Abstract
In southern Tunisia, oases represent a central component of the landscape. Different types of habitats make up this agroecosystem, hence the importance of investigating their value for local avifauna. In this study, we collected data on bird occurrence and vegetation structure and composition in 1398 plots within 53 oases. The principal component analysis showed significant differences in vegetation structure and composition among the studied oases. Two axes (PC1 and PC2) were identified: one (PC1) represents a gradient of fruit tree dominance, and the other (PC2) characterizes the nature and representation of the herbaceous stratum. High PC1 scores are associated with oases dominated by date palm trees, while low PC1 scores indicate oases dominated by fruit trees other than date palms. High PC2 scores describe oases with a natural herbaceous layer, while low PC2 scores embody those with a cultivated herbaceous layer. Then, using the generalized linear mixed model, we assessed the effects of PC1 and PC2 on the occurrence of 17 breeding bird species. Four groups of bird species with contrasted ecological affinities were thus identified. The first group (represented by two species) was exclusively affected by the PC1 axis. The second group (eight species) was affected by the additive effect of PC1 and PC2. The third group (five species) depended on the interactive effect of PC1 and PC2, and finally, two species (fourth group) were neither dependent on PC1 nor PC2. Overall, our results highlight the pertinence of oasis habitat composition for predicting breeding bird occurrence that should be considered by oasis managers to promote biodiversity conservation in these Mediterranean agroecosystems.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The desert is one of the most hostile environments for agricultural activities worldwide. This ecosystem is typically characterized by harsh conditions, such as high temperatures, evaporative water loss, water scarcity, and frequent wind and sand activities (Laity 2009; Sikka 1997). The local populations have succeeded in practicing agricultural activities in this fragile environment since ancient times. They have used innovative methods to extract and use rainwater and freshwater from superficial aquifer outcrops (Boualem et al. 2014; Faivre-Dupaigre 1957; Djellouli-Tabet 2010). Through their knowledge, local communities have developed agricultural areas dominated by date palms in association with several other fruit trees and vegetable crops. These densely vegetated areas resemble islands of greenery amid vast expanses of arid terrain and are called oases (Tengberg 2012). Nowadays, oases are spread in different countries, from Northern Africa to the Middle East, up to China and the Baja California peninsula of Mexico (Grenade 2013; Santoro 2023). Oases are vital livelihoods, providing food resources and socioeconomic benefits, such as date and fruit production, date by-products, beekeeping, recreation, and ecotourism activities (Santoro 2023). Oases also provide central environmental services, including soil conservation, carbon sequestration, climate change adaptation, and biodiversity conservation (Santoro 2023).
In North African countries such as Tunisia, oases represent a critical landscape component. These ecosystems are located mainly in the southern part of the country, mainly in Kébili (57% of oases), Tozeur (29%), Gabès (10%), and Gafsa (3%) governorates. Tunisian oases cover approximately 43,700 ha and are among the most prevalent agroecosystems (MEDD 2015). This agroecosystem generates a value of 400 Million Tunisian Dinars and about 60,000 jobs (MEDD 2015). Tunisian oases are composed of two types of oases: traditional and modern (Selmi and Boulinier 2003). Traditional oases were created before 1900; they cover around 15,051 ha (MEDD 2015). This oasis type features a three-tiered landscape, with palm trees forming the uppermost stratum. It is characterized by the predominance of common varieties. More than 45 date palm varieties can be observed on this floor, such as Bouhattam, Rochdi, Lemsi, Ammari, Eguiwa, Garn Ghazel, and Mattata (Salah 2015). This stratum acts as an umbrella which plays a crucial role in protecting cultivated land by creating a more humid microclimate during periods of heat stress (Ait-El-Mokhtar et al. 2022; Veyrac-Ben Ahmed and Abdedayem 2017). The second arboreal layer is composed of smaller fruit trees, such as pomegranate (Punica granatum), which has an important place on this floor. This stratum also contains other cultivated species, such as olive (Olea europaea), grapevine (Vitis vinifera), fig (Ficus carica), apricot (Prunus armeniaca), mulberry (Morus alba and M. nigra), Peach (Prunus persica), orange (Citrus sinensis), lemon (Citrus limon), common pear (Pyrus communis), and banana (Musa paradisiaca), many of which grow beyond their climatic zone (Ben Salah 2011). The last and lowest layer is mainly composed of alfalfa (Medicago sativa) and spontaneous plants, such as Bermuda grass (Cynodon dactylon). In some oases, the lowest layer is used to produce various vegetable crops (e.g., tomato (Lycopersicum esculentum), paprika (Capsicum annucim), barley (Hordeum annum), and maize (Zea mays) (Ben Salah 2011; Twiti et al. 2009). Traditional oases are mainly distributed between the governorates of Gabès and Gafsa.
