bassiana isolate, used as a standard in the experiments, yielded a mean corrected mortality of 9.7% on day 6 after application. For T. urticae, this isolate caused 22.5% mortality on the sixth day after application, thus being considered low virulent against this species (Tamai et al. 1999). In P. oleivora, however, this fungus caused 91.4% mortality when applied at a concentration of 1 × 108 conidia/ml (Alves et al. 2005). In some insect species, the same isolate presents good effectiveness and has been selected as promising in microbial control programs (Stimac et al. 1989; Alves 1998).
The M. anisopliae isolates ESALQ-1104, ESALQ-1203, and ESALQ-1204, and L. muscarum isolate ESALQ-972 caused a considerable increase in mite mortality after the third day from inoculation, but on day 6 live mites with infection symptoms could still be observed.
The main advantage of using M. anisopliae or L. muscarum to control B. phoenicis in the field is the ease of large-scale production, allowing the use of these agents in an inundative manner (Alves and Pereira 1998). Pathogenic but low-virulence lines can be used against mites that cause direct damage to the crop, as long as the interval between application and effective control does not compromise crop yield. However, the adoption of this strategy against B. phoenicis in citrus could be risky due to the mite’s capacity to transmit a rhabdovirus (Childers et al. 2003b). Because this is a persistent-circulative mode of transmission, the time between virus acquisition by the mite and virus transmission is shorter than the time required for mite infection and subsequent death, and this could compromise disease control effectiveness. On the other hand, the change in behavior of hosts infected by fungi, particularly in relation to reduced feeding, is an aspect that may reduce the rate of virus transmission by the mite (Hajek and Leger 1994). Therefore, the association of these fungi with compatible acaricides, as long as accompanied by a synergistic effect, could be evaluated for resistance management (Alves et al. 2000).
Even applied at a concentration 10 times lower than the other fungi (107 conidia/ml), the H. thompsonii isolates were effective to control B. phoenicis. This result indicates greater virulence of this species, per infective unit, compared to the others. The application of suspensions containing 106 conidia/ml of different Hirsutella spp. isolates against Varroa destructor, a parasitic mite on bees, provided mortality rates of 97% (7 days after application), similar to those obtained with the application of 108 conidia/ml of M. anisopliae, Lecanicillium spp., B. bassiana, and Paecilomyces spp. (Shaw et al. 2002). This result is interesting because it demonstrates the high specificity of the genus Hirsutella to Acarina.
With the exception of isolate ESALQ-1282 expressive mortality rate increases were observed on day 3 after application of the pathogen. On the last evaluation day, the total, cumulative, and corrected mortality indices were higher than 90% for isolates ESALQ-1221, ESALQ-1282, and ESALQ-1269, and 100% for ESALQ-1266 (Table 2).
The high virulence and specificity of H. thompsonii against mites could be related to a series of events resulting from the pathogen–host relations cycle. The action of secondary metabolites produced during the colonization process of Hirsutella spp. could be one of the factors responsible for the promptness with which this fungus is capable of killing B. phoenicis adults, as already observed for other organisms (Vey et al. 1993; Alves 1998; Omoto and McCoy 1998).
The mites infected by M. anisopliae, B. bassiana, B. brongniartti, Paecilomyces spp., and Sporothrix sp. showed alterations in integument color and movement, with conspicuous ventral stiffening of the legs. When disturbed by the touch of a brush or by direct incidence of light, the diseased individuals exhibited a behavior of “throwing” their bodies back, maintaining only the hind legs adhered to the substrate. For L. muscarum, on the fifth day from inoculation, the mites showed mycelial growth across the whole idiosoma region and sluggishness of movement, besides the previously described symptoms. All dead individuals showed ventrally stiffened legs and alterations in the color of the integument.
The symptoms and signs of the disease caused by H. thompsonii were also observed from the third day of inoculation in dying or dead mites, which were very similar to what was verified with the other fungus species. Under the optical microscope, the presence of hyphae could be observed inside and outside the body of the host, with the presence of conidiophores emerging through the mouth and hind openings. According to the literature, when infected by H. thompsonii, the eriophyid mites P. oleivora, Aceria vaccinii, and C. heveae show similar symptoms to those observed in B. phoenicis (Lipa 1971; Baker and Neunzig 1968; Tanzini et al. 2000).
Hirsutella thompsonii biological cycle stages on B. phoenicis
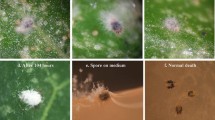
After applying a conidial suspension of H. thompsonii (ESALQ-1269), we observed some conidia attached to the citrus leaf and also to the dorsal part of the mite’s integument, especially on lateral depressions of the propodosoma and hysterosoma (Fig. 2a, b). The conidia were sphere-shaped, approximately 2.3 μm in diameter, marked with protuberances across the entire surface, similar to those observed by McCoy et al. (1984). Although conidia with these characteristics are considered “typical” of H. thompsonii, some variation may occur within the same species (Baker and Neuzing 1968). The conidia are covered with a mucilaginous substance that protects against desiccation, facilitates their adherence to the host surface, and helps the infection process (Van der Geest et al. 2000; Alves 1998).
