Abstract
In this work, we have used classical genetics techniques to find improved starter strains to produce cachaça with superior sensorial quality. Our strategy included the selection of yeast strains resistant to 5,5′,5″-trifluor-d,l-leucine (TLF) and cerulenin, since these strains produce higher levels of higher alcohols and esters than parental strains. However, no clear relationship was observed when levels of flavoring compounds were compared with the levels expression of the genes (BAT1, BAT2, ATF2, EEB1 genes) involved with the biosynthesis of flavoring compounds. Furthermore, we determined the stability of phenotypes considered as the best indicators of the quality of the cachaça for a parental strain and its segregants. By applying the principal component analysis, a cluster of segregants, showing a high number of characteristics similar to the parental strain, was recognized. One segregant, that was resistant to TLF and cerulenin, also showed growth stability after six consecutive replications on plates containing high concentrations of sugar and ethanol. “Cachaça” produced at laboratory scale using a parental strain and this segregant showed a higher level of flavoring compounds. Both strains predominated in an open fermentative process through seven cycles, as was shown by mitochondrial restriction fragment length polymorphisms analysis. Based on the physical chemical composition of the obtained products, the results demonstrate the usefulness of the developed strategies for the selection of yeast strains to be used as starters in “cachaça” production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cachaça, the sugar cane spirit, is the most popular distilled beverage produced in Brazil. It is obtained from the distillation of fermented sugarcane broth and may reach values of alcoholic graduation of 38–48% (vol/vol) at 20°C. The annual production is around 1.3 billion liters that makes cachaça the third distilled alcoholic beverage worldwide after vodka and soju (a distilled beverage native to Korea originally made from rice). Cachaça production is initiated with the sugarcane broth extraction using an electrical milling machine. After decantation, for the removal of small sugar cane pieces and other impurities, the broth is filtered and transferred to steel vats for fermentation. The majority of the distilleries still use a spontaneous fermentative process that relies on the microbiota present in the production environment (sugarcane and equipments) as starters. Therefore, cachaça production is characterized by mixed yeast fermentation with the predominance of Saccharomyces cerevisiae cells. Because cachaça production is a feed batch open fermentative process that takes place up to 6 months (corresponding to the sugar cane harvest), fermentative yeast populations are in constant change due to the continuous introduction of new strains from the sugarcane juice, and the non-sterile productive conditions (Guerra et al. 2001; Morais et al. 1999; Pataro et al. 1999, 2000).
Although, different factors interfere in the quality of distilled alcoholic beverages the yeast strain and the fermentation conditions have been indicated as the most important factors for the flavor of alcoholic beverages, since the majority of the flavoring compounds are synthesized during fermentation (Lambrechts and Pretorius 2000). S. cerevisiae produces volatile metabolites such as esters, carbonyls, volatile fatty acids, sulphur compounds and higher alcohols that derive from the sugar and amino acid metabolism. Different strains of S. cerevisiae producing variable amounts of secondary compounds, both desirable and undesirable flavor determinants, can affect the ultimate product quality (Swiegers et al. 2005).
A strategy widely used to improve the quality of beverages such as sake is to obtain mutants, induced or spontaneous, that do not present the feedback inhibition of synthesis of amino acids. Among strains resistant to drugs, such as 5,5′,5″-trifluor-d,l-leucine (TLF), is common to isolate those with a higher production of higher alcohols, such as isobutyl and isoamyl (Ashida et al. 1987; Yoshizawa 1999).
Higher alcohols are the largest group responsible for the organoleptic characteristics of beverages that are produced by two distinct processes: the catabolic pathway, also known as Ehrlich pathway, and the biosynthetic pathway (Dickinson et al. 1997; Eden et al. 2001). The extracellular amino acids transported by the high affinity Bap2p and low affinity Bap3p transporters are metabolized by the Ehrlich pathway. Then, there is a transamination step leading to the production to the correspondent α-keto acids, catalyzed by amino transferases encoded by the genes BAT1 and BAT2. The α-keto acids are decarboxylated and reduced to higher alcohols (isoamyl, n-propanol and isobutyl). It is already known that yeast strains with the highest expression of BAT1 and BAP2 genes produce higher concentrations of isoamyl and isobutyl alcohol in the fermentation process (Ashida et al. 1987; Casalone et al. 1997; Eden et al. 2001; Lilly et al. 2000, 2006; Pretorius 2000; Schoondermark-Stolk et al. 2005; Swiegers et al. 2005; Van Der Sluis et al. 2002; Yoshikawa et al. 1995; Yoshizawa 1999). In the biosynthetic pathway, amino acids as l-leucine are produced from glucose via α-isopropylmalate (α-IPM) by the action of α-IPM synthase, α-IPM isomerase and β-IPM dehydrogenase. The enzyme α-IPM synthase is inhibited by l-leucine through negative feedback and it can also be regulated by coenzyme-A (CoA) (Tracy and Kohlhaw 1977; Cavalieri et al. 1999; Kohlhaw 2003, Oba et al. 2006).