Since the 1970s, Tunisia’s agricultural policies have rapidly shifted focus towards intensification and economic profitability, hence the creation of modern oases, characterized by the monoculture of “Deglet Nour” (Phoenix dactylifera L.) variety and larger farm sizes (Hamza et al. 2015; MEDD 2015). According to Hamza et al. (2015) and MEDD (2015), more than 25,752 ha of agricultural land is devoted to the cultivation of this variety. It is also estimated that about 90% of the national production of dates comes mainly from Deglet Nour, the variety with the highest demand locally and globally (Ismail and Hassine 2021). Modern oases are mainly located in the southwest of Tunisia, in Djerid (Tozeur and Nefta) and Nefzaoua (Kébili and Douz) regions.
Southern Tunisia oases constitute hotspots of biodiversity that hold an exceptional richness in the middle of the desert (Abbes et al. 2020; Dhiab and Selmi 2021; Hamza et al. 2021, 2022). The dense woody vegetation structure of these oases provides different habitat conditions for a diverse fauna, including butterflies (Abbes et al. 2020), amphibians (Ben Hassine and Nouira 2012), reptiles (lizards, turtles, and snakes), and small and medium‐sized mammals (e.g., bats, rodents, Lagomorphs, etc.; Dhiab and Selmi 2021). Birds also constitute one of the most diverse groups inhabiting these agroecosystems (Hamza and Hanane 2021; Hamza et al. 2021, 2022, 2023). Many passerines and non-passerines species depend on agroecosystems in Tunisian oases during the breeding and non-breeding seasons (Hamza and Hanane 2021; Isenmann et al. 2005). Tunisian oases also play a fundamental role as key breeding quarters for the European turtle dove (Streptopelia turtur; Hamza et al. 2021), a vulnerable species according to the IUCN Red List (Birdlife International 2024). Over the last decades, research assessing avian species’ use of oasian habitats has increased extensively (e.g. Alaya-Ltifi and Selmi 2014; Hamza and Hanane 2021). Nevertheless, most studies have been developed locally (Gabes region) and had relatively small sample sizes (one to five oases) (e.g. Alaya-Ltifi and Selmi 2014; Hamza and Hanane 2021). To our knowledge, only one study conducted in 1999 by Selmi and Boulinier (2003) has covered a large scale of Tunisian oases. However, it investigated the relationship between the oasian vegetation structure and composition and the richness of breeding bird communities. Therefore, little is known of how breeding bird species (individual species) respond to oases vegetation structure and composition. Indeed, determining which vegetation structure and composition are the most important to breeding bird species occurrence can have implications for future oasis management strategies.
The main objective of this study was to determine the extent to which the habitat composition of Tunisian oases is determining in explaining the occurrence of bird species. It is commonly known that oasian bird species do not use oasis systems in the same way (Selmi and Boulinier 2003), Some species, such as Turdus merula, Sylvia hortensis, and Lanius senator, are attracted to traditional oases (Alaya-Ltifi and Selmi 2014; Selmi and Boulinier 2003), while others (e.g., Lanius meridionalis) are adapt to exploit the modern ones (Selmi and Boulinier 2003). We, therefore, hypothesized that the responses of birds would depending on their ecological characteristics and that some species would show higher attraction to traditional oases than modern ones.
