Abstract
Disengagement from care among people with HIV (PWH) and hepatitis C (HCV) increases the risks of adverse health outcomes and poses significant barriers to achieving global HIV and HCV elimination goals. In accordance with the Joanna Briggs Institute framework, a scoping review was conducted to synthesize and highlight existing gaps in the literature on (dis)engagement in care among PWH and HCV. We searched for original studies on (dis)engagement in care among PWH and HCV in high-income countries using eight electronic databases from inception to May 2023. Our search yielded 4462 non-duplicated records, which were scoped to 27 studies. Definitions of (dis)engagement in care were diverse, with considerable heterogeneity in how retention was operationalized and temporally measured. Studies identified predictors of (dis)engagement to be related to drug and substance use (n = 5 articles), clinical factors (n = 5), social and welfare (n = 4), and demographic characteristics (n = 2). When engagement in care was treated as an exposure, it was associated with HCV treatment initiation (n = 3), achieving sustained virological response (n = 2), and maintaining HIV viral suppression (n = 1). Interventions to improve care engagement among PWH and HCV were limited to five studies using cash incentives (n = 1) and individual case management (n = 4). (Dis)engagement in care is a dynamic process influenced by shifting priorities that may ‘tip the balance’ towards or away from regularly interacting with healthcare professionals. However, inconsistent definitions render cross-study comparisons and meta-analyses virtually impossible. Further research needs to establish a standardized definition to identify patients at high risk of disengagement and develop interventions that leverage the nested HIV/HCV care cascades to retain and recover patients lost from care.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus (HIV) and hepatitis C (HCV) co-infection is a global public health challenge. It is estimated that 2.3 million individuals are living with HIV/HCV—80% of whom are people who previously injected drugs (PWID) [1]. HIV/HCV co-infection exacerbates the natural history of both infections [2], accelerating the progression of HCV-related liver disease [3] and mortality risks [4, 5]. Significant advancements in HIV and HCV treatments underscore the capacity to address HIV/HCV co-infection effectively [6,7,8,9]. Following the advent of highly efficacious and safe direct-acting antiviral agents (DAAs) in 2013 [10, 11], the WHO announced an ambitious yet feasible goal towards eliminating viral hepatitis: reduce new viral hepatitis infections by 90% and mortality by 65% by 2030 [12]. Simultaneously, combined HIV antiretroviral therapy (cART) allows individuals to achieve undetectable HIV viral loads and become untransmissible (U = U) [13]. This progress is instrumental in realizing the UNAIDS 95-95-95 targets, which aim for 95% of people with HIV (PWH) to know their HIV status, 95% of those diagnosed to be on ART, and 95% of those on treatment to achieve viral suppression [14]. However, these international targets rely on people accessing and sustaining clinical care and treatment.
The cascade of care also referred to as care continuums, captures cross-sectional snapshots that describe clinical care milestones. PWH and HCV follow two nested care cascades that present unique healthcare challenges [2, 15]. The HIV care cascade is marked by five key stages—diagnosis, linkage to care, receipt of antiretroviral therapy, retention in care, and achievement of viral suppression—where the final stage requires continuous and consistent engagement in clinical care [16]. The International Antiviral Society-USA Panel (IAS-USA) recommends HIV virology tests every six months following viral suppression [17, 18], whereas the European AIDS Clinical Society (EACS) recommends HIV virology tests every 3–6 months [19]. Although the HCV care cascade follows similar sequential steps to the HIV care cascade [20, 21], it rarely includes steps following sustained virological response (SVR) [22], defined as undetectable HCV RNA 12–24 weeks after treatment completion [23].
In high-income settings, the majority of PWH and HCV are diagnosed and engaged in care, making them an ideal “micro-population” to target for HCV care and achieving WHO targets [24, 25]. However, the cascade of care PWH and HCV follow is dynamic, marked by changing clinical and social factors influencing patient engagement in different steps [26, 27]. For instance, dual HIV/HCV stigma, unmet mental health needs, and socio-structural complexities can function as pervasive barriers to care [28,29,30]. Thus, disengagement from care, or loss to follow-up (LTFU), can occur at any step of the care cascades, contributing to increased risks of HIV- [31,32,33] and HCV- [22] related morbidity and mortality.
Disengagement from care among PWH and HCV needs to be better understood. Varying definitions of disengagement/LTFU and retention in care exist across the literature [22, 34,35,36,37]. This ambiguity hinders the development of patient-centred interventions that ensure retention in care and optimal health outcomes. The paucity of synthesized information covering the topic necessitated a scoping review. Our primary objective was to examine the scope of literature on (dis)engagement from care among PWH and HCV in high-income countries, highlighting existing gaps in the literature and clarifying concepts regarding the research topic. Our research sub-questions were:
-
How was (dis)engagement in care defined in the context of care cascade for PWH and HCV?
-
What key factors influence (dis)engagement in care among PWH and HCV?
-
What are the health outcomes associated with (dis)engagement in care?
-
What are the existing interventions aimed at addressing (dis)engagement/LTFU among HIV/HCV co-infected individuals?
Methods
The scoping review was conducted in accordance with the Joanna Briggs Institute framework [38], originally described by Arksey and O’ Malley [39]. The scoping review included the following stages: (i) identifying the research questions; (ii) information sources and search strategy; (iii) study selection; (iv) data extraction and synthesis; and (v) collating, summarizing, and reporting the results [38]. The protocol was registered in the Open Science Framework [40]. Modifications to the protocol included (i) the consolidation of the data extraction tools, (ii) data extraction simplifications, and (iii) the omission of grey literature from the summary of factors, outcomes, and interventions.
Eligibility Criteria
Eligible studies provided insight into retention in clinical care and disengagement/LTFU among PWH and HCV [41]. Due to the variations in health services between high and low- and middle-income countries, particularly concerning HIV [42] and HCV [43] infections, the review focused solely on high-income countries. This scoping review considered peer-reviewed journal articles and grey literature sources (e.g., conference abstracts, preprints, and graduate theses), contributing primary empirical data. To prevent data duplication, secondary sources of information, including systematic reviews, opinion papers and grey literature that were subsequently peer-reviewed/published, were not considered.
Guided by a conceptualization proposed by the WHO [44] for HIV care, we adopted a pragmatic definition of retention as continuous engagement (i.e., no documented interruptions) in the care cascade(s) following linkage to care. Conversely, disengagement from care was conceptualized as discontinuing care (i.e., ceasing to interact with healthcare professionals or access care) for any reason following linkage to care.
Search Strategy
A health sciences librarian assisted in conducting the search strategy, which involved locating published and unpublished studies on the topic.