By its turn, esters are produced in very low concentrations, far below the limit of human olfactory perception. Nevertheless, due to the “matrix effect”, a synergy occurs between the substances causing a pleasant olfactory sensation to the consumer. There are two groups of esters: acetate esters and medium chain fatty acid esters (Lambrechts and Pretorius 2000). The acetate esters are those who have received greater attention, since they are found in higher concentration, they are more easily characterized by the detection methods available and more easily excreted by yeast cells. From the sensory point of view, the most important are ethyl acetate, isoamyl acetate, phenylethyl acetate. The production of acetate esters occurs by the transfer of acetyl group (from acetyl-CoA) to a higher alcohol in a reaction catalyzed by alcohol acetyl transferases Atf1 and Atf2 (Mason and Dufour 2000; Verstrepen et al. 2003a, b). More recently, the medium-chain fatty acids esters have attracted attention because the description of the genes encoding for enzymes involved their production. They are produced in very low concentrations, and its excretion by the yeast is strongly influenced by the length of the fatty acid chain constituent. The main representatives are: ethyl hexanoate, ethyl octanoate and ethyl decanoate. The enzymes involved in the biosynthesis of these compounds are Eth1 and Eeb1 (Saerens et al. 2006, 2008a, b). The synthesis of fatty acids in yeast is catalyzed by a multifunctional fatty acid synthase. The change in the balance of the catalytic activity of this enzyme affects the long-chain fatty acids synthesized. Thus, the use of inhibitors, such as cerulenin, is a proposed strategy to select strains with improved production and accumulation of medium-chain fatty acids esters (Ichikawa et al. 1991).
The use of selected yeast starters is a well-known practice used in the wine and beer industries, but not yet established for production of cachaça with enhanced and more reproducible quality attributes; in that cases, the yeast starters belong, with a few exceptions, to the species S. cerevisiae. Natural strains of S. cerevisiae, as those found in cachaça production, are predominantly diploid, homothallic and mostly homozygous (65%), with low (Bakalinsky and Snow 1990; Barre et al. 1992; Guijo et al. 1997) and to high (>85%) sporulation capacity (Mortimer 2000). Moreover, it is known that wine yeast strains are characterized by high karyotype instability that is believed to be a potential source of genetic variability (Carro et al. 2003; Lopez et al. 2001; Nadal et al. 1999). On the other hand, a mechanism called “genome renewal” (Marullo et al. 2004) has been proposed for natural wine yeast strains that undergo mating among their progeny cells and thereby change a multiple heterozygote into completely homozygous diploids, leading to gradual replacement of heterozygous diploids and the elimination of deleterious mutations.
A methodology was developed for the isolation of yeast strains showing the characteristics required for the production of a good quality cachaça (Vicente et al. 2006; Oliveira et al. 2008): low or null H2S production, positive flocculation, higher fermentation capacity, tolerance to high ethanol and sugar concentrations, high temperatures, and resistance to drugs.
The aim of the present work was to establish correlations between the levels of both higher alcohols and esters (1-propanol, isobutanol, isoamyl alcohol, ethyl octanoate, ethyl decanoate) produced by the selected strains to study the expression of genes (genes BAT1, BAP2, ATF2, EEB1) that encode for enzymes of metabolic routes involved with of the formation of these by-products. In addition, the stability of the select strains and their segregants was evaluated by assays of the set of physiological and biochemical phenotypes applied to samples withdrawn from the process over consecutive fermentation cycles.
Materials and methods
Yeast strains, maintenance and growth media used
Saccharomyces cerevisiae strains LBCM 422 and LBCM 427 were isolated from a spontaneous fermentation carried out in a cachaça distillery located in Ouro Preto, Minas Gerais, Brazil (Oliveira et al. 2008). Yeast cells were propagated under laboratory conditions at 30°C in YPD medium, which contained yeast extract (1%, w/v), peptone (1%, w/v) and glucose (2%, w/v) (Difco® Laboratories). Solid medium was supplemented with agar (2%, w/v) (Difco® Laboratories). All yeast isolates obtained throughout the present work were frozen in glycerol (30%, v/v) and kept at −80°C for long-term storage, whereas short-term storage was at 4°C on YPD plates. As a control, commercial S. cerevisiae wine yeast was used, that is occasionally utilized for cachaça production. As a control, commercial S. cerevisiae wine yeast was used, that is occasionally utilized for cachaça production.
Sporulation and isolation of spores
The standard protocol used here was described by Sherman (2002). The yeast strain LBCM 427 was inoculated in YPD medium and incubated overnight at 30°C. Sporulation was induced on acetate medium [sodium acetate (1%, w/v), potassium acetate (1%, w/v) and agar (2% w/v)] during 3 days at 24°C. Ascus wall was digested using snail intestinal juice (Sigma®) and ascospores were isolated by micromanipulation. Germination efficiency was expressed as the percentage of colony forming spores after incubation (3 days, 30°C). Thirty-six ascospores were analyzed and ten presented four viable spores. These spores were further analyzed to evaluate the physiological stability of spores.
Physiological and biochemical characteristics of the segregants
The segregants were submitted to the following tests: H2S production, growth in YPD at 37°C, growth in YP media supplemented with sucrose or ethanol, flocculation, killer behavior, sensitivity to mycocins, TFL and cerulenin resistance. Each segregant was previously inoculated in tubes containing 3 ml YPD medium and incubated overnight at 30°C. The suspension was used to inoculate plates and tubes in all tests described below.
H2S production was examined by inoculation on plates containing bismuth sulfite agar (Merck). Strains were incubated at 30°C for 3 days and H2S producing strains were identified by the brown or black color of their colonies. Segregant colonies were also tested for their ability to grow in stressing conditions such as high temperature (37°C, YPD agar medium, 3 days incubation) or high alcohol concentration (YP medium supplemented with ethanol 10%, v/v). Growth on YP media (yeast extract, 1% and peptone, 1%, w/v) containing ethanol or glucose was scored by visual inspection after 3 days of incubation at 30°C (Ribeiro and Horii 2004).
Flocculation capacity was evaluated during growth in YPD liquid medium (peptone, 2% w/v yeast extract 1% w/v). Cells were inoculated into test tubes containing 4 ml of medium and grown at 200 rpm and 30°C until absorbance at 600 nm reached 1.0. Flocculent strains were then selected by their capacity to sediment at the bottom of the tube at the end of fermentation as described before (Verstrepen et al. 2001).