Materials and methods
Study area
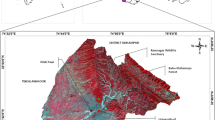
Fieldwork was conducted in the northern edge of the Grand Erg Oriental in the Sahara Desert (Fig. 1). It covers an area of 27,890 km2. This pre-Saharan area is characterized by an arid to hyper-arid climate (El-Fahem 2003; Mamou 1989; Zammouri et al. 2007). The rainfall is scant and irregular when it occurs, with an annual value of approximately 75–200 mm (Jemai et al. 2017; Zammouri et al. 2007). Daily mean temperatures vary between 10 °C in winter and 40 °C in summer, with August being the hottest month (Jemai et al. 2017; Zammouri et al. 2007). The yearly open water evaporation exceeds 1700 mm (El-Fahem 2003; Mamou 1989; Jemai et al. 2017; Zammouri et al. 2007). The landscape of this pre-Saharan region is marked by the presence of two types of oases, namely traditional and modern oases (MEDD 2015). The former is characterized by low density of palm trees, which is less than 70 trees/ha with the predominance of common varieties. This type of oasis is also characterized by a high density of fruit trees with a great diversity of species, such as pomegranate, olive, common fig, grape, and apricot (Rhouma et al. 2020). In addition, the herbaceous layer of traditional oases consists of various vegetable crops, fodder crops, and industrial crops (Rhouma et al. 2020). Unlike traditional oases, modern ones are oriented towards monoculture with the predominance of profitable date varieties (Deglet Nour), while little importance was given to fruit trees and herbaceous plants (MEDD 2015).
In this part of Southern Tunisia, we initially identified 86 representative oases. Among them, 53 were selected randomly using the QGIS random selection tool (Quantum GIS Development Team 2020) to carry out our monitoring. Out of these 53 oases, 21 were traditional (mean surface areas [ha] 459.80 ± 445.67 SD, min. = 34.02 ha; max. = 1698.04 ha) and 32 modern (270.68 ± 303.87 SD, min. = 31.55 ha; max. = 1414.67 ha) (Fig. 1). The studied oases belong to four different pre-Saharan regions, namely Gabes (33° 53′ N 10° 5′ 30 E), Gafsa (34° 23′ N 8° 46′ E), Djerid (i.e., Tozeur (33° 54′ N 8° 8′ E) and Nefta (33° 52′ N 7° 52′ E)), and Nefzaoua (i.e., Kebili (33° 41′ N 8° 58′ E) and Douz (33° 26′ N 8° 48′ E); Fig. 1).
Data collection
Bird surveys were carried out between May and June in 2021 and 2022. This period was chosen as it coincides with annual peaks in activities of breeding birds (Hamza and Hanane 2021; Hamza et al. 2021, 2022, 2023). A total of 1398 point counts were performed in the 53 oases during two visits (699 per year), which were, in a first step, selected randomly using the QGIS random selection tool (Quantum GIS Development Team 2020). These coordinates were subsequently entered into a handheld GPS to determine their location in the field. The point counts method (Bibby et al 2000) was used to survey the occurrences of breeding birds in each oasis. The number of point counts was proportional to the size of the oases, with 8point counts sampled in oases of 10–30 ha, 14 points in oases of 30–50 ha, and 25 points in oases of > 50 ha. The minimum distance between two points was set to at least 200 m in oases of small size and 400 m in oases of large size to cover the maximum potential area of studied oases. In each sampled point count we recorded all birds seen or heard during a 10 min period. At each point count, we recorded all birds through visual and auditory records within a radius of 50 m (Anjos et al 2011; Hamza et al. 2021, 2022, 2023; Hamza and Hanane 2021). Observations were made in the morning (5:30–11:00 h) and in the afternoon (16:00–18:30 h). The surveys were performed only on calm days (i.e. no observations were made on windy or rainy days) to ensure bird detectability.Data in all point counts was collected by at least two observers of the field team (ME, FH, MAC).
In each point count, we recorded six vegetation variables to assess their potential role as determinant factors of the occurrence of the recorded bird species. We visually estimated the covers (%) of the date palm trees, fruit trees (other than date palms), and natural and cultivated herbaceous layer, as well as the number of date palm trees and fruit trees (Hamza et al. 2021, 2022). To match the biodiversity data, vegetation was also characterized within the same plots of 50 m radius (Alaya-Ltifi and Selmi 2014). To avoid observer-related biases in vegetation sampling, all vegetation parameter estimations were conducted by the same observer (ME; Prodon and Lebreton 1981).