This scoping review searched eight online databases (MEDLINE (Ovid), Embase (Ovid), Cochrane CENTRAL (Ovid EBM Reviews), Global Health (Ovid), Web of Science Core Collection, CINAHL, ProQuest Dissertation and Theses Global, and the Preprint Citation Index) from database inception through May 2023. Relevant keywords and database-specific subject headings (MeSH) for HIV, HCV, co-infection, and LTFU [45,46,47,48] were used to develop a comprehensive search strategy (see Supplemental File #S1).
Following the database searches, the reference lists of all included articles were screened for additional studies. Cited reference searching was also performed for all included studies using Google Scholar.
Study/Source of Evidence Selection
All identified citations were collated and uploaded into the systematic review software Covidence (Veritas Health Innovation, Melbourne, Australia), and duplicates were removed. Prior to screening, the research team conducted meetings and pilot testing [38] to ensure consistency and reliability in the screening process. Pilot testing involved an initial screening of a random sample of 25 titles/abstracts, assessed by two reviewers (DAD, YT) against the inclusion criteria. Interrater reliability was measured, and conflicts were discussed with another reviewer (SS) to achieve consensus. The described pilot testing was repeated until an interrater reliability metric of ≥ 75% was achieved. Following pilot testing, the reviewers began the independent title/abstract screening process.
Two reviewers (DAD, YT) then assessed the full text of selected citations in detail against the inclusion criteria. Conflicts were resolved by the third reviewer (SS).
Data Extraction
One independent reviewer (DAD) extracted data from the included studies and verified it by another reviewer (SS). Conflicts were resolved through discussion until consensus was achieved.
We classified papers as defining (dis)engagement as the outcome, the exposure, and/or interventions related to (dis)engagement. Initially, the protocol included three separate extraction sheets for each category. However, recognizing the potential overlap among articles in these categories (e.g., an intervention article that describes factors of (dis)engagement), we utilized a single consolidated extraction sheet (see Supplemental File #S2).
Data extracted included study characteristics, such as author(s), year, study timeframe (i.e., during the interferon-era (January 2002 - December 2013), DAA-era (January 2014-Present), or both) [49], primary objectives, country/study setting, study population characteristics (e.g., relevant demographic characteristics and sample size), and the focal care cascade (i.e., HIV, HCV, or both); conceptualizations of key terms; and reported outcomes pertaining to the research question (i.e., factors and outcomes associated with (dis)engagement). To facilitate the synthesis and comparison of data, a protocol modification was made to simplify factors associated with (dis)engagement into categories based on studies that have identified patient-, provider-, and system factors that influence retention in care [28, 30, 50]. These categories included the following: demographics (e.g., age, race, gender), substance use (i.e., any substance misuse or illicit drug use), social/welfare (i.e., psychosocial influences such as employment status, housing stability, incarceration, discrimination), clinical (e.g., HIV/HCV disease progression, treatments, and physical comorbidities), mental health (i.e., diagnoses of any mental health disorders), and other factors that do not fit into the pre-specified categories. For studies focused on describing an intervention or care programme, additional data was abstracted on the engagement method, facilitators involved in the intervention, and relevant outcomes.
Data Analysis and Presentation
Quantitative descriptive analyses involving frequency counts and proportions of study characteristics were conducted. A narrative summary of results was produced to describe and summarize the extracted data. Given the lack of specificity in definitions of (dis)engagement and description of statistical methodologies in included grey literature, only peer-reviewed articles were included to summarize factors, outcomes, and interventions. Data extracted from the grey literature articles are detailed in a supplementary file for readers (See Supplement #S3). Consistent with best-practice scoping review methodology [39], the scope of the data was mapped descriptively without assessing the source’s quality. The results of this scoping review are reported following the PRISMA-ScR guidelines (see Supplemental File #S4) [51, 52].
Results
Characteristics of Included Studies
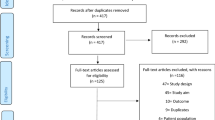
The database search yielded a total of 6682 records, with 4462 titles and abstracts screened for after removal of duplicates. 152 database-searched articles were identified for full-text screening. Of these, 25 met the inclusion criteria. An additional two sources were identified through citation searching. In total, 27 studies were identified for final review [20, 25, 53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. The PRISMA flow diagram (Fig. 1) depicts the process of identifying records that meet the inclusion criteria.
Table 1 summarizes the demographic information and characteristics of included studies. Among the 27 studies included, 19 (70.4%) were peer-reviewed articles, and eight (29.6%) were grey literature. Sample sizes ranged from n = 46 to n = 5114, with a majority of included studies (n = 23, 85.2%) consisting entirely of PWH and HCV [25, 53,54,55,56,57,58,59,60,61,62,63, 65,66,67,68,69, 71, 72, 74,75,76,77]. 16 studies were retrospective observational studies [20, 53, 55,56,57,58,59,60,61, 63,64,65, 67, 72,73,74], five were prospective observational [25, 54, 62, 66, 69], four experimental (e.g., randomized controlled trials) [68, 75,76,77], and two were cross-sectional surveys [70, 71]. 16 studies were conducted in the United States [20, 53, 55, 57, 60, 62, 63, 67, 68, 70, 72,73,74,75,76,77], five in Canada [25, 59, 61, 64, 69], one each in the UK [71], Austria [58], Taiwan [65], and the Netherlands [66]; and two in multiple countries [54, 56] (i.e., HCV-Tren and EuroSIDA cohort studies).
Four studies examined (dis)engagement in clinical care during the interferon-era (January 2002 - December 2013) [20, 61, 70, 74], 14 during the DAA-era (January 2014-Present) [53, 55,56,57, 60, 62, 63, 66,67,68, 71, 72, 75, 76], and six during both [25, 54, 58, 65, 69, 73]; three studies did not specify their study period [59, 64, 77]. Based on the primary objective of the studies, 22 were focused on the HCV care cascade [20, 25, 53,54,55,56,57,58,59,60, 62, 63, 65,66,67, 70,71,72,73, 75,76,77], two on the HIV care cascade [61, 64], and three on both [68, 69, 74]. 16 sources (12 peer-reviewed [20, 56,57,58, 61, 62, 65, 69, 70, 72, 73, 75] and 4 grey literature [54, 60, 74, 77]) identified factors associated with retention or disengagement in care; 10 (6 peer-reviewed [25, 55, 62, 63, 66, 71] and 4 grey literature [53, 59, 64, 67]) measured (dis)engagement as an exposure and identified outcomes; and 6 identified an intervention (5 peer-reviewed [63, 68, 72, 75, 76] and one grey literature [77]) to improve engagement across the care cascade.
Conceptualization of Key Terms
Of the 27 included studies, 18 conceptualized retention or disengagement in care (Table 1). Definitions of (dis)engagement in care were diverse, with considerable heterogeneity in how retention was operationalized and temporally measured (Fig. 2).
