Abstract
The heightened risk of persons with serious mental illness (SMI) to contract and transmit human immunodeficiency virus (HIV) is a public health problem. Our objective was test the effectiveness of a community-based advanced practice nurse intervention to promote adherence to HIV and psychiatric treatment regimens call Preventing AIDS Through Health for Positives (PATH+). We enrolled 238 HIV-positive subjects with SMI who were in treatment at community HIV provider agencies from 2004 to 2009. Participants in the intervention group were assigned an advanced practice nurse who provided community-based care management at a minimum of one visit/week and coordinated their medical and mental healthcare for 12 months. A parallel process latent growth curve model using three data points for biomarkers (baseline, 12 and 24 months) and five data points for health related quality of life (baseline, 3, 6, 12, and 24 months) showed moderate to excellent fit for modeling changes in CD4, viral load, and mental and physical SF-12 subscales. Results suggest that positive effects for PATH+ persisted at 24 months; 12 months after the intervention ended. This project demonstrates the effectiveness of a nurse-led, community-based, individually tailored adherence intervention. We demonstrated improved outcomes in individuals with HIV/SMI and regarding health-related quality of life and reductions in disease burden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Persons with serious mental illness (SMI) are at increased risk to contract and transmit human immunodeficiency virus (HIV) [1–3]. Estimates of the prevalence of HIV among persons with SMI range from 4 to 23 %, as compared to 0.4–0.6 % in the general population [4, 5]. These percentages may still underestimate the true prevalence and signal a hidden epidemic because studies have identified undetected cases of HIV in inpatient psychiatric populations that are missed by care providers [6, 7]. Early detection and treatment of HIV reduces the risk of transmission and secondary infections although the SMI population is a challenging population to treat because of cognitive dysfunction and poor medication adherence [8]. Non-adherence to antiretroviral therapy (ART) poses an additional problem due to the risk of development of treatment-resistant HIV strains because ART regimens are difficult to maintain even among persons without SMI [9]. For these reasons, persons with SMI are potentially important vectors of HIV and development of effective interventions to improve adherence to treatment for them should be a high priority.
Treatment programs that offer integrated physical and mental healthcare show promise for persons with comorbid HIV and SMI [10]. The Program of Assertive Community Treatment (PACT) model is one example used with the SMI population [11]. PACT is a multi-disciplinary model of care in which services are delivered in the community instead of clinics or offices. Advanced practice nurses (APNs) are well-suited to lead these programs because APNs have been found to provide equivalent or better care than physicians on important dimensions of HIV/AIDS care [12, 13]. APN’s have post baccalaureate clinical training, in most cases a masters in nursing science (MSN), which may or may not meet criteria for licensure as a nurse practitioner depending on state regulations. The benefits of adding APNs to PACT programs have been documented in both SMI [14] and HIV positive persons [13, 15, 16] populations but not among HIV positive persons with SMI.
The PATH+ Intervention
Preventing AIDS through Health for HIV Positive persons (PATH+) was a HIV regimen management study carried out by APNs who provided in-home services and coordinated participants’ care. There were a total of four APNs who implemented PATH+ over the study period, and each had a caseload that varied between 12 and 20 persons at any given point in time. In PATH+ an adaptive treatment design [17] implemented through a “treatment cascade” was used to titrate the intensity (and expense) of the intervention to actual adherence outcomes. As described by Blank and Eisenberg [18], the PATH+ intervention cascade is an individually tailored intervention to promote adherence in HIV positive persons with co-occurring mental illnesses. The PATH+ intervention consisted of assignment to an APN who provided in-home consultations and coordinated medical and mental health services for 1 year. The APNs collaborated with prescribing providers, pharmacists, and case managers to organize medication regimens and help participants cope with barriers to medication adherence and promote the participant’s ability to self-care. The basic intervention included a meeting with the participant at a minimum of once a week. These face-to-face meetings were at the participants’ homes or at another location of their choosing, and that the face-to-face contacts were weekly at a minimum, but could be more frequent. The basic intervention consisted of psycho-education along with pillboxes and beeping watches and was provided to all participants in the intervention group. In addition, the APN coordinated physician appointments for the client and would also attend them with the patients when there was a problem with a medication, communication, or other issues needing physician attention.