Statistical analyses
Given that some of the vegetation variables were inter-correlated (Fig. S1), we summarized variation using a principal components analysis (PCA). Orthogonal variables were ranked by the product of their eigenvalues and the percent of variation explained by the principal component axes. Only Principal components (PCs) with eigenvalues exceeding 1.0 were considered as main factors (Jayathunga et al. 2020). A varimax rotation was applied to the retained PCAs to obtain an optimal distribution of variance in the various components (Legendre and Legendre 1998). Then, we performed Kaiser–Meyer–Olkin test (KMO) to check data robustness for the PCA.
In order to evaluate the effects of oasis habitat descriptors derived from the PCA (PCAs_scores as fixed effects) and spatial structure on the occurrence of 17 breeding bird species, we used General Linear Mixed Models (GLMMs) with a binomial error (logistic regression). In these analyses we considered bird occurrence (0/1) as a response variable and the study oases and point identities as random factors to account for the potential non-independence of multiple observations at the same oasis, as well as at the same plot within an oasis. All GLMMs analyses were performed with lme4 (Bates et al. 2014). The full model (FM) below was developed to test the effects of PC1, PC2, and PC1*PC2 on the probability of occurrence of each breeding bird species studied.
FM: glmer (species occurrence (0/1) ~ PC1*PC2 + (1|oasis/ID), family = binomial).
The marginal R2 describes the variance explained by fixed effects, and the conditional R2 describes the variance explained by the full model. Using information-theoretic approach (Burnham and Anderson 2002), we developed an all-inclusive design. For each species, the models were then ordered by using Akaike’s Information Criterion for small sample sizes (AICc; Burnham and Anderson, 2002) and using the package “MuMIn” (Bartoń 2015). All models with ΔAICc < 2 were considered equally good (Burnham and Anderson 2002). The variance explained was calculated using the methods of Nakagawa and Schielzeth (2013). The package “MuMIn” and the function “rsquared.glmm” were used to calculate these variances.
To evaluate the degree of spatial dependence (autocorrelation), we applied the nugget-to-sill ratio (NSR) of residuals to the best AICc-based models. When spatial autocorrelation was found (NSR < 0.25 (Cambardella et al. 1994), we utilized the statistical approach of generalized linear mixed model penalized quasi-likelihood (glmmPQL; Dormann 2007). In glmmPQLs, we adopted three different spatial structures (i.e. Gaussian, spherical and exponential) because the real correlation structure is unknown. We generated spatial vectors via Moran's eigenvector map (MEMs) method (Dray et al. 2006) that produces spatial predictors by performing principal coordinate analysis of a truncated geographic distance matrix between various points while measuring spatial effects at multiple scales. To test for overdispersion in the residuals of the best AICc models of the seventeen FM models, diagnostics were performed using the ‘DHARMa' R package (Hartig 2020).
We used the package ‘visreg’ (Breheny and Burchett 2012) to plot the relationship between the predicted occurrences and the covariates included in the best AICc models. Means are shown ± SE.
Results
A total of 17 breeding bird species belonging to 12 families were recorded across all plots in Southern Tunisian oases (Table 1). The most widely distributed species are Laughing dove, Rufous-tailed Scrub-Robin, Western Olivaceous Warbler, and European Turtle-dove (Table 1). The species with restricted distributions are Crested lark, Sardinian Warbler, House bunting, and European serin (Table 1). The mean values of the variables measured (i.e., date palm tree cover, fruit tree cover, number of date palm trees, number of fruit trees, natural herbaceous layer cover, and cultivated herbaceous layer cover) at each point count are summarized in Table 2.