The most common definition of (dis)engagement in care was based on scheduling and attending appointments over a six-month period [20, 55, 57, 62, 63, 74, 75] (e.g., “Having two or more visits with an HIV primary care provider in our clinic separated by ≥ 3 months in each calendar year“ [55]). Other conceptualizations of engagement in care were operationalized based on records of blood biomarker testing [54, 58, 77] (e.g., LTFU defined as being “without any visits and/or HCV-PCR test result at 24/12 weeks after the end of treatment or afterward“ [58]) or treatment access [60, 72] (e.g., LTFU defined as “no evidence of treatment or cure and no longer in care at practice” [60]). Five studies clearly distinguished between disengagement and other clinical events such as death [25, 66, 72], clinic transfers or study withdrawals [20, 25, 55, 66, 72], and incarceration [20, 55]. Furthermore, five studies [54, 55, 57, 62, 63] conceptualized (dis)engagement in care based on HIV care guidelines, such as by the EACS [19], Infectious Diseases Society of America (IDSA) [78], and US Department of Health and Human Services (DHHS) [79, 80], whereas the others did not specify their basis. One definition based on EACS guidelines (version 10.1) defined lost to follow-up as “Not having a CD4 count, HIV-RNA, or visit data for 15 months” to account for any deferrals in blood biomarker testing or visits [54].
Factors Associated with Retention in Care or Disengagement in Care
Twelve peer-reviewed studies identified factors associated with retention in care or disengagement from care (Table 2).
Demographics
Demographic characteristics affect retention in the HCV/HIV care cascades [61, 72]. Factors associated with poor retention in care included male sex [61], female sex [72], and younger age [72]. The underlying reasons for gender-based differences in HIV/HCV care retention are not well understood [61], with speculations including women deferring treatment due to family obligations or other traditionally gendered domestic responsibilities [72]. Three peer-reviewed studies evaluated the role of race on (dis)engagement in care [62, 69, 72], but none found a statistically significant association.
Substance Use
Substance use emerged as a significant factor affecting retention in care among PWH and HCV in five studies [20, 56, 57, 62, 75]. Generally, injection drug use [56, 57, 62] and alcohol misuse [56, 57] were associated with poor retention in care. In a bivariate analysis from an HCV-TREN cohort study, ongoing injection drug use and alcohol misuse were key predictors of HCV treatment challenges, manifesting as premature treatment discontinuation due to any cause, including losses to follow-up [56]. However, a counterintuitive positive association between alcohol use and engagement in HCV care (OR = 3.79, 95% CI [1.14–12.61]) was observed in one study, suggesting motivation among some to achieve HCV cure to reduce the synergistic effects of alcohol use [75].
Social/Welfare
Social and welfare factors also affect disengagement from care. Experiences of unstable housing have been linked to non-engagement in HIV care [57] and HCV clinical failures [56]. Moreover, social isolation—reflective of experiences of stigma —can negatively influence maintaining liver clinic appointments among both HCV and HIV/HCV co-infected patients [70]. To further explore these individual experiences, the Canadian Co-infection Cohort study developed a deprivation index with social/lifestyle variables (income >$1500/month, education > high school, employment, identifying as gay or bisexual, Indigenous status, injection drug use in last 6 months, injection drug use ever, past incarceration, and past psychiatric hospitalization) and found that higher deprivation scores were linked to increased odds of failing to attend a second care appointment (OR = 1.17, 95% CI [1.02–1.34]), suggestive of greater propensity for disengagement from care [69].
Clinical-Related Factors
The introduction of DAAs has had a transformative effect on retention in HCV care [58, 65, 73]. A study conducted at a viral hepatitis clinic in Austria indicated reduced odds of LTFU during the restricted DAA era (January 2014-September 2017; OR = 0.11, 95% CI [0.03–0.46]) and unrestricted DAA era (October 2017-December 2020; OR = 0.47, 95% CI [0.24–0.92]), compared to the interferon era (before December 2013) [58]. Disease progression in PWH and HCV, along with co-morbidities, can also influence retention in HCV care [56, 58, 75]. Clinical indicators such as advanced liver fibrosis [58] and higher CD4 cell count [75] have been associated with improved retention in care, suggesting that advanced disease may facilitate shifts in individual risk behaviour [58]. In direct contrast, another study indicated that advanced liver fibrosis was associated with HCV treatment failures [56], but the reason for the discrepancy remains elusive.
Other
Other factors, including treatment location, mental illness, and peer influence, have also been recognized for their role in HCV care retention [56, 58, 75]. Having personal connections to individuals who have been cured of HCV infection [75] and participation in dedicated treatment programs [58] are associated with greater odds of engagement in care (OR = 5.25, 95% CI [1.40-19.55]) and reduced risk of loss to follow-up (OR = 0.26, 95% CI [0.13–0.52]), respectively. In contrast, mental illness has been associated with any clinical HCV failure (OR = 2.78, 95% CI [1.71–4.52]) [56].
Outcomes
Six peer-reviewed studies identified outcomes associated with disengagement (Table 2).
HCV Treatment Initiation
Three studies cited treatment initiation as an outcome associated with retention in care [62, 66, 71]. A prospective observational study from Baltimore reported a negative association between missing a majority of HIV primary care appointments and HCV treatment initiation (HR = 0.39, 95% CI [0.25–0.60]) [62].
HIV- and HCV-Treatment Success
Three studies also drew a connection between successful HIV and HCV treatment outcomes and retention in care [25, 62, 63]. In line with existing research [25], a retrospective chart review of a hospital-based clinic in an underserved community indicated that patients who had a lapse in care for > 12 months were at reduced odds of achieving SVR (OR = 0.069, 95% CI [0.014–0.337]) [63]. Likewise, individuals poorly engaged in HIV care were less likely to achieve HIV viral suppression [62].
Interventions Addressing Engagement in Care
Five peer-reviewed articles detailed interventions to increase engagement among PWH and HCV in HCV care (Table 3) [63, 68, 72, 75, 76]. Interventions included contingency measures [76] (n = 1), such as peer support and contingent financial incentives, and individual case management [63, 68, 72, 75] (n = 4). The case management approach—facilitated by various healthcare professionals, including pharmacists [63], nurses [75], care facilitators with a social work background [68], or a multidisciplinary team of health professionals [72]—focused on providing HCV treatment education and assistance in appointment scheduling and attendance.
Integrated HCV and HIV care services with a multidisciplinary team can improve the care cascades via sustained patient engagement and decreased barriers to care [72, 76]. However, the efficacy of specific interventions to increase HCV care engagement among PWH was considered modest. Individual case management by an individual healthcare professional improved rates of linkage to HCV care [75], reduced clinic prevalence of HCV infection [63], and overall progress along the HCV care cascade [68], but did not improve rates of achieving HCV SVR [68, 75]. Further, there were no significant differences in engagement in care in the intervention based on peer support and contingent cash incentives [76].