(a) Hirsutella thompsonii conidia attached to the integument of Brevipalpus phoenicis. (b) Germinated conidium attached to a citrus leaf. (c) Conidium attached to the gnathosoma region of B. phoenicis with apparent formation of appressorium. (d) Conidium between 6 h and 12 h from inoculation, with the presence of germ tube. (e) Conidiogenesis and horizontal fungus dissemination 120 h after inoculation
Germination started between 0 h and 6 h from inoculation. At this stage, we observed some conidia attached near the marginal setae of the mite’s hysterosoma, and also some germinated conidia with the apparent formation of an appressorium (Fig. 2c). These structures are considered adaptations of the microorganism, which concentrates physical energy and high metabolic and enzymatic activity over a small area of the host to make the penetration process more effective (Hajek and Leger 1994). Because the B. phoenicis integument is thick and quite coarse throughout, the formation of these structures during conidium germination may have been stimulated. This process has been demonstrated for M. anisopliae infecting Manduca sexta larvae (Lepidoptera: Sphingidae) (Leger et al. 1991).
The main sites of penetration of H. thompsonii on B. phoenicis were the base of the setae on the hysterosoma, due to the large number of germinated conidia present at those locations. Between 6 h and 12 h from inoculation, most conidia attached to the mite’s integument showed the characteristic formation of germ tubes (Fig. 2d). Germination time and velocity are variable traits among different species of entomopathogenic fungi, and may occur in at least 12 h at temperatures between 23°C and 30°C for most Deuteromycetes (Alves 1998).
Mycelial and hyphal growth were observed between 24 h and 48 h from inoculation at some regions of the dorsum of B. phoenicis, especially on the intersegmental setae, foreleg, and gnathosoma regions. During this period, the mean cumulative mortality indices were 24.5% on the third day (72 h) and 74.5% on the fourth day (96 h) after application of the pathogen, indicating that intense invasion by the fungus in the mite’s hemocoel and events that culminated in mite death occurred during that period (24 h). These events are described as a combination of mechanical damages that occur due to fungus growth, host nutrient depletion, and action of toxic metabolites (Hajek and Leger 1994). Colonization time also varies between 72 h to 120 h depending on host, pathogen, and environmental conditions (Alves 1998).
The extrusion and conidiogenesis of H. thompsonii occurred 120 h after application (Fig. 2e). Many hyphae were observed emerging from the gnathosoma and opisthosoma of B. phoenicis, probably from the oral, anal, and/or genital openings. The dead mites showed all legs clearly stiffened ventrally, with a “wilted” appearance. The H. thompsonii conidiophores formed during the conidiogenesis process consisted of a single phialide of approximately 270 μm to which the conidium was attached. Non-typical phialides may also occur in this fungus (McCoy et al. 1984). Once out of the body of the host, the pathogen showed intense growth throughout the substrate, with the formation of mycelium that extended up to 0.5 cm from the mite practically in all directions. In the eriophyid mite C. heveae, the hyphae and conidiophores formed from dead mites were found at distances up to 15 times the body length (Tanzini et al. 2000). These characteristics of the pathogen can favor the dissemination of its reproductive structures in the environment, facilitating the horizontal transmission of the disease in a population.
Greater data precision about the biological cycle of the fungus can only be obtained with the combined use of electron/fluorescence microscopy techniques.
Concluding remarks
In laboratory bioassays, the false spider mite, B. phoenicis, presented low susceptibility to several species of mitosporic fungi frequently used as microbial control agents, such as B. bassiana and M. anisopliae. However, isolates of H. thompsonii were highly virulent to B. phoenicis adults. These observations indicate the potential of H. thompsonii as a sustainable environment friendly alternative to chemical acaricides for the control of B. phoenicis in citrus orchards.