The PCA on the oases habitat variables gave two independent factors (PC1 and PC2) with eigenvalues > 1, respectively 2.50 and 1.40, which accounted for 77.94% of the variance. PC1 (50% of the original variance) was positively correlated with palm tree cover (r = 0.92, p < 0.0001) and number of palm trees (r = 0.88, p < 0.0001) but negatively with the cover (r = − 0.74, p < 0.0001) and number (r = − 0.86, p < 0.0001) of fruit trees other than date palms. High PC1 scores describe oases dominated by palm trees, while low PC1 scores describe those dominated by fruit trees other than date palms. PC2 (27.94%) was positively correlated with natural herbaceous cover (r = 0.80, p < 0.0001) but negatively with cultivated herbaceous cover (r = − 0.71, p < 0.0001). Therefore, the high PC2 scores distinguish oases with natural herbaceous layer, while those with low PC2 scores are for cultivated herbaceous layer. The Kaiser–Meyer–Olkin (KMO) measure of sampling adequacy indicated that our data were suitable for the PCA (PCA: KMO = 0.694; Bartlett test for sphericity, χ2 = 4887.06, p < 0.001).
The glmmPQL results allowed identifying four groups of bird species according to the PC1 axis, the additive effect of PC1 and PC2, and the interactive effect of PC1 and PC2 (Table 3).
PC1
Two species, namely the Laughing Dove (LD) and the Crested Lark (CL), were exclusively affected by the PC1 axis. While LD had high occurrences in oases dominated by palm trees (positive effect of PC1; Table 3), CL showed high occurrences in oases dominated by fruit trees other than date palms (negative effect of PC1; Table 3).
PC1 + PC2
Statistical analyses showed that the occurrences of eight bird species, namely European Greenfinch (EG; Chloris chloris), WOW, Common Blackbird (CB; Turdus merula), RSR, Eurasian Hoopoe (EH; Upupa epops), Woodchat Shrike (WS; Lanius senator), Great Grey Shrike (GGS; Lanius excubitor), and Spotted Flycatcher (SF; Muscicapa striata), were affected by both PC1 and PC2 axes (Table 3). Among these species, five (i.e., EG, WOW, CB, RSR, and SF) were mainly attracted by the oases dominated by fruit trees other than date palms (negative effect of PC1) and endowed with a natural herbaceous layer (positive effect of PC2) (Table 3). One species, namely WS, used the oases dominated by fruit trees with a cultivated herbaceous layer (Table 3). The remaining two species (i.e., GGS and EH) exhibited contrasting preferences for oases dominated by palm trees depending on the herbaceous layer. Indeed, while GGS used palm tree oases with a cultivated herbaceous layer (Table 3), EH favored palm tree oases endowed with natural herbaceous layers (Table 3).
PC1*PC2
Our results also individualized a group of bird species that the occurrence depended on the interaction between PC1 and PC2 (Table 3, Fig. 2). This group is composed of five species: ES, Common Chaffinch (CC; Fringilla coelebs), Orphean Warbler (OW; Curruca hortensis), Sardinian Warbler (SW; Curruca melanocephala), and Zitting Cisticola (ZC; Cisticola juncidis) (Table 3, Fig. 2). The occurrence probability of the bird species mentioned above in palm and fruit tree (other than date palms) oases is influenced by the cover level of the natural herbaceous layer. This effect was especially recorded for ZC, where higher probabilities of occurrence were documented in fruit tree oases with high cover of cultivated herbaceous layer (Table 3, Fig. 2e) and in palm tree oases endowed with significant natural herbaceous layer cover (Table 3, Fig. 2e). A second occurrence pattern was observed for ES, with a relatively high presence recorded in fruit tree oases featuring a weak cover of natural herbaceous layer (Table 3, Fig. 2a). However, in palm tree oases, the ES occurrence increased dramatically in the presence of a high cover of natural herbaceous layer (Table 3, Fig. 2a). For OW and SW, a third pattern of occurrence is recorded, showing an apparent attractiveness for fruit tree oases (other than date palms) compared to palm tree oases, whatever the type and cover of the herbaceous layer (Table 3, Fig. 2c and d). It is worth noting that the occurrence probability of these two species increased significantly in palm tree oases with high cover of natural herbaceous layer (Table 3, Fig. 2c and d). Nonetheless, this increase remains below that recorded in fruit tree oases with high cover of natural herbaceous layer (Table 3, Fig. 2c and d). For CC, the effect of increasing natural herbaceous layer cover was more important, and even exclusive, in fruit tree oases compared to date palm tree oases (Table 3, Fig. 2b).