Discussion
The present scoping review examined 27 sources, shedding light on the complexity of disengagement from care among PWH and HCV in high-income countries. While several reviews exist in the context of HIV and HCV mono-infection [22, 37, 46, 47, 50, 81], to our knowledge, this is the first review to synthesize literature on disengagement from HIV and HCV co-infection care.
Our scoping review highlights heterogeneity in the conceptualization of (dis)engagement in care among PWH and HCV. Most included studies characterize engagement through patients’ adherence to appointment schedules within a specific timeframe, ranging from three to over 12 months, while others encompass aspects such as accessing treatment and reports of laboratory blood work. Five studies adopted conceptualizations of (dis)engagement in care based on national HIV guidelines (e.g., the EACS and DHHS guidelines); however, the remaining 22 failed to explicitly state or justify the specific definitions employed. Furthermore, a minority of studies differentiated LTFU from other clinical events, such as death, incarceration, and clinic transfers.
This lack of uniformity in definitions renders cross-study comparisons and meta-analyses virtually impossible. Similar challenges were described in existing systematic reviews on retention in care and LTFU among HIV and HCV mono-infected populations [22, 36, 50]. Moreover, this variability in defining engagement in care distorts measures of SVR success rates, complicating our ability to track progress towards the WHO goals. Failures to appropriately identify and account for individuals’ LTFU in the HCV care cascade may lead to inaccurate and incomparable SVR success rates. Definitions of disengagement from care should be standardized to HIV and HCV clinical care guidelines, with specific operationalizations and timeframes. Disengagement from care should also be clearly distinguished from permanent clinical events, such as mortality and clinic transfers. To account for variations in engagement timeframes and ensure the robustness of findings, sensitivity analyses with varying definitions of disengagement from care (e.g., with varying timeframes) should be provided whenever possible [82].
While disengagement from care does not necessarily imply medication non-adherence, health outcomes associated with disengagement are conspicuous. In the context of HIV and HCV co-infection, poor engagement in care impedes rates of HCV treatment initiation [62, 66, 71] and achieving both HCV SVR [25, 63] and HIV viral suppression [62]. However, the link between disengagement from care and risks of adverse long-term health outcomes, such as HCV reinfection and mortality, among PWH and HCV were not identified.
Furthermore, a majority of included studies (n = 18 (66.7%)) were cross-sectional in design (i.e., retrospective cohort studies or cross-sectional surveys), failing to capture patients’ actual experiences in care [83]. Retention is a multi-factorial concept [26, 27], influenced by shifting priorities that may ‘tip the balance’ towards or away from regularly interacting with healthcare professionals [84]. PWH and HCV may exit and re-enter care at various points along the care continuum, exhibiting dynamic transition patterns that significantly impact mortality and treatment outcomes [82]. Thus, this reliance on cross-sectional studies limits our ability to track and accurately understand (dis)engagement in care.
In the present scoping review, several factors of (dis)engagement in care among PWH and HCV were identified, including substance use-, social/welfare-, clinical-, and demographic-related factors. Congruent with findings from a systematic review on retention in HIV care [50], injection drug use [56, 57, 62] emerged as a primary factor influencing poor retention in care among PWH and HCV in high-income countries. PWID face substantial socio-structural barriers to care [2], including fractured social networks, competing priorities such as attaining stable housing, and experiences of stigma in healthcare settings [85, 86]. These barriers contribute to institutional mistrust [87] among PWID and may lead to poor engagement in clinical care. Even following HCV treatment success, mortality rates among PWID remain disproportionately high [88], underscoring the need for tailored and continued care for this group. Interestingly, our search highlighted a significant gap in the literature on the associations of race/ethnicity, socioeconomic status, and supervised consumption services (SCS) with (dis)engagement in care. Understanding the impact of race/ethnicity and socioeconomic status is crucial, given the profound effects of systemic inequities and discrimination on healthcare access and outcomes [28, 89, 90]. Furthermore, SCS often employ multidisciplinary teams that can facilitate testing, linkage to care, and referrals to other harm reduction services [91,92,93,94], making them a promising avenue for future interventions to enhance engagement in care for PWH and HCV. Further research should continue to elucidate factors associated with (dis)engagement in care, including the role of race/ethnicity, socioeconomic status, and SCS.
While limited engagement interventions were identified, the findings from this scoping review corroborate existing research on the effectiveness and practicality of HIV/HCV co-located clinics [72, 76]. Given the complex needs of PWH and HCV, employing multidisciplinary teams of healthcare professionals may be fruitful in engaging patients, particularly in earlier stages of care. Considering overlaps in the HIV and HCV care cascades, integrating HCV care into established HIV clinics has the potential to reduce accessibility barriers, extend the reach of resources, and streamline patient-centred care [95].
The continuous nature of the HIV care continuum can help healthcare professionals retain individuals with HCV post-SVR [95]. However, this scoping review reveals a lack of studies that include post-SVR care as part of the HCV care continuum. Unlike HIV care, in which life-long engagement is required to achieve viral suppression [16], HCV care often concludes upon achieving SVR [22]. While SVR is a cure, concluding engagement in care at this point is suboptimal, overlooking the need for ongoing health monitoring for PWH and HCV [22]. HCV reinfection following SVR is a substantial concern, particularly among high-risk persons, including PWH, men who have sex with men, and PWID [96, 97]. Despite achieving SVR, competing co-morbidities may put people at high mortality risk [98], underscoring the need for ongoing support. The interconnected nature of the HIV and HCV care cascade provides an opportunity for continual engagement in care and intervention. Future research should focus not only on engaging PWH and HCV in care until treatment success but also beyond—especially those with advanced liver fibrosis, who may still face long-term liver outcomes after achieving SVR [22], and those considered at high-risk of reinfection.
Findings from this scoping review should be interpreted within several limitations. Firstly, terminology and conceptualizations used to describe (dis)engagement varied markedly, restricting comparisons between studies and complicating the inclusion/exclusion criteria. Secondly, the present scoping review is not intended to assess the validity of study results or the efficacy of interventions; instead, it serves as a synthesis of existing literature. Interpretations of factors and outcomes should be cautiously approached, as they do not encompass an exhaustive list of all possible variables associated with (dis)engagement in care. Furthermore, the findings discussed in this scoping review are specific to high-income settings, where access to HIV and HCV care and sociocultural beliefs are not generalizable to low- or middle-income settings [42, 43]. Nonetheless, this scoping review is a comprehensive literature synthesis, providing valuable insights to inform future research and interventions for PWH and HCV.
Conclusion
Despite recent breakthroughs in HIV and HCV treatments, disengagement from care remains a pressing public health concern. Our scoping review highlights significant gaps in the literature on disengagement from care among PWH and HCV. Future research should address (i) a standardized definition of disengagement from care, (ii) interventions that capitalize on the nested HIV and HCV care continuums, and (iii) longitudinal study designs to analyze diverse factors and outcomes associated with disengagement. Eliminating HIV and HCV infections extends beyond medicine. It demands a holistic and coordinated approach to ensuring retention in care—vital to moving global efforts of HIV and HCV eradication toward success.