References
Almeida JEM, Alves SB, Pereira RM (1997) Selection of Beauveria spp. isolates for control of the termite Heterotermes tenuis (Hagen, 1858). J Appl Entom 121:539–543
Alves SB (1998) Fungos entomopatogênicos. In: Alves SB (ed) Controle microbiano dos insetos. FEALQ, Piracicaba, SP, Brazil, pp 637–711
Alves SB, Pereira RM (1998) Produção de fungos entomopatogênicos. In: Alves SB (ed) Controle microbiano dos insetos. FEALQ, Piracicaba, SP, Brazil, pp 289–370
Alves EB, Omoto C, Franco CR (2000) Bases for an acaricide resistance management program of Brevipalpus phoenicis (Acari: Tenuipalpidae) to the acaricide dicofol in brazilian citrus. International Congress Of Entomology. Foz do Iguaçu, RS. 4 (abstract)
Alves SB, Tamai MA, Rossi LS, Castiglioni E (2005) Beauveria bassiana pathogenicity to the citrus rust mite Phyllocoptruta oleivora. Exp App Acarol 37:117–122
Baker JR, Neunzig HH (1968) Hirsutella thompsonii as a fungus parasite of the blueberry bud mite. J Econ Entom 61(4):1117–1118
Chagas CM, Kitajima EW, Rodrigues JCV (2003) Coffee ringspot virus vectored by Brevipalpus phoenicis (Acari: Tenuipalidae) in coffee. Exp App Acarol 30:203–213
Chandler D, Davidson G, Pell JK, Ball BV, Shaw K, Sunderland KD (2000) Fungal biocontrol of acari. Bioc Sci Techn 10:357–384
Childers CC, Rodrigues JCV, Welbourn C (2003a) Host plants of Brevipalpus californicus, B. obovatus and B. phoenicis (Acari: Tenuipalpidae) and their potencial involvement in the spread of viral diseases vectored by these mites. Exp App Acarol 30:29–105
Childers CC, French JV, Rodrigues JC (2003b) Brevipalpus californicus, B. obovatus, B. phoenicis and B. lewisi (Acari: Tenuipalpidae): a review of their biology, feeding injury and economic importance. Exp App Acarol 30:5–28
Gonzáles RH (1975) Revision of the Brevipalpus phoenicis “complex” with descriptions of new species from Chile and Tailand (Acarina, Tenuipalpidae). Acarology 17(1):81–91
Guinado N, Silvério JL (1992) Leprose e declínio: problemas sérios na citricultura paulista. Laranj 13(2):541–552
Hajek AE, Leger RJ (1994) Interactions between fungal pathogens and insects hosts. Ann Rev Entomol 39:293–322
Kitajima EW, Rezende JAM, Rodrigues JCV, Chiavegato LG, Piza CT Jr, Morozini W (1997) Green spot of passion fruit, a possible viral disease associated with infestation by the mite Brevipalpus phoenicis. Fitopatol Bras 22(4):555–559
Leger RJ, Goettel M, Roberts DW, Staples RC (1991) Penetrations events during infection of host cuticle by Metarhizium anisopliae. J Inv Pathol 58:168–179
Lipa JJ (1971) Microbial control of mites and ticks. In: Burges HD, Hussey NW (eds) Microbial control of insects and mites. Academic Press, London, United Kingdom, p 373
McCoy CW, Stamper DH, Tuveson RW (1984) Conidiogenous cell differences among mutant and wild-type pathotypes of Hirsutella thompsonii var. thompsonii. J Inv Pathol 43:414–421
Moino Junior A, Alves SB, Pereira RM (1998) Efficacy of Beauveria bassiana (Balsamo) Vuillemin isolates for control of stored-grain pests. J App Entom 122:301–305
Neves EM (2000) Economia da produção citrícola e efeitos alocativos. Pr Agr 162:9–12
Omoto C (1998) Acaricide resistance management of leprosis mite (Brevipalpus phoenicis) in brazilian citrus. Pest Sci 52:189–191
Omoto C, McCoy C (1998) Toxicity of purified fungal toxin hirsutellin A to the citrus rust mite Phyllocoptruta oleivora (Ash.). J Inv Pathol 72:319–322
Oomen PA (1982) Studies on population dynamics of the scarlet mite, Brevipalpus phoenicis, a pest of tea in Indonesia. Veenman & Zonen, Wageningen, 88pp
Rodrigues JCV, Nogueira NL, Prates HS, Freitas DS (1994) Leprose dos citros: importância, histórico, distribuição e relações com o ácaro vetor. Laranj 15:123–138
Shaw KE, Davidson G, Clark SJ, Ball BV, Pell JK, Chandler D, Sunderland KD (2002) Laboratory bioassays to assess the pathogenicity of mitosporic fungi to Varroa destructor (Acari: Mesostigmata) an ectoparasite mite of the honeybee, Apis mellifera. Biol Contr 24:266–276
Schneider-Orelli O (1947) Entomologisches Praktikum: Einfuehrung in die land – und forstwirtschaftliche Insektekunde. Zweite Aufl., Aarau, p 237
Stimac JL, Alves SB, Camargo MTV (1989) Controle de Solenopsis spp. (Hymenoptera: Formicidae) com Beauveria bassiana (Bals.) Vuill. em condições de laboratório e campo. An Soc Entomol Bras 8:95–103
Tamai MA, Alves SB, Neves PJ (1999) Patogenicidade de Beauveria bassiana (Bals.) Vuill. ao ácaro Tetranychus urticae. Koch Sci Agr 56(1):285–288
Tanzini MR, Alves SB, Moraes GJ, Tamai MA, Ferla NJ (2000) An epizootic of Calacarus heveae (Acari: Eriophyidae) caused by Hirsutella thompsonii F. on rubber trees. Exp App Acarol 24(2):141–144
Van der Geest LPS, Elliot SL, Breeuwer JAJ, Beerling EAM (2000) Disease of mites. Exp App Acarol 24:497–560
Vey A, Quiot JM, Mazet I, McCoy CW (1993) Toxicity and pathology of crude broth filtrate produced by Hirsutella thompsonii var thompsonii in a shake culture. J Inv Pathol 61:131–137
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi-Zalaf, L.S., Alves, S.B. Susceptibility of Brevipalpus phoenicis to entomopathogenic fungi. Exp Appl Acarol 40, 37–47 (2006). https://doi.org/10.1007/s10493-006-9024-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-006-9024-3