Killer toxin production and resistance to mycocins was assessed using Candida glabrata NCYC 388 and S. cerevisiae NCYC 1006, respectively, as references strains (Morais et al. 1999; Pataro et al. 1999). Briefly, YM agar (yeast extract, 0.3% w/v, malt extract, 0.3% w/v, peptone, 0.5% w/v, glucose 1% w/v and 1.5% w/v agar), supplemented with 0.003% (w/v) methylene blue (Sigma,) and buffered to pH 4.2 with sodium acetate (Sigma) was previously seeded with C. glabrata and S. cerevisiae. Segregant strains were inoculated and identified as killer (if they produced a halo of dark blue dead cells of the sensitive strain) or resistant (if they grew in the presence of the killer strain).
Segregant strains were also transferred to plates containing minimal medium (SD) containing glucose (2%, w/v) and 1 mM TLF (Ashida et al. 1987) or 25 μM cerulenin (Ichikawa et al. 1991) and incubated at 30°C for 3 days. Strains were considered as resistant when colonies were observed within 3 days of incubation at 30°C.
To evaluate the physiological stability of the segregants, the plates were successively replica-plated to other plates containing YPD medium and incubated at 30°C for 48 h. Six replications were performed and the colonies resulting of first, second, fourth and sixth replications were tested for the characteristics described above.
Fermentation procedures
In order to study gene expression and production of flavoring compounds by strains LBCM 427 and its segregant RB-23A, the cells stored at −80°C were grown in liquid YPD medium (2% glucose) by two consecutive cultivations (72 h culturing at 30°C starting with an initial cell density of 107 cells/ml, followed by a 24 h growth period on a fresh YPD medium). Then the cells were washed (a threefolds washing) with sterile water, prior to be used as inoculum for 300 ml of minimal medium (15% sucrose; 0.2% yeast nitrogen base) added to the 500 ml bottles, which were locked up with a locking air system. The fermentation was carried out for 36 h at 30°C during which five samples were withdrawn for volatile assays and RNA extraction for analysis.
The cells previously grown in YPD medium were also used to produce cachaça at laboratorial scale; in this case a sufficient amount of yeast cells was obtained through adaptation to the fermentative environment by subsequent inoculations (28°C, 24 h) and up-scaling (20 ml, 8°Brix; 100 ml, 12°Brix; 1,000 ml, 15°Brix), always using sterilized sugarcane must. The 1,000 ml-cultures were used as inoculum for small-scale fermentations in cylindrical stainless steel tanks (20 l) filled with 12 l of “non-sterilized” sugar cane juice (15°Brix). The initial cell concentration was adjusted to 2.4 × 107 cells ml−1. After the end of a 24-hours fermentative cycle (28°C), musts were distilled and the stainless steel tanks were again filled with 12 l of sugar cane juice (15°Brix) for a new cycle. The predominance of LBCM 427 and RB-23A strains during the fermentative process was evaluated in must samples that were collected at the moment of inoculation of the 12 l fermenters and after the end (day 7) of seven fermentative cycles. The samples were serially diluted, and two dilutions (10−4 and 10−5) were plated on YPD medium. After incubation (28°C, 24 h), 30 colonies were randomly chosen from each sample and submitted to mitochondrial DNA RFLP analysis (see below).
The ethanol production was evaluated after must distillation (Distillatore Elettronico Enochimico Gibertini) by densitometry with a glass picnometer of 20 ml. The temperature of the sugarcane wine was maintained between 90 and 95°C and the distillation rate set to about 1 l h−1. Cachaça distillation produced three fractions: the first one (“head fraction”) was discarded and corresponds to about 5% of the total volume of cachaça. The main (“heart”) fraction was collected until an ethanol concentration of about 42–44°GL. The last (“tailing”) fraction was also discarded. The obtained cachaça samples from the main fraction was then stored in glass bottles with caps and kept at room temperature.
Gas chromatography analysis
In order to measure higher alcohols and esters the organic compounds were previously extracted in a liquid–liquid system. To be identified and quantified by gas chromatography, 25 ml of fermented broth at indicated time were collected in conical glass tubes of 50 ml, previously identified. The samples were centrifuged at 1,000×g for 5 min. The resulting supernatant was transferred to new 50 ml conical tubes containing 5 g NaCl and 3 ml of a mixture of diethyl ether/hexane (1:1) and the mixture stirred in vortex for 2 min. Then, the mixture was centrifuged at 1,000×g for 3 min and the organic phase collected and stored in 15 ml tube. A re-extraction was performed by adding a new amount of 3 ml mixture of ethyl ether/hexane (1:1) to the fermentation broth and the other procedures were repeated in the same manner as described above as well as the collection and transfer of organic phase in 15 ml tube. Subsequently, 3 ml of this organic phase were transferred to new tubes, and concentrated under nitrogen gas until completely dry. Then, it was added 490 μl of ethyl ether/hexane (1:1) and the desired final volume of 500 μl was corrected with a solution of 4 methyl 2-pentanol diluted 500 times. The samples were analyzed with a Shimadzu Shimadzu GCMS-QP2010 Plus is equipped with a quadrupole mass detector operating in electron impact mode at 70 eV, scan and selective ion monitoring (SIM). The chromatographic column used was a capillary column of polyethylene glycol (J & W Scientific) 30 m, 0.25 mm internal diameter and 0.5 mm thick film. The chromatographic conditions used were as follows: The temperature of injector and the interface of the gun was equal to 240°C, raised to 10°C min−1–120°C, followed by an elevation of 20°C min−1–240°C and held for 5 min. Helium was used as carrier gas with a flow rate of 1.24 ml/min and a rate of split of 1:50. The injection of samples was done in direct mode with 2 μl of each sample. The injections were performed in triplicate.