Sankey diagram showcasing heterogeneity in the definitions of (dis)engagement from care (n = 18). Note Of the 27 included studies, 18 defined (dis)engagement in care based on how it was operationalized (records of scheduling and attending appointments, blood biomarker testing, and/or treatment access) and their timeframes. Five of these 18 studies specified definitions were based on HIV clinical care guidelines, such as by the European AIDS Clinical Society (EACS) [19], Infectious Diseases Society of America (IDSA) [78], and US Department of Health and Human Services (DHHS) [79, 80]
References
Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808. https://doi.org/10.1016/S1473-3099(15)00485-5.
Taylor LE, Swan T, Matthews GV. Management of hepatitis C virus/HIV coinfection among people who use drugs in the era of direct-acting antiviral-based therapy. Clin Infect Dis. 2013;57(Suppl 2):S118–24. https://doi.org/10.1093/cid/cit326.
Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–91. https://doi.org/10.1097/QAD.0b013e32830e6d51.
Hernando V, Perez-Cachafeiro S, Lewden C, Gonzalez J, Segura F, Oteo JA, et al. All-cause and liver-related mortality in HIV positive subjects compared to the general population: differences by HCV co-infection. J Hepatol. 2012;57(4):743–51. https://doi.org/10.1016/j.jhep.2012.06.010.
van der Helm J, Geskus R, Sabin C, Meyer L, Del Amo J, Chene G, et al. Effect of HCV infection on cause-specific mortality after HIV seroconversion, before and after 1997. Gastroenterology. 2013;144(4):751–. https://doi.org/10.1053/j.gastro.2012.12.026. 60 e2.
Hearn B, Delbello D, Lawler J, Ng M, Harty A, Dieterich DT. Hepatitis C Virus Treatment in HIV-Coinfected patients: no longer different from Monoinfection Treatment. Gastroenterol Hepatol (N Y). 2014;10(11):706–15. PMID: 28435407.
Naggie S, Cooper C, Saag M, Workowski K, Ruane P, Towner WJ, et al. Ledipasvir and Sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med. 2015;373(8):705–13. https://doi.org/10.1056/NEJMoa1501315.
Patel SV, Jayaweera DT, Althoff KN, Eron JJ, Radtchenko J, Mills A, et al. Real-world efficacy of direct acting antiviral therapies in patients with HIV/HCV. PLoS ONE. 2020;15(2):e0228847. https://doi.org/10.1371/journal.pone.0228847.
Wyles DL, Sulkowski MS, Dieterich D. Management of Hepatitis C/HIV Coinfection in the era of highly effective Hepatitis C Virus Direct-acting antiviral therapy. Clin Infect Dis. 2016;63(Suppl 1):S3–11. https://doi.org/10.1093/cid/ciw219.
Geddawy A, Ibrahim YF, Elbahie NM, Ibrahim MA. Direct acting anti-hepatitis C virus drugs: clinical pharmacology and future direction. J Transl Int Med. 2017;5(1):8–17. https://doi.org/10.1515/jtim-2017-0007.
Zoratti MJ, Siddiqua A, Morassut RE, Zeraatkar D, Chou R, van Holten J, et al. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: a systematic literature review and meta-analysis. EClinicalMedicine. 2020;18:100237. https://doi.org/10.1016/j.eclinm.2019.12.007.
World Health Organization. Global health sector strategy on viral Hepatitis 2016–2021. Geneva, Switzerland: WHO; 2016.
Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA. 2019;321(5):451–2. https://doi.org/10.1001/jama.2018.21167.
Frescura L, Godfrey-Faussett P, Feizzadeh AA, El-Sadr W, Syarif O, Ghys PD, et al. Achieving the 95 95 95 targets for all: a pathway to ending AIDS. PLoS ONE. 2022;17(8):e0272405. https://doi.org/10.1371/journal.pone.0272405.
Chen JY, Feeney ER, Chung RT. HCV and HIV co-infection: mechanisms and management. Nat Rev Gastroenterol Hepatol. 2014;11(6):362–71. https://doi.org/10.1038/nrgastro.2014.17.
Kay ES, Batey DS, Mugavero MJ. The HIV treatment cascade and care continuum: updates, goals, and recommendations for the future. AIDS Res Ther. 2016;13:35. https://doi.org/10.1186/s12981-016-0120-0.
Gandhi RT, Bedimo R, Hoy JF, Landovitz RJ, Smith DM, Eaton EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the International Antiviral Society-USA panel. JAMA. 2023;329(1):63–84. https://doi.org/10.1001/jama.2022.22246.
Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–33. https://doi.org/10.1001/jama.2010.1004.
Ambrosioni J, Levi L, Alagaratnam J, Van Bremen K, Mastrangelo A, Waalewijn H, et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023;24(11):1126–36. https://doi.org/10.1111/hiv.13542.
Cachay ER, Hill L, Wyles D, Colwell B, Ballard C, Torriani F, et al. The hepatitis C cascade of care among HIV infected patients: a call to address ongoing barriers to care. PLoS ONE. 2014;9(7):e102883. https://doi.org/10.1371/journal.pone.0102883.
Fursa O, Mocroft A, Lazarus JV, Amele S, Lundgren J, Matulionyte R, et al. The hepatitis C cascade of care in HIV/hepatitis C virus coinfected individuals in Europe: Regional and intra-regional differences. AIDS. 2022;36(3):423–35. https://doi.org/10.1097/QAD.0000000000003112.
van Dijk M, Drenth JPH. HepNed study g. loss to follow-up in the hepatitis C care cascade: a substantial problem but opportunity for micro-elimination. J Viral Hepat. 2020;27(12):1270–83.
Jacobson IM, Poordad F, Brown RS Jr., Kwo PY, Reddy KR, Schiff E. Standardization of terminology of virological response in the treatment of chronic hepatitis C: panel recommendations. J Viral Hepat. 2012;19(4):236–43. https://doi.org/10.1111/j.1365-2893.2011.01552.x.
Sacks-Davis R, Doyle JS, Rauch A, Beguelin C, Pedrana AE, Matthews GV, et al. Linkage and retention in HCV care for HIV-infected populations: early data from the DAA era. J Int AIDS Soc. 2018;21(2):e25051. https://doi.org/10.1002/jia2.25051.
Saeed S, Strumpf E, Moodie EEM, Wong L, Cox J, Walmsley S, et al. Eliminating structural barriers: the impact of unrestricted access on hepatitis C treatment uptake among people living with human immunodeficiency virus. Clin Infect Dis. 2020;71(2):363–71. https://doi.org/10.1093/cid/ciz833.
Ehrenkranz P, Rosen S, Boulle A, Eaton JW, Ford N, Fox MP, et al. The revolving door of HIV care: revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLoS Med. 2021;18(5):e1003651. https://doi.org/10.1371/journal.pmed.1003651.