Adherence to HIV and psychiatric medications was calculated weekly. Adherence was assessed by self-report and verified by pill count. If either of those criteria fell below an 80 % threshold the next step in the intervention cascade was implemented. The treatment cascade represented a gradual increase in intensity and included activation of social networks, the use of reminder beepers with alphanumeric displays, and then prepaid cellular phones to encourage participants to follow their treatment regimens. Other phone contacts and text messages to participants and their social network members were utilized as needed. The final step in the cascade was directly observed therapy. The intervention cascade was implemented until adherence was maintained equal to or above 80 % for 3 weeks and then reverted back to the basic intervention.
The analysis here analyzes the effects of PATH+ on HIV biomarkers and behavioral quality of life indicators simultaneously. We utilize three waves of biomarker data (baseline, 12, and 24 months) and five waves of behavioral indicators (baseline, 3, 6, 12, and 24 months) for growth curve modeling. Although the PATH+ intervention lasted for 12 months, the 24 month data collection was intended to assess behavioral or biological decay of the treatment effects after the intervention was completed.
Methods and Measures
The study design was a longitudinal randomized trial using the PATH+ intervention group and an enhanced treatment- as-usual control group (eTAU). Participants receiving eTAU engaged in the structured interviews enhanced by providing biomarker results (viral load and CD4) and to treating physicians at each assessment. The PATH+ intervention group was assigned an APN who provided in-home services and coordinated care among their other service providers. Health care providers included infectious disease clinicians, mental health service providers including psychiatrists and case managers, substance abuse counsellors, primary care physicians, dentists, and any other providers of health services that PATH+ recipients encountered in the treatment of their co-occurring disorders. The study took place from September 2004 to April 2009. Oversight for the trial was provided by the University of Pennsylvania Institutional Review Board as well by the Philadelphia Department of Public Health Institutional Review Board. Inclusion criteria for the study required that the participant be age 18 or older, be able to understand spoken English, have a diagnosed SMI, demonstrate sufficient competency to provide informed consent, and be HIV seropositive.
Participants were recruited from HIV treatment sites throughout the city of Philadelphia. Inclusion criteria were that they self-identified as being HIV positive and were also receiving case management for a serious mental illness. These criteria were later confirmed by HIV testing and by contacting their mental health service providers. Any participant who was not currently receiving treatment for HIV was referred to the outpatient clinic at the Hospital of the University of Pennsylvania. All participants were paid $40 for each of four interviews over the 12 month study period, as well as one 24 month follow-up. A bonus of $100 was paid to participants who provided data at all study time points to incentivize provision of complete data. Eligible consenting patient participants were randomized on a 1:1 basis to the intervention and control groups. Randomization was employed to ensure that approximately equal numbers of patients were assigned to each of the two groups that were balanced with respect to observed and unmeasured baseline factors. Experimental status was blinded so that the study investigators and research staff did not know the treatment status of participants, however participants sometimes disclosed information which made it possible identify experimental status.
PATH+ Participants
PATH+ enrolled 238 HIV-positive participants with SMI of which 128 were randomized to the intervention group while 110 received treatment as usual. 54 % of participants were male and 81 % were African American. The average age of the participant was 43 (SD = 7.25, range was 18–63). Figure 1 gives a graphical presentation of the patient processing and the prospective data available for analysis at each wave. For both the biomarker and the SF-12 data, there was no statistically significant difference in data availability between experimental groups over time: for the biomarker data, χ2 = 0.15, df = 2, p = 0.93, for the SF-12 data, χ2 = 0.18, df = 4, p = 0.99.
Outcome Measures
The SF-12 is a common battery of health-related quality of life items [19] that has been validated for use in populations of persons with SMI [20]. Six items produce a mental health score and six produce a physical health score, normed to an average of 50. Standard HIV testing and viral load assays were provided from the clinical laboratories at the Hospital of the University of Pennsylvania. Because of the associated expense, HIV viral load and CD4 counts were measured only at baseline, 12 and 24 months. Results of the blood tests at each assessment were shared with all participants’ treating physicians.
Statistical Analyses Using Growth Curves
We use growth curve modeling because it constitutes a flexible longitudinal data analysis strategy [21, 22] relevant to the two research issues relevant here: estimating experimental/control differences in change over time [23, 24] and computing associations between temporal changes in one data series and temporal changes in another [25]. Both of these purposes are important because we want to estimate treatment effect of PATH+ both on social and biomarker outcomes simultaneously.