The compounds were identified by comparison with retention times of standards and monitoring of selected ions. Initially the standards were injected separately to determine retention times. The method of internal standardization was used for quantification of compounds in samples using the 4-methyl-2-pentanol as internal standard for all analysis. The calibration curves for the compounds under analysis were constructed by plotting the area ratio of the composite pattern and the area of internal standard versus the concentration that, for the following compounds: n-propanol, isobutanol, isoamyl alcohol, ethyl hexanoate, ethyl octanoate and ethyl decanoate. To construct the calibration curve, different known amounts of each standard compound were added to 25 ml of minimal medium and after this addition, all steps of extraction, liquid–liquid were performed as described previously. The calibration curves were constructed using five concentration levels as described for each compound: n-propanol (50, 100, 150, 200, 300 g/ml), isobutanol (50, 100, 200, 300, 500 mg/ml), IAA (50, 500, 750, 1,000, 1,500 mg/ml), ethyl hexanoate (500, 1,000, 1,500, 2,000, 2,500 ng/ml), ethyl octanoate (500, 1,500, 2,500, 3,500, 5,000 ng/ml) and ethyl decanoate (100, 500, 1,500, 2,500, 3,500 ng/ml). We used the following standards Merck: isoamyl alcohol (PA grade), 1-propanol (PA grade), isobutanol (PA grade), ethyl hexanoate (PA grade), ethyl octanoate (PA grade) and ethyl decanoate (grade PA). To prepare the alcoholic solution (ethanol/water) 40% v/v ethyl alcohol was used HPLC grade (Fisher Scientific) and distilled deionized water in MilliQ system (Millipore). We used water Purification System Milli-Q in all the steps when necessary. The experiments were performed three times with less than 10% of standard deviation for each point.
Real time PCR
For RNA extraction, six independent experiments were done. The cells were collected by centrifugation and washed twice with 2 ml of Milli Q water at 4°C (3,000×g for 10 min, 4°C). The pellets were suspended in 1 ml of phenol/water 3.75:1 and 1 ml of TES (10 mM Tris HCl; 10 mM EDTA; 0.5% SDS; pH 7.5) followed by vigorous agitation in Vortex. Then, the tubes were incubated at 70°C for 1 h, and agitated vigorously in Vortex at intervals of 5 min. The tubes were centrifuged (3,000×g for 10 min 4°C) and the superior phase of the suspension was transferred to microcentrifuge tubes. After that, 400 μl of phenol/water (3.75:1) were added to the suspension, and after homogenization, the tubes were again centrifuged (3,000×g for 10 min 4°C). The supernatant was collected and 300 μl of chloroform were added, following new agitation in Vortex and centrifugation (3,000×g for 10 min 4°C). RNA was precipitated with 70% ethanol, washed once with 70% ethanol, suspended with 50-μl DEPC-treated water and stored at −70°C until use.
Then three sets of two RNA samples were pooled; from each pool 4 μg of pooled RNA were treated with DNase (Promega, Madison, WI) and Reverse transcription was carried out using M-MLV Reverse Transcriptase (Promega) and oligo-dT(18) primer (Promega) according to the manufacturer’s instructions. Real-time PCR reactions were performed on an ABI7500 instrument (Applied Biosystems, Foster City, CA), using Platinum® qPCR SuperMixes (Invitrogen). The amplification reactions were performed as follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles of 94°C for 15 s and 59°C for 1 min. To confirm primer specificity, the dissociation curves of all amplification products were analyzed in the ABI7500 instrument. Fold variation in gene expression was quantified using the comparative Ct method: 2^-(ΔCt treatment–Δct control), which is based on the comparison of expression of the target gene (normalized to the endogenous control) between experimental and control samples (Livak and Schimittgen 2001). RDN1 mRNA was used as endogenous control to normalize all values in the real-time PCR assays, since it exhibited no significant variation in expression values among treatments. The results express means of three RNA pools each one originated from two samples of experiments performed six times. Standard deviations are indicated in figure.
Mytochondrial DNA restriction fragment analysis
For mitochondrial DNA RFLP analysis, total DNA isolation was carried out as previously described (Querol et al. 1992) with some minor modifications (Shuller et al. 2005). From the total genomic DNA extracted, about 200–300 ng, diluted in 16.5 μl deionized water, were digested with 1.0 μl of the restriction endonucleases HinfI (10 U/μl, MBI Fermentas) in presence of 2 μl of the corresponding 10× buffer (MBI Fermentas) and 0.5 μl of RNAse (10 mg/ml, MBI Fermentas), overnight at 37°C. The mtDNA restriction fragments were separated by electrophoresis in a 1.5% (w/v) agarose gel, GelRed™ stained and photographed.
Statistical analysis
The experiments were performed at least three times with consistent results. Statistics analysis was done by using the Student’s t test. Differences were considered statistically significant when the P value was smaller than 0.05.
Principle component analysis (PCA) was used to choose the segregants that present similar behavior to the parental strain (no H2S production; tolerance to ethanol and temperatures; fermentative capacity; ability to flocculate and to produce mycocins; resistance to the drugs TFL and cerulenin). The selected segregants were also subjected to PCA to find the strain with the highest physiological stability regarding stressing growth conditions (37°C, 20% (w/v) sucrose, 10% (v/v) ethanol) as well as TFL and cerulenin resistance. The analyses were carried out using statistical software MINITAB for Windows (release 14).