Keene CM, Euvrard J, Amico KR, Ragunathan A, English M, McKnight J, et al. Conceptualising engagement with HIV care for people on treatment: the indicators of HIV Care and AntiRetroviral Engagement (InCARE) Framework. BMC Health Serv Res. 2023;23(1):435. https://doi.org/10.1186/s12913-023-09433-4.
Ortiz-Paredes D, Amoako A, Lessard D, Engler K, Lebouche B, Klein MB. Barriers and facilitators related to HCV treatment uptake among HIV coinfected populations in Canada: patient and treatment provider perceptions. Can Liver J. 2022;5(2):124–43. https://doi.org/10.3138/canlivj-2021-0020.
Lekas HM, Siegel K, Leider J. Felt and enacted stigma among HIV/HCV-coinfected adults: the impact of stigma layering. Qual Health Res. 2011;21(9):1205–19. https://doi.org/10.1177/1049732311405684.
Grebely J, Oser M, Taylor LE, Dore GJ. Breaking down the barriers to hepatitis C virus (HCV) treatment among individuals with HCV/HIV coinfection: action required at the system, provider, and patient levels. J Infect Dis. 2013;207(Suppl 1):S19–25. https://doi.org/10.1093/infdis/jis928.
Giordano TP, Gifford AL, White AC Jr., Suarez-Almazor ME, Rabeneck L, Hartman C, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44(11):1493–9. https://doi.org/10.1086/516778.
Yehia BR, French B, Fleishman JA, Metlay JP, Berry SA, Korthuis PT, et al. Retention in care is more strongly associated with viral suppression in HIV-infected patients with lower versus higher CD4 counts. J Acquir Immune Defic Syndr. 2014;65(3):333–9. https://doi.org/10.1097/QAI.0000000000000023.
Ulloa AC, Puskas C, Yip B, Zhang W, Stanley C, Stone S, et al. Retention in care and mortality trends among patients receiving comprehensive care for HIV infection: a retrospective cohort study. CMAJ Open. 2019;7(2):E236–45. https://doi.org/10.9778/cmajo.20180136.
Font H, Rollins N, Essajee S, Becquet R, Foster G, Mangwiro AZ, et al. Retention-in-care in the PMTCT cascade: definitions matter! Analyses from the INSPIRE projects in Malawi, Nigeria and Zimbabwe. J Int AIDS Soc. 2020;23(10):e25609. https://doi.org/10.1002/jia2.25609.
Granozzi B, Guardigni V, Badia L, Rosselli Del Turco E, Zuppiroli A, Tazza B, et al. Out-of-hospital treatment of Hepatitis C increases retention in care among people who inject drugs and homeless persons: an observational study. J Clin Med. 2021;10(21). https://doi.org/10.3390/jcm10214955.
Grimsrud AT, Cornell M, Egger M, Boulle A, Myer L. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66(9):1006–13. https://doi.org/10.1016/j.jclinepi.2013.03.013.
Palacio-Vieira J, Reyes-Uruena JM, Imaz A, Bruguera A, Force L, Llaveria AO, et al. Strategies to reengage patients lost to follow up in HIV care in high income countries, a scoping review. BMC Public Health. 2021;21(1):1596. https://doi.org/10.1186/s12889-021-11613-y.
Peters MDJGC, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E MZ, editor. JBI Manual for Evidence Synthesis: JBI; 2020. https://doi.org/10.46658/JBIMES-20-12.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. https://doi.org/10.1080/1364557032000119616.
Dinh D, Tan Y, Saeed S. Factors and outcomes associated with disengagement from care among people co-infected with HIV and HCV: a scoping review protocol. Open Sci Framew. 2023. https://doi.org/10.17605/OSF.IO/YU8S4.
World Bank Group. World Bank Country and Lending Groups. 2023. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups. Accessed May 20, 2024.
Shao Y, Williamson C. The HIV-1 epidemic: low- to middle-income countries. Cold Spring Harb Perspect Med. 2012;2(3):a007187. https://doi.org/10.1101/cshperspect.a007187.
Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Res. 2015;119:89–96. https://doi.org/10.1016/j.antiviral.2015.01.004.
World Health Organization. Retention in HIV programmes: defining the challenges and identifying solutions. Geneva, Switzerland: WHO; 2011.
Bar N, Bensoussan N, Rabinowich L, Levi S, Houri I, Ben-Ami Shor D, et al. Barriers and facilitators of hepatitis C care in persons coinfected with human immunodeficiency virus. Int J Environ Res Public Health. 2022;19(22). https://doi.org/10.3390/ijerph192215237.
Bauermeister JA, Bonett S, Rosengren AL, Choi SK, Watson D. Approaches to promoting linkage to and retention in HIV care in the United States: a scoping review. Curr HIV/AIDS Rep. 2021;18(4):339–50. https://doi.org/10.1007/s11904-021-00557-y.
Hall BJ, Sou KL, Beanland R, Lacky M, Tso LS, Ma Q, et al. Barriers and facilitators to interventions improving retention in HIV care: a qualitative evidence meta-synthesis. AIDS Behav. 2017;21(6):1755–67. https://doi.org/10.1007/s10461-016-1537-0.
Ma J, Non L, Amornsawadwattana S, Olsen MA, Garavaglia Wilson A, Presti RM. Hepatitis C care cascade in HIV patients at an urban clinic in the early direct-acting antiviral era. Int J STD AIDS. 2019;30(9):834–42. https://doi.org/10.1177/0956462419832750.
Manns MP, Maasoumy B. Breakthroughs in hepatitis C research: from discovery to cure. Nat Rev Gastroenterol Hepatol. 2022;19(8):533–50. https://doi.org/10.1038/s41575-022-00608-8.
Bulsara SM, Wainberg ML, Newton-John TRO. Predictors of adult retention in HIV care: a systematic review. AIDS Behav. 2018;22(3):752–64. https://doi.org/10.1007/s10461-016-1644-y.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. https://doi.org/10.7326/M18-0850.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Ajose TA, Chan A. Barriers to initiating DAA therapy in HCV/HIV coinfected patients. Top Antivir Med. 2019;27(SUPPL 1):215s.
Amele S. The impact of DAA treatment on HIV/HCV co-infected individuals across Europe: analyses of co-infection data from a European cohort study. England: University of London, University College London (United Kingdom); 2021.
Cachay ER, Hill L, Torriani F, Ballard C, Grelotti D, Aquino A, et al. Predictors of missed hepatitis C intake appointments and failure to establish hepatitis C care among patients living with HIV. Open Forum Infect Dis. 2018;5(7):ofy173. https://doi.org/10.1093/ofid/ofy173.