Growth curves typically have two different forms: unconditional and conditional. The unconditional equation predicts an individual respondent’s values of the social and biomarker outcomes as a function of (1) the initial value of outcome at the beginning of the intervention and (2) the slope of change over time. In equation form, the unconditional growth model is:
The “i” subscript reflects individual observations, the Time Metric is the time scale, the η i0 is the value of the latent intercept when the Time Metric is zero, the η i1 is the regression coefficient indicating the latent slope of time for each individual, and the “t” subscript represents the ordering of the observations. Thus, the error term defines the individual (e.g., “within subject”) measurement errors of the outcomes for each observation.
The advantage of the growth curve approach is that because the intercept (η i0) and slope (η i1) parameters vary across individuals, the parameters can be treated as dependent variables in auxiliary equations that predict the initial value and slope of the outcome: the “conditional growth model” [26]. Here we use the experimental status of the PATH+ participants as the conditional predictor of the slope and intercept we estimate the growth curves simultaneously for the SF-12 outcomes and the biomarker outcome—a “parallel process” model [27]. In other words, this type of growth model allows for the simultaneous estimation of the correlated trajectories of change for quality of life outcomes and medical biomarkers.
Estimating the Best Fitting Time Metric
Analyses of the unconditional models for the biomarkers and the SF-12 measures (not shown) with a time metric free to vary [28] indicated that a log linear model for time (in months after the initiation of the intervention) is an excellent fitting time metric for all the outcomes except for the mental health score. Thus, for all but this equation the time metric is defined as the log of the number of months after program commencement (e.g., 0, 1.098, 1.79, 2.48, 3.18). Using this metric, the change from baseline to month 6 is 1.79 times as large as the change from baseline to month 3, the change from baseline to month 12 is 2.48 times as large as the change from baseline to month 3, and the change from baseline to month 24 is 3.18 times as large as from baseline to month 3. In this non-linear case, if the slope change over time is positive, this time metric produces a positive slope that flattens with time and if the slope is negative, a negative slope that flattens with time. For the mental health summary score, we use a data derived time metric that has a non-linear form (0, 1, 2.55, 3.23, 2.87) that decreases in slope after month 12. This makes sense in this situation because at 24 months the intervention component of PATH+ had ended.
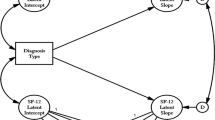
Figure 2 presents the generic analysis model. Note that the focus of the prediction is on the slopes of the two series. Because PATH+ was a randomized trial, the intercept differences between the experimental and the control condition at baseline data collection are universally non-significant as they should be given an initial equivalence of the experimental and control groups.
Results
Table 1 shows the characteristics of the patients and outcome measures by experimental status at baseline. There were no statistically significant differences at baseline between the intervention and control group on any of these demographic characteristics or outcome variables.
Table 2 shows the results of the growth curve analysis. There are four models. Model A estimates PATH+ treatment effects on log HIV viral load and the SF-12 mental health scores simultaneously. Model B uses the CD4 % and the SF-12 mental health score. Model C uses the log HIV viral load biomarker and the SF-12 physical health score. Model D uses the CD4 % biomarker and the SF-12 physical health score. We have the following expectations. For Models A and C, PATH+ should reduce the slope of change over time in log HIV viral load and increase the slope of change over time in the SF-12 outcomes. For Models B and D, PATH+ should increase the slope of change over time for CD4 % and increase the slope of change over time in the SF-12 outcomes.
Only Model A fully meets these expectations. Model B treatment estimates all have the correct sign, but only the PATH+ change in mental health is discernible from zero. Model C shows a significant decline in viral load, but no change in perceptions of physical health status due to PATH+. Model D shows no significant change in either CD4 % or perceptions of physical health status. Note that no model shows a significant change in slope for any outcome in the “wrong” direction. PATH+ did not make participants “worse” on any outcome measure. The global measures of goodness of fit suggest good to excellent fit of all the pairs of equations [29].
To better display the effects of each model, we plot the predicted outcomes by experimental status and time for each model in Fig. 3. Note that the change in the SF-12 mental health score occurs early in the PATH+ life-cycle. With an intercept of 40.6, the estimate of the change at 6 months (43.2) is 76 % of the total change from baseline to month 12 (44.0). After month 12, the SF-12 physical health summary score declines because all PATH+ intervention activities had been concluded by the last data collection point.
Parallel process growth curves for all analysis models. Constructed from Models A–D (Table 1)
Discussion
We found that the use of PATH+, a nurse-led community-based intervention to promote medication and treatment regimen management, improved both biomarker and health-related quality of life indicators in persons with HIV/SMI. The intervention group exhibited greater reductions in mean log viral load and improvements in immune functioning as indicated by CD4 % from baseline to 24 months. Results demonstrated significant improvements in health related quality of life for the mental health subscale. Interpretation of findings associated with physical health requires a more nuanced perspective.