Results and discussion
Production of higher alcohols and esters by yeast selected cachaça strains
Since higher alcohols and esters have been detected in cachaça as compounds that incorporate the aroma and taste (Nonato et al. 2001, Oliveira et al. 2005), we measured the level of higher alcohols and esters produced by the strains LBCM 422 and LBCM 427 that are, respectively, sensitive and resistant strains to both TFL and cerulenin (Vicente et al. 2006). The results shown in Fig. 1 indicate that only the production of isoamyl alcohol was clearly higher in the strain LBCM 427 when compared to the strain LBCM 422. Related to the production of esters, it was observed that for both ethyl octanoate and decanoate, higher levels were also observed in the cerulenin-resistant strain LBCM 427 (Fig. 2).
It has been demonstrated that in yeast strains used to produce beers or wine the production of acetate esters correlates with the expression of ATF genes (Verstrepen et al. 2003b; Saerens et al. 2008b). A positive correlation has been also found for higher alcohols and the expression of BAT1 and BAT2 genes (Kodama et al. 2001; Yoshimoto et al. 2002; Lilly et al. 2006; Saerens et al. 2008b). It is also known that the expression of specific gene that encode for the amino acid transporter Bap2 is also associated to a higher production of higher alcohols, particularly isoamyl alcohol (Lilly et al. 2006). Only in the case of ethyl esters there is no correlation between the expression of the genes EEB1 and EHT1. In this case, the fatty acid precursor level is likely the major limiting factor for ethyl ester production rather than the activity of the biosynthetic enzymes (Saerens et al. 2008a, b).
Therefore, we also measured the level of expression of different genes that encode for proteins that are involved in the production of flavoring compounds in the cachaça yeast strains. However, if we compare the data related to the quantification of isoamyl alcohol (Fig. 1) with the level of expression of the genes BAT1 and BAP2 (Fig. 3–Panel A), there is not clear relationship between the expression of these genes with the production of isoamyl alcohol. Even, the expression of BAT2 gene was higher in the strain LBCM 422, compared to the strain LBCM 427 (Fig. 3–Panel A).
Levels of relative expression of genes that encode for amino acid carriers and enzymes involved in the biosynthesis of higher alcohols—BAT1 and BAP2 (Panel A) or esters—ATF2 and EEB2 (Panel B) of strains LBCM 422 (black bars) and LBCM 427(grey bars). The levels of all RNAs were normalized by using RDN1 gene that encodes for 18s region of the rDNA. The results express means of three RNA pools each one originated from two samples of experiments performed six times
On the other hand, we measured the level of expression of ATF2 and EEB1 genes that are related to the production of acetate and ethyl esters, respectively. Again, the data indicate that there is any relationship between gene expression and ester production during the fermentation process. In fact, we observed that the expression of ATF2 was higher in LBCM 427 strain and the expression of EEB1 is more significant in LBCM 422 strain (Fig. 3–Panel B). Nevertheless, in our experimental conditions, it was not detected any measurable level of acetate ester and the quantification of octanoate and decanoate ethyl esters showed an higher production in LBCM 427 than in LBCM 422 strain.
This apparent contradiction could be related to the fact that the expression of such genes has never been studied in conditions where the main carbon source was sucrose and/or in sugar cane fermentations. In this sense, it was already seen that ATF1 gene is induced by glucose and nitrogen compounds [as a target of the Ras/cAMP/PKA and the fermentable growth medium-induced pathway] (Thevelein and de Winde 1999; Verstrepen et al. 2003a). On the other hand, Molina et al. (2007) found no correlation between expression of ATF1 and ATF2 genes and the production of any ester compound during wine fermentation suggesting that under wine fermentation conditions, ester production was largely regulated by other mechanisms. We believe that this point must be further studied in the cachaça conditions in order to clarify the situation.
Our data suggest that there is a reasonable relationship between the resistance to TFL and cerulenin and production of higher alcohols and ethyl esters. Nevertheless, when we measured the level of the expression of genes that encode for enzymes involved in the synthesis of these flavoring compounds any relationship could be established. Therefore, these results suggest that resistance to drugs such as TFL and cerulenin be a good strategy to select yeast strains with a higher capacity to produce flavoring compounds. However, it cannot be discarded that the conditions of the fermentation may play an important role in the regulation of the expression of genes encoding for enzymes involved in the production of higher alcohols and esters.
Segregant analysis and evaluation of physiological stability
Routine observations in our laboratories have shown that yeast strains isolated directly from different cachaça distilleries present a clear phenotypic instability, even when manipulated in laboratory conditions (not shown). Indeed, it is already known that yeast strains may present genetic instability through different mechanisms (Carro et al. 2003; Lopez et al. 2001; Nadal et al. 1999; Marullo et al. 2004).
Based on these considerations, our interest was also to obtain cachaça yeast starters that present a higher phenotypic stability after successive sub-culturing, mainly the potential to produce flavoring compounds. Therefore, we evaluated the possibility to get particular more stable segregants. This is a frequently used strategy for genetic studies as far as the parental strain produces a reasonable number of asci with viable spores (Johnston et al. 2000; Marullo et al. 2004; Romano et al. 1998; Sipiczki et al. 2004).
As shown in Table 1, forty segregants from asci with four viable spores from strain LBCM 427 were chosen and further characterized (growth in YPD at 37°C, YP supplemented with sucrose—20%, w/v, YP supplemented with ethanol—10%, v/v, flocculation, killer behavior, sensitivity to mycocins, H2S production, TFL and cerulenin resistance) in order to obtain strains with improved properties for cachaça production (Vicente et al. 2006). All segregants differed from strain LBCM 427 regarding their phenotypic profile. Growth in YP medium containing sucrose (20%, w/v) and absence of H2S production was maintained for all segregants, whereas growth at 37ºC, mycocin resistance, flocculation, resistance to ethanol, TFL and cerulenin and killer activity was maintained by 29 (73%), 22 (55%), 16 (40%), 14 (35%), 14 (35%), 14 (35%), and 4 (10%) of the segregants, respectively. The data were then subjected to PCA to verify differences between segregants of the 10 tetrads in different growth conditions and to choose the segregant that presents the closest behavior to the parental strain LBCM 427.