Cachay ER, Mena A, Morano L, Benitez L, Maida I, Ballard C, et al. Predictors of hepatitis C treatment failure after using direct-acting antivirals in people living with human immunodeficiency virus. Open Forum Infect Dis. 2019;6(3):ofz070. https://doi.org/10.1093/ofid/ofz070.
Cachay ER, Torriani FJ, Hill L, Jain S, Del Real A, Qin H, et al. The role of barriers to care on the propensity for HCV non-referral among people living with HIV. AIDS. 2020;34(11):1681–3. https://doi.org/10.1097/QAD.0000000000002610.
Chromy D, Bauer D, Simbrunner B, Jachs M, Hartl L, Schwabl P, et al. Progress of Hepatitis C elimination in viennese people living with HIV after two decades of increasing cure rates. Infect Dis (Lond). 2023;55(3):189–98. https://doi.org/10.1080/23744235.2022.2153914.
Conway B. Correlates of successful HCV treatment in HIV co-infected vulnerable populations. Hepatology. 2015;62(SUPPL. 1):1131A-2A. https://doi.org/10.1002/hep.28236.
Elion R, Althoff K, Eron J, Gillman J, Haubrich R, Huhn G, et al. Untreated HCV in HIV/HCV-coinfected US population remains common despite the availability of curative therapies. HIV Med. 2019;20(Supplement 9):194. https://doi.org/10.1111/hiv.12815.
Emery J, Pick N, Mills EJ, Cooper CL. Gender differences in clinical, immunological, and virological outcomes in highly active antiretroviral-treated HIV-HCV coinfected patients. Patient Prefer Adherence. 2010;4(101475748):97–103. https://doi.org/10.2147/ppa.s9949.
Falade-Nwulia O, Sutcliffe CG, Mehta SH, Moon J, Chander G, Keruly J, et al. Hepatitis C elimination in people with HIV is contingent on closing gaps in the HIV continuum. Open Forum Infect Dis. 2019;6(10):ofz426. https://doi.org/10.1093/ofid/ofz426.
Hanna J, Sufian J, Suh JS, Jimenez HR. Hepatitis C virus micro-elimination within a clinic for people with HIV: challenges in Thehomestretch. HIV Med. 2022;23(7):801–6. https://doi.org/10.1111/hiv.13241.
Holeksa J, Magel T, Truong D, Yung R, Thiam A, Chu L, et al. Prevention of HIV transmission and optimization of HIV therapy among HCV-infected people who inject drugs (PWID) by engagement in long-term medical care. Hepatol Int. 2019;13(Supplement 1):S16. https://doi.org/10.1007/s12072-019-09936-5.
Huang M-H, Sun H-Y, Ho S-Y, Chang S-Y, Hsieh S-M, Sheng W-H, et al. Recently acquired hepatitis C virus infection among people living with human immunodeficiency virus at a university hospital in Taiwan. World J Gastroenterol. 2021;27(37):6277–89. https://doi.org/10.3748/wjg.v27.i37.6277.
Isfordink CJ, Smit C, Boyd A, De Regt MJA, Rijnders BJA, Van Crevel R, et al. Low hepatitis C virus-viremia prevalence yet continued barriers to direct-acting antiviral treatment in people living with HIV in the Netherlands. AIDS. 2022;36(6):773–83. https://doi.org/10.1097/QAD.0000000000003159.
Jain MKC, Sanders C, Vysyaraju J, Hepatitis K. Eradication: who is being left behind in the HIV population? Open Forum Infect Dis. 2018;5:S660. https://doi.org/10.1093/ofid/ofy210.1886.
Metsch LR, Feaster DJ, Gooden LK, Masson C, Perlman DC, Jain MK, et al. Care facilitation advances movement along the hepatitis C care continuum for persons with human immunodeficiency virus, hepatitis C, and substance use: a randomized clinical trial (CTN-0064). Open Forum Infect Dis. 2021;8(8):ofab334. https://doi.org/10.1093/ofid/ofab334.
Palayew A, Schmidt AM, Saeed S, Cooper CL, Wong A, Martel-Laferriere V, et al. Estimating an individual-level deprivation index for HIV/HCV coinfected persons in Canada. PLoS ONE. 2021;16(4):e0249836. https://doi.org/10.1371/journal.pone.0249836.
Pundhir P, North CS, Fatunde O, Jain MK. Health beliefs and co-morbidities Associated with appointment-keeping behavior among HCV and HIV/HCV patients. J Community Health. 2016;41(1):30–7. https://doi.org/10.1007/s10900-015-0059-4.
Raya RP, Curtis H, Kulasegaram R, Cooke GS, Burns F, Chadwick D, et al. The British HIV Association national clinical audit 2021: management of HIV and Hepatitis C coinfection. HIV Med. 2023;24(4):471–9. https://doi.org/10.1111/hiv.13417.
Rizk C, Miceli J, Shiferaw B, Malinis M, Barakat L, Ogbuagu O, et al. Implementing a comprehensive hepatitis C virus (HCV) clinic within a human immunodeficiency virus clinic: a model of care for HCV microelimination. Open Forum Infect Dis. 2019;6(10). https://doi.org/10.1093/ofid/ofz361.
Roberson JL, Lagasca AM, Kan VL. Comparison of the hepatitis C continua of care between hepatitis C virus/HIV coinfected and hepatitis C virus mono-infected patients in two treatment eras during 2008–2015. AIDS Res Hum. 2018;34(2):148–55. https://doi.org/10.1089/AID.2017.0092.
Schnee A, Bean M, Salgado C, Richey L. Pill burden, drug use, mental illness, and imprisonment and the impact on retention rates for human immunodeficiency virus (HIV)/ hepatitis C co-infected patients in South Carolina. Open Forum Infect Dis. 2016;3(Supplement 1). https://doi.org/10.1093/ofid/ofw172.344.
Starbird LE, Budhathoki C, Han H-R, Sulkowski MS, Reynolds NR, Farley JE. Nurse case management to improve the hepatitis C care continuum in HIV co-infection: results of a randomized controlled trial. J Viral Hepat. 2020;27(4):376–86. https://doi.org/10.1111/jvh.13241.
Ward KM, Falade-Nwulia O, Moon J, Sutcliffe CG, Brinkley S, Haselhuhn T, et al. A randomized controlled trial of cash incentives or peer support to increase HCV treatment for persons with HIV who use drugs: the CHAMPS study. Open Forum Infect Dis. 2019;6(4):ofz166. https://doi.org/10.1093/ofid/ofz166.
Wegener MD, Brooks RP, Speers S, Gosselin D, Villanueva M. Efficacy of using disease intervention specialists (DIS) to re-engage out of care HIV/HCV co-infected persons into HCV treatment. Open Forum Infect Dis. 2020;7(SUPPL 1):S509–10. https://doi.org/10.1093/ofid/ofaa439.1143.
Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):e1. https://doi.org/10.1093/cid/cit665.