It is notable that the positive effects for PATH+ began early and plateaued to 24 months, decaying less than might have been expected after the intervention was withdrawn at 12 months. In fact, biomarkers for viral load and CD4 did not decay at all after PATH+ concluded and positive effects continued to 24 months. It may be that better adherence behaviors were integrated into standing behavior patterns for these individuals and were habitualized and integrated into their daily routines. These adherence behaviors may be learned and practiced after the intervention period. This is a positive finding that suggests that it may be possible to shorten PATH+ to contain costs and still see positive effects, and these effects may persist well after the end of the formal intervention and the additional adherence supports are withdrawn.
As expected, the PATH+ group displayed improvement from baseline to the 24 month assessment, but contrary to expectations, the eTAU group actually reported better perceptions of physical health on the SF-12 than the PATH+ group. The difference between groups was not significant but this trend may indicate the potential power of a self-assessment component to influence perceptions of risk behavior and suggests the possibility for adaptive behavior change when individuals are provided with the opportunity for self-reflection and self-monitoring. The opportunity for self-reflection and monitoring may have been less instrumental in the PATH+ group’s assessment of physical health because their APNs also provided objective feedback about non-adherence and instructions for corrective action through the intervention cascade in PATH+ that was not provided to the eTAU participants. In other words, the eTAU participants relied only on their own subjective behavioral assessments to evaluate progress over the follow-up period, while PATH+ participants were constantly reminded by the APNs when their adherence to treatment regimens were compromised. As a result PATH+ participants may have perceived their physical health status to be more precarious than participants receiving eTAU, when in fact the biomarkers indicate that they were healthier.
Adaptive behavior change in control group participants has long been observed in studies of HIV risk behavior as demonstrated by risk reduction in condom use and unprotected anal sex [30, 31] well as reductions in drug risk behavior [32–35]. Without diminishing the meaningful impact our PATH+ intervention played in risk reduction and quality of life assessments, we also believe our eTAU group findings reveal the potential for interventions of a lower intensity that include opportunities for repeated self-reflection and self-monitoring [36, 37]. The positive functions of assessments within an intervention may operate to serve as a cue to risk reduction and adaptive behavior change.
Very few studies have examined the use of regimen management interventions for the HIV population using a RCT design, while fewer have used a nurse-led intervention. To our knowledge, this is the first study to simultaneously evaluate longitudinal biological and health-related quality of life outcomes in this population. Results of these prior studies are inconclusive. For example, Mannheimer et al. [38] found that patients assigned to a medication manager, who was often a research nurse, had a higher mean increase in CD4 count by an average of 22.4 at the end of 36 months which is consistent with our findings. Viral load differences were not statistically significant. However, Holzemer et al. [39] found no significant effect of a nurse-led intervention on adherence, CD4 count and viral load over a six month period. Inconsistencies in intervention content/approach, differences in outcome time points, and the definitions of adherence in these and similar studies hinder the comparison of findings and effect sizes. We note however that the intervention tested here had multiple components, was individually tailored, was manualized and highly structured, and included both didactic and cognitive components. All of these features are characteristics of interventions positively associated with improved adherence and outcomes [40]. While an economic analysis such as cost effectiveness or cost threshold analysis is beyond the scope of the current report, such analyses will be critical to evaluating scalability of PATH+ and other intensive adaptive treatments in order to guide translation and dissemination into other service settings and systems.
Conclusion
As healthcare reform is implemented, an opportunity presents itself to ensure system changes are made in the provision of care for patient populations with complex co-occurring conditions. In the current health care system, the SMI patient would most likely be referred to a HIV medical provider in a location separate from their mental health care, requiring the patient to be responsible for arranging and keeping the appointment, as well as finding transportation. Such a fragmented health care system does not promote optimal outcomes for the HIV/SMI population. The concept of the “medical home” that promotes collaborative care among specialties could be translated into nurse-led treatment centers in the community that provide cost-effective and quality care specifically to this and other similar populations [41].
We note some study limitations. The APNs used in this study were university-based and had training in research. Therefore, results might be different for community-based nurses. Also, the difference in CD4 counts between the control and intervention groups was not statistically significant at 12 months. We attribute this in part to the control group also having a positive change in CD4 that we speculate is due to enhanced reflection due to assessment and increases in subsequent self-monitoring of adherence. It is also worth noting that there may be a lagged effect of CD4 count relative to viral load, since it takes some time for immune functioning to improve after viral load has been suppressed. In fact, in some people who have been immune-compromised for a long period of time, suppressing viral load later in the course of disease may not result in any substantial improvements in immune functioning [42].