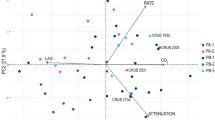
The Fig. 4 (Panel A) shows the score plot of the principal component 1 versus principal component 2 (PC1 vs. PC2) for all strains. PCA shows one cluster with four segregants (RB-8B, RB-23A, RB-23C, RB-27D) with a high number of similar characteristics compared to the parental LBCM 427 strain, including the resistance to TFL and cerulenin. All four segregants lost flocculation capacity and ethanol resistance, whereas three segregants (RB-8B, RB-23A, RB-27D) lost killer activity.
PCA of segregants of the parental strain LBCM 427 tested for different growth conditions. Panel A score plot of PC1 versus PC2 for cluster classification of segregants regarding to the selected characteristics found in the parental strain LBCM 427. Panel B score plot of PC1 versus PC2 for identification of the segregant regarding to the maintenance of physiological stability of different characteristics through successive replications
Considering the continuous process of cachaça production, it seems logical to us to evaluate the stability of phenotypic characteristics during successive sub-culturing of strains. This was estimated by assessing the amount of mitotic variability of the four segregants (RB-8B, RB-23A, RB-23C, RB-27D), during several rounds of replication in YPD medium. As shown in Table 2, all segregants keep their phenotype regarding growth in sucrose containing medium during 6 rounds of sub-culturing. Ethanol resistance tended to increase with increasing rounds of sub-cultivation while the opposite was observed for TFL resistance and growth at 37°C. All data were used for PCA (Fig. 4, Panel B), including also a hypothetical “ideal” strain considered as being stable throughout the whole replication process. Segregant RB-23A presented a higher tendency to stability, since it was found to be closest to the hypothetical ideal strain. Besides, it was the only strain that maintained TFL and cerulenin resistance during the successive replications (Table 2). Taking in account that the main focus of our selective methodology was to find yeast strains with the best potential to produce flavoring compounds (Vicente et al. 2006; Oliveira et al. 2008), the segregant RB-23A was chosen to be used in fermentation trials in sugar cane juice.
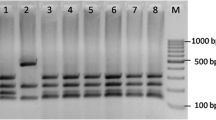
During the fermentative process, mtDNA-RFLP was used to monitor the predominance of strains LBCM 427 and RB-23A. Thirty colonies of the yeast strains were obtained from the freshly inoculated must and from the end of seven successive fermentation cycles and submitted to mtDNA-RFLP analysis. The strains predominated at 100% throughout 7 days of fermentation, since all of the 30 isolates obtained from the beginning and end of fermentations corresponded to the mtDNA RFLP profile of the inoculated strain (Fig. 5).
Strains LBCM 427 and RB-23A produced similar ethanol levels, significantly higher compared to the cachaça produced with the commercial strain suggesting a best fermentative power. Cachaça produced with LBCM 427 and RB-23A strains had also higher isobutyl and isoamyl alcohol content compared to that produced with a commercial strain (Table 3).
Since our results also showed an absence of 2:2 segregation in tetrads, the majority of the phenotypical characteristics used for yeast strains selection seem to be of polygenic origin with independent segregation. The segregants did also not maintain their phenotypic stability when replicated through successive generations (Tables 1 and 2). This is in agreement with previous studies showing that segregants present a significant variation regarding galactose and maltose utilization, resistance to sulphur dioxide and ethanol as well as synthesis of compounds such as n-propanol, isobutanol, isoamyl alcohol, acetaldehyde and ethyl acetate (Cavalieri et al. 2000; Sipiczki et al. 2004).
Conclusions
In this work, we studied some characteristics of the cachaça produced by a selected strain and one more stable segregant in comparison to cachaça produced by a commercial wine strain and in the same production conditions. Our data related to the predominance of the selected strain in an open fermentative process (Fig. 4) and to the chemical composition (Table 3) indicate that the strategies used here can contribute to improve the quality of the cachaça, since in general both strains—LBCM 427 (parental strain) and RB-23A (segregant)—produced higher concentrations of higher alcohols when compared to the values found in commercial cachaça.
Our results point out to the possibility to obtain stable yeast strains that present a clear high potential to produce flavoring compounds. However, it seems clear that natural strains isolated from cachaça distilleries present different alleles that cause modification in the global gene expression. Physiological instability during successive replications might be related to different mechanisms of genomic reorganizations. Therefore, the data presented in this study are the basis for further strategies in order to get still better strains to be used as starters to the production of cachaça.