U.S. Department of Health & Human Services. HIV Care Continuum. HIV.gov. 2022. https://www.hiv.gov/federal-response/policies-issues/hiv-aids-care-continuum/. Accessed 20 May 2024.
HHS Panel on Antiretroviral Guidelines for Adults and Adolescents—A Working Group of the Office of AIDS Research Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Volume 27. Rockville (MD): US Department of Health and Human Services; 2024 Feb. https://www.ncbi.nlm.nih.gov/books/NBK586306/.
Mirzazadeh A, Eshun-Wilson I, Thompson RR, Bonyani A, Kahn JG, Baral SD, et al. Interventions to reengage people living with HIV who are lost to follow-up from HIV treatment programs: a systematic review and meta-analysis. PLoS Med. 2022;19(3):e1003940. https://doi.org/10.1371/journal.pmed.1003940.
Saeed S, Thomas T, Dinh D, Moodie E, Cox J, Cooper C, et al. Frequent disengagement and subsequent mortality among people living with HIV and Hepatitis C in Canada: a prospective cohort study. Open Forum Infect Dis. 2024;ofae239. https://doi.org/10.1093/ofid/ofae239.
Hogg RS. Understanding the HIV care continuum. Lancet HIV. 2018;5(6):e269–70. https://doi.org/10.1016/S2352-3018(18)30102-4.
Fuente-Soro L, Iniesta C, Lopez-Varela E, Cuna M, Guilaze R, Maixenchs M, et al. Tipping the balance towards long-term retention in the HIV care cascade: a mixed methods study in southern Mozambique. PLoS ONE. 2019;14(9):e0222028. https://doi.org/10.1371/journal.pone.0222028.
Harris M, Rhodes T. Hepatitis C treatment access and uptake for people who inject drugs: a review mapping the role of social factors. Harm Reduct J. 2013;10:7. https://doi.org/10.1186/1477-7517-10-7.
Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88(2–3):188–96. https://doi.org/10.1016/j.drugalcdep.2006.10.014.
Muncan B, Walters SM, Ezell J, Ompad DC. They look at us like junkies: influences of drug use stigma on the healthcare engagement of people who inject drugs in New York City. Harm Reduct J. 2020;17(1):53. https://doi.org/10.1186/s12954-020-00399-8.
Mortality rates among. Patients successfully treated for hepatitis C in the era of interferon-free antivirals: population based cohort study.
Freeman R, Gwadz MV, Silverman E, Kutnick A, Leonard NR, Ritchie AS, et al. Critical race theory as a tool for understanding poor engagement along the HIV care continuum among African American/Black and hispanic persons living with HIV in the United States: a qualitative exploration. Int J Equity Health. 2017;16(1):54. https://doi.org/10.1186/s12939-017-0549-3.
Napoles TM, Batchelder AW, Lin A, Moran L, Johnson MO, Shumway M, et al. HCV treatment barriers among HIV/HCV co-infected patients in the US: a qualitative study to understand low uptake among marginalized populations in the DAA era. J Public Health (Oxf). 2019;41(4):e283–9. https://doi.org/10.1093/pubmed/fdz045.
Greenwald ZR, Bouck Z, McLean E, Mason K, Lettner B, Broad J, et al. Integrated supervised consumption services and hepatitis C testing and treatment among people who inject drugs in Toronto, Canada: a cross-sectional analysis. J Viral Hepat. 2023;30(2):160–71. https://doi.org/10.1111/jvh.13780.
Laniece Delaunay C, Maheu-Giroux M, Marathe G, Saeed S, Martel-Laferriere V, Cooper CL, et al. Gaps in hepatitis C virus prevention and care for HIV-hepatitis C virus co-infected people who inject drugs in Canada. Int J Drug Policy. 2022;103:103627. https://doi.org/10.1016/j.drugpo.2022.103627.
Rudzinski K, Xavier J, Guta A, Chan Carusone S, King K, Phillips JC, et al. Feasibility, acceptability, concerns, and challenges of implementing supervised injection services at a specialty HIV hospital in Toronto, Canada: perspectives of people living with HIV. BMC Public Health. 2021;21(1):1482. https://doi.org/10.1186/s12889-021-11507-z.
Socias ME, Karamouzian M, Parent S, Barletta J, Bird K, Ti L. Integrated models of care for people who inject drugs and live with hepatitis C virus: a systematic review. Int J Drug Policy. 2019;72:146–59. https://doi.org/10.1016/j.drugpo.2019.05.023.
Clement ME, Collins LF, Wilder JM, Mugavero M, Barker T, Naggie S. Hepatitis C virus elimination in the human immunodeficiency virus-coinfected population: leveraging the existing human immunodeficiency virus infrastructure. Infect Dis Clin North Am. 2018;32(2):407–23. https://doi.org/10.1016/j.idc.2018.02.005.
Hosseini-Hooshyar S, Hajarizadeh B, Bajis S, Law M, Janjua NZ, Fierer DS, et al. Risk of hepatitis C reinfection following successful therapy among people living with HIV: a global systematic review, meta-analysis, and meta-regression. Lancet HIV. 2022;9(6):e414–27. https://doi.org/10.1016/S2352-3018(22)00077-7.
Simmons B, Saleem J, Hill A, Riley RD, Cooke GS. Risk of late relapse or reinfection with hepatitis C virus after achieving a sustained virological response: a systematic review and meta-analysis. Clin Infect Dis. 2016;62(6):683–94. https://doi.org/10.1093/cid/civ948.
Hamill V, Wong S, Benselin J, Krajden M, Hayes PC, Mutimer D, et al. Mortality rates among patients successfully treated for hepatitis C in the era of interferon-free antivirals: population based cohort study. BMJ. 2023;382:e074001. https://doi.org/10.1136/bmj-2022-074001.
Acknowledgements
We thank Sandra McKeown (Bracken Health Sciences Library, Queen’s University) for her assistance in developing the literature search strategy.
Funding
DAD was supported by a summer student fellowship from the Canadian Network on Hepatitis C (CanHepC) and the CIHR; CanHepC is funded by a joint initiative of the CIHR [NPC-178912] and the Public Health Agency of Canada. The funders had no role in the production of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: DAD, YT & SS; Methodology: DAD & SS; Formal analysis and investigation: DAD, YT & SS; Visualization: DAD; Writing - original draft preparation: DAD; Writing - review and editing: DAD, YT, & SS; Supervision: SS. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Financial Interests
DAD reports a stipend from the Canadian Network on Hepatitis C (CanHepC) as part of a summer student fellowship. SS reports consulting fees from Novo Nordisk (unrelated to this study). YT declares they no financial interests.
Non-Financial Interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dinh, D.A., Tan, Y. & Saeed, S. Disengagement from Care Among People Co-Infected with HIV and HCV: A Scoping Review. AIDS Behav (2024). https://doi.org/10.1007/s10461-024-04436-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s10461-024-04436-6