For the seventeen breeding bird species, model dispersion tests showed no significant overdispersion in the residuals of the best AICc models (all P values greater than 0.05; Table S1). For almost all bird species (except EG, ES, WOW, and SW; Table 3), our statistical analyses resulted in a low marginal R2 (the unique contribution explained by the fixed effects) but a higher conditional R2 (the variance explained by the fixed and random effects together). This finding suggests that the 1398 plots across the 53 oases also contribute to explaining the occurrences of breeding birds.
Discussion
Our study represents the first attempt to assess the occurrence of breeding bird species in a large-scale survey of southern Tunisian oases. Our results support our hypothesis that breeding birds respond differently to the composition of oasis habitats. We outlined the oasis types that are important for breeding birds and delineated four groups of bird species: species dependent on the fruit tree layer (PC1), species dependent on both fruit trees and herbaceous layers (PC1 + PC2), species dependent on the interaction between fruit trees and herbaceous layers (PC1*PC2), and species dependent on neither fruit trees (PC1) nor herbaceous layers (PC2).
The first group includes two species (LD and CL), which are exclusively affected by the PC1 axis. We found that the probability of the presence of the LD increases in oases dominated by palm trees (positive effect of PC1). This result is consistent with that of Saâd et al. (2020, 2021), who showed that LD selected date palm farms during the breeding season in the arid region of Biskra (southern Algeria). This finding is not surprising, as this species uses the base of leaves of the top palms as nesting sites (El-Shafie and Abdel-Banat 2018; Saâd et al. 2020, 2021). Furthermore, the rigidness of palm tree stems, at least compared with other fruit trees (e.g., pomegranate), makes date palms more attractive for LD. Indeed, arid environments, like south Tunisia, are typically characterized by frequent wind activity during spring periods. Palm trees can provide suitable and stable anchorage points for LD nests, reducing the risk of them falling to the ground. Furthermore, nesting in date palms may also provide some security for LDs against terrestrial predators such as the domestic cat (Felis domestica), Egyptian cobra (Naja haje), viperine snake (Natrix maura) and desert horned viper (Cerastes cerastes), which may find some palm trees difficult to climb (Sol et al. 1997).
Our statistical analyses show that contrary to LD, CL uses oases dominated by fruit trees other than date palms. This ground-nesting bird is often associated with non-irrigated and open landscapes, such as steppes (Chiatante 2022; Heatwole and Muir 1982; Hamza and Hanane 2021). It selects these ecosystems as primary habitats for nesting and/or feeding (Heatwole and Muir 1982; Hamza and Hanane 2021). Surprisingly, in our study area, CL selects sites localized in oases with dense fruit trees. Seemingly, this lark species uses these oases as alternative feeding habitats. It seems that some specific and exclusive resources in these oases could attract CL from adjacent habitats. Furthermore, the high complexity of vegetation in oases dominated by fruit trees can create a 'microclimate' that is very different from the climatic conditions in modern oases and the surrounding desert environment. Such conditions offer CL the opportunity to mitigate unfavorable thermal conditions.Our results highlight the importance of the additive effect of PC1 and PC2 axes as predictors of the occurrence of the second group of bird species (i.e., EG, WOW, CB, RSR, EH, WS, GGS, and SF). We found that the occurrence of five species (i.e. EG, WOW, CB, RSR and SF) was negatively correlated with PC1 and positively correlated with PC2, suggesting that these species were attracted to oases dominated by fruit trees with a natural herbaceous layer compared to oases dominated by palms. This finding can potentially be explained by three reasons: (i) oases dominated by fruit trees have more complex oasis architectures, at least compared to homogenous palm trees oases, making sheltered nesting sites available to these tree-dwelling birds (Alaya-Ltifi and Selmi 2014; Hamza and Hanane 2021; Hamza et al. 2021, 2022), (ii) the intermediate arboreal layer can also provide more song posts or hunting perches for these species (Alaya-Ltifi and Selmi 2014; Hamza and Hanane 2021; Hamza et al. 2022). Furthermore, the presence of rich foliage in the arboreal layer leads to an increased abundance of foliage insects, which serve as a food source for these bird species like, for instance, the WOW and SF (Alaya-Ltifi and Selmi 2014; Snow and Perrins 1998), and (iii) high fruit trees cover may also provide thermal refugium to avoid the desert’s heat stress (Hamza et al. 2022; Zwarts et al. 2023), which may sometimes exceed 50 °C. Indeed, oases with a high cover of fruit trees can retain higher humidity and lower temperatures, thus buffering birds and their nestlings against mortality. Finally, the result can be attributed to human frequentation and disturbance, which are most intense in oases dominated by cultivated grounds than in those with a high natural herbaceous layer (Hamza et al. 2022). Daily agricultural activities, such as irrigation, mowing (the case of Alfalfa Medicago sativa), and weeding, are known to significantly disturb birds (Gabriel et al. 2010; Jeliazkov et al. 2016).