These results show that persons with SMI can achieve lower viral load with appropriate supportive services. Implementation of community-based nurse disease management for this population and other complex patient populations may have significant impact on viral load, immune functioning, and health-related quality of life. Consistent with innovative efforts toward case finding and early initiation of treatment, reductions in viral load in bridge populations can help contain the epidemic. Our findings also can inform the redesign of health care delivery to the population of persons with SMI to slow the spread of HIV.
References
Gottesman I, Groome CS. HIV/AIDS risks as a consequence of schizophrenia. Schizophr Bull. 1997;23(4):675–84.
Walkup J, Blank MB, Gonzalez JS, et al. The impact of mental health and substance abuse factors on HIV prevention and treatment. J Acquir Immune Defic Syndr. 2008;47:S15–9.
Blank MB, Eisenberg MM. HIV and mental illness: opportunities for prevention. Journal of prevention & intervention in the community. J Prev Interv. 2007;33(1–2):1–4.
Susser E, Valencia E, Conover S. Prevalence of HIV-infection among psychiatric patients in a New York City men’s shelter. Am J Public Health. 1993;83(4):568–70.
Blank MB, Mandell DS, Aiken L, Hadley TR. Co-occurrence of HIV and serious mental illness among medicaid recipients. Psychiatric services. 2002;53(7):868–73.
Himelhoch S, McCarthy JF, Ganoczy D, Medoff D, Dixon LB, Blow FC. Understanding associations between serious mental illness and HIV among patients in the VA health system. Psychiatr Serv. 2007;58(9):1165–72.
Rothbard A, Blank M, Staab J, et al. Previously undetected metabolic syndromes and infectious diseases among psychiatric inpatients. Psychiatr Serv. 2009;60(4):534–7.
Parry CD, Blank MB, Pithey AL. Responding to the threat of HIV among persons with mental illness and substance abuse. Curr Opin Psychiatr. 2007;20(3):235–41.
Blank MB, Hanrahan NP, Fishbein M, et al. A randomized trial of a nursing intervention for HIV disease management among persons with serious mental illness. Psychiatr Serv. 2011;62(11):1318–24.
Walkup J, Blank MB, Gonzalez JS, et al. The impact of mental health and substance abuse factors on HIV prevention and treatment. J Acquir Immune Defic Syndr. 2008;47:S15–9.
Drake RE. Brief history, current status, and future place of assertive community treatment. Am J Orthopsychiatry. 1998;68(2):172–5.
Lee AK, Hanrahan NP, Aiken LH, Blank MB. Perceived facilitators and barriers to the implementation of an advanced practice: nursing intervention for HIV regimen adherence among the seriously mentally ill. J Psychiatr Ment Health Nurs. 2006;13(5):626–8.
Morgan BD, Rossi AP. Difficult-to-manage HIV/AIDS clients with psychiatric illness and substance abuse problems: a collaborative practice with psychiatric advanced practice nurses. J Assoc Nurses AIDS Care. 2007;18(6):77–84.
Kane CF, Blank MB. NPACT: enhancing programs of assertive community treatment for the seriously mentally Ill. Community Ment Health J. 2004;40(6):549–59.
Aiken L, Lake E, Semaan S, et al. Nurse practitioner managed care for persons with HIV infection. J Nurs Scholarsh. 1993;25(3):172–7.
Battaglioli-DeNero AM. Strategies for improving patient adherence to therapy and longterm patient outcomes. J Assoc Nurses AIDS Care. 2007;18(1 Suppl):S17–22.
Collins L, Murphy S, Bierman K. A conceptual framework for adaptive preventive interventions. Prev Sci. 2004;5(3):185–96.
Blank MB, Eisenberg MM. Tailored treatment for HIV + persons with mental illness: the intervention cascade. J Acquir Immune Defic Syndr. 2013;63:S44–8.
Ware JE Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of Scales and Preliminary Tests of Reliability and Validity. Med Care. 1996;34(3):220–33.
Salyers MP, Bosworth HB, Swanson JW, Lamb-Pagone J, Osher FC. Reliability and Validity of the SF-12 Health Survey among People with Severe Mental Illness. Med Care. 2000;38(11):1141–50.