References
Ashida S, Ichikawa E, Suginami K, Imayasu S (1987) Isolation and application of mutants producing sufficient isoamyl acetate, a sake flavor component. Agric Biol Chem 51:2061–2065
Bakalinsky AT, Snow R (1990) Conversion of Wine Strains of Saccharomyces cerevisiae to Heterothallism. Appl Environ Microbiol 56:849–857
Barre P, Vezinhet F, Dequin S, Blondin B (1992) Genetic improvement of wine yeasts. In: Fleet GH (ed) In book wine microbiology and biotechnology. Harwood Academic Publishers, Chur, pp 265–289
Carro D, García-Martinez J, Pérez-Ortín JE, Piña B (2003) Structural characterization of chromosome I size variants from a natural yeast strain. Yeast 20:171–183
Casalone E, Fia G, Barberio C, Cavalieri D, Turbanti L, Polsinelli M (1997) Genetic and biochemical characterization of Saccharomyces cerevisiae mutants resistant to trifluoroleucine. Res Microbiol 148:613–623
Cavalieri D, Casalone E, Bendoni B, Fia G, Polsinelli M, Barberio C (1999) Trifluoroleucine resistance and regulation of alpha-isopropyl malate synthase in Saccharomyces cerevisiae. Mol Gen Genet 261:152–160
Cavalieri D, Townsend JP, Hartl DL (2000) Manifold anomalies in gene expression in a vineyard isolate of Saccharomyces cerevisiae revealed by DNA microarray analysis. Proc Natl Acad Sci USA 97:12369–12374
Dickinson JR, Lanterman MM, Danner DJ, Pearson BM, Sanz P, Harrison SJ, Hewlins MJE (1997) A C-13 nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J Biol Chem 272:26871–26878
Eden A, Van NL, Drukker M, Benvenisty N, Debourg A (2001) Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl Microbiol Biotechnol 55:296–300
Guerra JB, Araújo RCA, Pataro C, Franco GR, Moreira ESA, Mendonça-Hagler LC, Rosa CA (2001) Genetic diversity of Saccharomyces cerevisiae strains during the 24 h fermentative cycle for the production of the artisanal “cachaça”. Lett Appl Microbiol 33:106–111
Guijo S, Mauricio JC, Salmon JM, Ortega JM (1997) Determination of the relative ploidy in different Saccharomyces cerevisiae strains used for fermentation and ‘flor’ film ageing of dry sherry-type wines. Yeast 13:101–117
Ichikawa E, Hosokawa N, Hata Y, Abe Y, Suginami K, Imayasu S (1991) Breeding of sake yeast with improved ethyl caproate productivity. Agric Biol Chem 55:2153–2154
Johnston JR, Baccari C, Mortimer RK (2000) Genotypic characterization of strains of commercial wine yeasts by tetrad analysis. Res Microbiol 151:583–590
Kodama Y, Omura F, Miyajima K, Ashikari T (2001) Control of higher alcohol production by manipulation of the BAP2 gene in brewing yeast. J Am Soc Brew Chem 59:157–162
Kohlhaw GB (2003) Leucine biosynthesis in fungi: entering metabolism through the back door. Microbiol Mol Biol Rev 67:1–15
Lambrechts MG, Pretorius IS (2000) Yeast and its importance to wine aroma–a review. S Afr Enol Vitic 21:97–129
Lilly M, Lambrechts MG, Pretorius IS (2000) Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl Environ Microbiol 66:744–753
Lilly M, Lambrechts MG, Pretorius IS (2006) The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res 6:726–743
Livak KJ, Schimittgen (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta-Delta C(T)) method. Methods 25:402–408
Lopez V, Querol A, Ramon A, Fernandez-Espinar MT (2001) A simplified procedure to analyse mitochondrial DNA from industrial yeasts. Int J Food Microbiol 68:75–81
Marullo P, Bely M, Masneuf-Pomarede I, Aigle M, Dubourdieu D (2004) Inheritable nature of enological quantitative traits is demonstrated by meiotic segregation of industrial wine yeast strains. FEMS Yeast Res 4:711–719
Mason AB, Dufour JP (2000) Alcohol acetyltransferases and the significance of ester synthesis in yeast. Yeast 16:1287–1298
Molina AM, Swiegers JH, Varela C, Pretorius IS, Agosin E (2007) Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl Microbiol Biotechnol 77:675–687
Morais PB, Rosa CA, Linardi VR, Pataro C, Maia ABRA (1999) Characterization and succession of yeast populations associated with spontaneous fermentations during the production of sugarcane aguardente. World J Microbiol Biotech 13:241–243
Mortimer RK (2000) Evolution and variation of the yeast (Saccharomyces) genome. Genome Res 10:403–409
Nadal D, Carro D, Fernández-Larrea J, Piña B (1999) Analysis and dynamics of the chromosomal complements of wild sparkling-wine yeast strains. Appl Environ Microbiol 65:1688–1695
Nonato EA, Carazza F, Silva FC, Carvalho CR, Cardeal ZL (2001) A headspace solid-phase microextraction method for the determination of some secondary compounds of Brazilian sugar cane spirits by gas chromatography. J Agric Food Chem 49:3533–3539
Oba T, Yamamoto Y, Nomiyama S, Suenaga H, Muta S, Tashiro K, Kuhara S (2006) Properties of a trifluoroleucine-resistant mutant of Saccharomyces cerevisiae. Biosci Biotechnol Biochem 70:1776–1779
Oliveira ES, Cardello HMAB, Jeronimo EM, Souza ELR, Serra GE (2005) The influence of different yeasts on the fermentation, composition and sensory quality of cachaça. World J Microbiol Biotechnol 21:707–715
Oliveira VA, Vicente MA, Fietto LG, Castro IM, Coutrim MX, Schüller D, Alves H, Casal M, Santos JO, Araújo LD, Silva PHA, Brandão RL (2008) Biochemical and molecular characterization of Saccharomyces cerevisiae strains obtained from sugar-cane juice fermentations and their impact in “cachaca” production. Appl Environ Microbiol 74:693–701