In the same group, glmmPQL analyses show a higher EH occurrence in oases dominated by palm trees with a natural herbaceous layer. Being a cavity-nesting bird (Barbaro et al. 2009; Nuhlíčková et al. 2021), this species uses old palm trees with plenty of cavities and holes for nest construction. Furthermore, oases dominated by palm trees also feature some falling palms with portions of their trunks embedded in the ground. Dead stems, whether upright or fallen, are characterized by the presence of holes that serve as nesting sites for EH. Finally, the high cover of the natural herbaceous layer in the selected oases also seems to meet the dietary needs (arachnids, annelids, crustaceans, crickets and reptiles) of this bird species. Also, our results suggest that the GGS avoids oases dominated by fruit trees and uses date palm oases. This shrike species is largely known as a sit-and-wait predator that hunts prey from elevated perches (Paczuska et al. 2021; Steinmetz et al. 2019). The configuration of palm tree oases favors this avian species’ hunting/foraging strategy by providing semi-open habitats. Unlike oases with dense arboreal habitats, these areas contribute to (i) ensuring higher visibility for this species and (ii) enhancing prey detectability and catchability. Interestingly, our results also show that contrary to GGS, WS avoid palm tree oases; they select oases dominated by fruit trees with a cultivated herbaceous layer. Like other shrike species, WS avoids the densest tree land and uses open and semi-open areas (Alaya-Ltifi and Selmi 2014; Brambilla et al. 2017; Chiatante 2018). This segregation in the use of habitats may be due to interspecific competition with GGS. Our results also show that the two shrike species were attracted to oases with a higher cultivated herbaceous layer. These species occupy a higher position in the food web; their diet includes relatively large prey. In our studied oases, the cultivated herbaceous layer is mainly dominated by alfalfa crops. This forage crop is known to harbor a wide variety of vertebrates and invertebrate species, including small mammals, reptiles, beetles, orthopterans, and crickets (del Portillo et al. 2022). Previous works have shown that these organisms are the main prey for these Lanius species (e.g. Hódar 2006; Kočí and Krištín 2020; Nikolov et al. 2004).
Regarding the third group of five species (i.e., ES, CC, OW, SW, and ZC), our results show that the occurrence of these species depends on the interaction between PC1 and PC2. Moreover, ZC occurrence probabilities are higher in fruit tree oases with high cover of cultivated herbaceous layer and in palm tree oases with important natural herbaceous layer. The high occurrence of this species in palm tree oases with a natural herbaceous layer is not surprising, given that this species is known to select open and semi-open habitats (Gregory et al. 2007; Godinho and Rabaça 2011; Katayama et al. 2021). In southern Tunisian oases, many date palm oases are characterized by semi-open habitats with a high cover of Phragmites communis, especially those old and abandoned (MEDD 2015). This small bird uses the natural herbaceous layer to build their nests, especially in the Phragmite stems (FH per. obs.). Such a layer is also used as a feeding place, where they can take advantage of abundant insect prey as these habitats are less disturbed by human activities. During the breeding period, Fenech (2012) showed that ZC fed their nestlings on a wide variety of insects and arachnids, such as for ficula decipiens, Ameles sp., Platycleis sp., Tipula sp., Eupeodes corollae, Synthymia fixa, Autographa gamma, Pisaura mirabilis, and Micrommata ligurina. The increased occurrence of ZC in fruit tree oases with a high cover of the cultivated herbaceous layer can be explained by the availability of food resources in these habitats. Indeed, as mentioned above, the cultivated herbaceous layer in the studied oases is mainly dominated by alfalfa crops and is among the richest habitat in arthropods, hosting from 250 to 1000 arthropod species (del Portillo et al. 2022), including many species of orthopteran, spiders, and hemipterans (del Portillo et al. 2022; Rutledge and O’Neil 2005).