Curran P, Hussong A. Structural equation modeling of repeated measures data: latent curve analysis. In: Moskowitz D, Hershberger S, editors. Modeling intraindividual variability with repeated measures data. Mahwah: Lawrence Erlbaum; 2002. p. 59–85.
Curran P, Muthén B. The application of latent curve analysis to testing developmental theories in intervention research. Am J Community Psychol. 1999;27:567–95.
Fergus S, Zimmerman M, Caldwell CH. Growth trajectories of sexual risk behavior in adolescence and young adulthood. Am J Public Health. 2007;97:1096–101.
Barnes G, Reifman A, Farrell M, Dintcheff B. The effects of parenting on the development of adolescent alcohol misuse: a six-wave latent growth model. J Marriage Family. 2000;62:175–86.
Fishbein M, Hennessy M, Kamb M, et al. Using intervention theory to model factors influencing behavior change. Project RESPECT. Eval Health Prof. 2001;24(4):363–84.
Bollen K, Curran P. Latent curve models. New York: Wiley; 2006.
Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Struct Equ Modeling. 2003;10(2):238–62.
Biesanz J, Deeb-Sossa N, Papadakis A, Bollen K, Curran P. The role of coding time in estimating and interpreting growth curve models. Psychol Methods. 2004;9(1):30–52.
Hu L-T, Bentler P. Evaluating model fit. In: Hoyle R, editor. Structural equation modeling: Concepts, issues and applications. Newbury Park: Sage; 1995. p. 76–99.
Koblin B, Chesney M, Coates T. Effects of a behavioral intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomized controlled study. Lancet. 2004;364:41–50.
Belcher L, Kalichman S, Topping M, et al. A randomized trial of a brief HIV risk reduction counseling intervention for women. J Consult Clin Psychol. 1998;66(5):856–61.
Cottler L, Compton W, Abdahllah A, Cunningham-Williams R, Abram F, Fichtenbaum C. Peer delivered interventions reduce HIV risk behaviors among out-of-treatment drug abusers. Public Health Rep. 1998;113:58–66.
Stephens RC, Simpson DD, Coyle SL, McCoy CB. Comparative effectiveness of NADR interventions. In: Brown BS, Beschner GM, editors. Handbook on risk of AIDS. Westport: Greenwood Press; 1993. p. 519–56.
Friedman SR, Jose B, Deren S, Des Jarlais DC, Neaigus A. Risk factors for human immunodeficiency virus seroconversion among out-of-treatment drug injectors in high and low seroprevalence cities. Am J Epidemiol. 1995;142(8):864–74.
Gibson DR, McCusker J, Chesney M. Effectiveness of psychosocial interventions in preventing HIV risk behaviour in injecting drug users. AIDS. 1998;12(8):919–29.
Coyle S, Needle R, Normand J. Outreach based HIV prevention for injecting drug users: a review of published outcome data. Public Health Rep. 1998;113(Supplement 1):19–30.
Lightfoot M, Rotheram-Borus MJ, Comulada S, Gundersen G, Reddy V. Self-monitoring of behaviour as a risk reduction strategy for persons living with HIV. AIDS Care. 2007;19(6):757–63.
Mannheimer SB, Morse E, Matts JP, et al. Sustained benefit from a long-term antiretroviral adherence intervention: results of a large randomized clinical trial. J Acquir Immune Defic Syndr. 2006;43:S41–7.
Holzemer WL, Bakken S, Portillo CG. Testing a nurse-tailored HIV medication adherence intervention. Nurs Res. 2006;55:189–97.
Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143.
Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611–20.
Marziali M, De Santis W, Carello R, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006;20(16):2033–41.
Acknowledgments
This research was supported by grants from National Institute on Drug Abuse (RO1-DA-015627 ‘‘HIV Prevention Program among Substance Abusing SMI”), the National Institute for Nursing Research (RO1-NR-008851 ‘‘Nursing Intervention for HIV Regimen Adherence among SMI’’, the Penn Center for AIDS Research (P30AI045008), and the Penn Mental Health AIDS Research Center (P30MH097488)). Trial registration: clinicaltrials.gov identifier NCT00264823. The authors would like to thank William W. Thompson., Ph.D. of the Health-Related Quality of Life and Well-Being Team, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention for his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blank, M.B., Hennessy, M. & Eisenberg, M.M. Increasing Quality of Life and Reducing HIV Burden: The PATH+ Intervention. AIDS Behav 18, 716–725 (2014). https://doi.org/10.1007/s10461-013-0606-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-013-0606-x