Pataro C, Santos A, Correa SR, Morais PB, Linardi VR, Rosa CA (1999) Physiological characterization of yeasts isolated from artisanal fermentation in an aguardente distillery. Rev Microbiol 29:104–108
Pataro C, Guerra JB, Petrillo-Peixoto ML, Mendonça-Hagler LC, Linardi VR, Rosa CA (2000) Yeast communities and genetic polymorphism of Saccharomyces cerevisiae strains associated with artisanal fermentation in Brazil. J Appl Microbiol 88:1–9
Pretorius IS (2000) Tailoring wine yeast for the new millennium: novel approaches to the ancient art of winemaking. Yeast 16:675–729
Pretorius IS (2003) The genetic analysis and tailoring of wine yeasts. In: de Winde JH (ed) Genetics and genomics of industrial yeasts, vol 2. Heidelberg, Germany
Querol A, Barrio E, Ramón D (1992) A comparative study of different methods of yeast strain characterization. Syst Appl Microbiol 15:439–446
Ribeiro CAF, Horii J (2004) Negative H2S character and flocculation as yeast strain markers for inoculum recovery. Sci Agric 61:292–297
Romano P, Paraggio M, Turbanti L (1998) Stability in by-product formation as a strain selection tool of Saccharomyces cerevisiae wine yeasts. J Appl Microbiol 84:336–341
Saerens SMG, Verstrepen KJ, Van Laere SDM, Voet ARD, VanDijck P, Delvaux FR, Thevelein JM (2006) The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem 281:4446–4456
Saerens SMG, Delvaux F, Verstrepen KJ, Van Dijck P, Thevelein JM, Delvaux FR (2008a) Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Micobiol 74:454–461
Saerens SMG, Verbelen PJ, Vanbeneden N, Thevelein JM, Delvaux FR (2008b) Monitoring the influence of high-gravity brewing and fermentation temperature on flavour formation by analysis of gene expression levels in brewing yeast. Appl Microbiol Biotech 80:1039–1051
Schoondermark-Stolk SA, Tabernero M, Chapman J, Ter Schure EG, Verrips CT, Verkleij AJ, Boonstra J (2005) Bat2p is essential in Saccharomyces cerevisiae for fusel alcohol production on the non-fermentable carbon source ethanol. FEMS Yeast Res 5:757–766
Sherman F (2002) Getting started with yeast. Methods Enzymol 350:3–41
Shuller D, Alves H, Dequin S, Casal M (2005) Ecological survey of Saccharomyces cerevisiae strains from vineyards in the Vinho Verde region of Portugal. FEMS Microbiol Ecol 51:167–177
Sipiczki M, Romano P, Capece A, Paraggio M (2004) Genetic segregation of natural Saccharomyces cerevisiae strains derived from spontaneous fermentation of Aglianico wine. J Appl Microbiol 96:1169–1175
Swiegers JH, Bartowsky EJ, Henchke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 2:139–173
Thevelein JM, de Winde JH (1999) Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol 33:904–918
Tracy JW, Kohlhaw GB (1977) Evidence for two distinct CoA binding sites on yeast α-isopropylmalate synthase. J Biol Chem 252:4085–4091
Van Der Sluis C, Rahardjo YSP, Smit BA, Kroon PJ, Hrtmans S, Ter Schure EG, Tramper J, Wijffels RH (2002) Concomitant extracellular accumulation of alpha-keto acids and higher alcohols by Zygosaccharomyces rouxii. J Biosci Bioeng 93:117–124
Verstrepen KJ, Derdelinckx G, Delvaux FR, Winderickx J, Thevelein JM, Bauer FF, Pretorius IS (2001) Late fermentation expression of FLO1 in Saccharomyces cerevisiae. J Am Soc Brew Chem 59:69–76
Verstrepen KJ, Derdelinckx G, Dufour JP, Winderickx J, Pretorius IS, Thevelein JM, Delvaux FR (2003a) The Saccharomyces cerevisiae alcohol acetyl transferase gene ATF1 is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res 4:285–296
Verstrepen KJ, Van Laere SD, Vanderhaegen BM, Derdelinckx G, Dufour JP, Pretorius IS, Winderickx J, Thevelein JM, Delvaux FR (2003b) Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microbiol 69:5228–5237
Vicente MA, Fietto LG, Castro IM, Santos ANG, Coutrim MX, Brandão RL (2006) Isolation of Saccharomyces cerevisiae strains producing higher levels of flavoring compounds for production of “cachaça” the Brazilian sugarcane spirit. Int J Food Microbiol 108:51–59
Yoshikawa S, Oguri I, Kondo K, Fukuzawa M, Shimosaka M, Okazaki M (1995) Enhanced formation of isoamyl alcohol in Zygosaccharomyces rouxii due to elimination of feedback inhibition of alpha-isopropylmalate synthase. FEMS Microbiol Lett 127:139–143
Yoshimoto H, Fukushige T, Yonezawa T, Sone H (2002) Genetic and physiological analysis of branched-chain alcohols and isoamyl acetate production in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 59:501–508
Yoshizawa K (1999) Sake: production and flavor. Food Rev Int 15:83–107
Acknowledgments
This work was supported by grants from Fundação de Capacitação de Pessoal de Nível Superior from Ministry of Education (CAPES); from Universidade Federal de Ouro Preto, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (Process EDT-2080/03 and Process CBB 2422-401/07), Program POCI 2010 (FEDER/FCT, POCI/AGR/56102/2004, Fundação para a Ciência e Tecnologia) and a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico–CNPq (Brazil) Process 300998/89-9 (RLB), Process 490717/04-7 (CNPq/Grices) and Process 301255/01-6 (LGF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, A.P.G., Vicente, M.d.A., Klein, R.C. et al. Strategies to select yeast starters cultures for production of flavor compounds in cachaça fermentations. Antonie van Leeuwenhoek 101, 379–392 (2012). https://doi.org/10.1007/s10482-011-9643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-011-9643-5