OW and SW show a distinct attractiveness for fruit tree oases compared to date palm oases, whatever the type and cover of the herbaceous layer. Although these two species increase significantly in palm tree oases with a high cover of natural herbaceous layer, this increase remains below that recorded in fruit tree oases with a high cover of natural herbaceous layer. This high attractiveness of OW and SW to fruit tree oases can be explained by the presence of a high variety of fruits (e.g., fig, mulberry, apricot, and grapevine), which can provide a concentrated and energical-rich food source for these omnivore bird species (Snow and Perrins 1998). Additionally, the habitat structures within oases dominated by fruit trees increase the availability of suitable nesting sites for these species and sufficient shelter against predators, at least compared with palm tree oases. Finally, oases with a high cover of natural herbaceous layer can provide a rich food base in the undergrowth and better possibilities to hide from predators.
For CC, the effect of increasing natural herbaceous layer cover is more important and even exclusive in fruit tree oases compared to palm tree oases. This result is in line with Holland et al. (2006), who found that the CC forages mainly in areas dominated by weeds. In our study area, ground-layer vegetation growing naturally in oases dominated by fruit trees can provide seeds and invertebrates for this bird species. It is also possible that the CC can benefit from removing natural grass by accessing new food resources.
In addition, a relatively high presence of ES is recorded in fruit tree oases with a weak cover of the natural herbaceous layer. However, in palm tree oases, the ES occurrence has increased dramatically with a high cover of natural herbaceous layer. ES is a granivorous species characterized by a high degree of diet specialization during the breeding season (Valera et al. 2005). In the studied oases, natural herbaceous layer comprises several spontaneous species. It seems that this stratum provides a wide variety of seeds for this breeding bird.
None of the oasis habitat compositions has a significant effect on the occurrences of the remaining species (TD and HB), which seem less sensitive to vegetation structure and composition and occur in different types of oases. Other factors not considered in this study (i.e., growing urbanization) may force these species to have such a response. Previous studies on birds in southern Tunisia have shown that HB selects urban environments (Alaya-Ltif and Selmi 2014). Additionally, in studying the occurrence of TD in southeastern Tunisian oases, Hamza et al. (2021) demonstrated that the occurrence of this species depends on the cover of urban areas, being high when it is weak. Eddajjani et al. (2022) have also demonstrated that the occurrence of TD is negatively affected by the cover of built-up areas in the capital city of Morocco, Rabat.
Limitations
The present study is the first attempt to assess the effects of vegetation structure and composition in shaping the distribution of breeding bird species through a wide-scale survey covering Tunisian oasian agroecosystems. A potential caveat of this study is not including the landscape components in the modeling process (e.g., Normalized Difference Vegetation Index, oasis connectivity and isolation, oasis fragmentation, edge density, and roads and urban proportion), which are known to influence bird habitat use (e.g., Anjos et al 2011; Hamza et al. 2021, 2022, 2023; Mahmoudi et al 2016; Vetter et al 2011). We leave these covariates as interesting directions for future work.
Implications, recommendations, and prospects
Overall, the results obtained in the present study improve our understanding of how bird species respond to variation in oases habitat composition. Bird community inhabiting southern Tunisian oases has different oasis preferences.
This study also suggests that the oases dominated by date palm trees are less attractive to breeding birds. We found that bird species occurrence varied between one and two species in the different types of palm tree oases (i.e., date palm monoculture oases and oases dominated by date palm trees with natural and cultivated herbaceous layer, respectively).
In Tunisia, for economic reasons, the current trend is to extend date palm oases (modern oases; Rhouma 1996). However, such a trend is not favorable for the diversity of bird communities, as proven by this study. Initiating a dialogue with oasis owners is essential to create a suitable nesting habitat for birds. This dialogue should aim to persuade them to implement beneficial changes that would not only benefit the breeding birds, but also increase the income of local people. An actionable step involves enhancing the structural complexity of oases. It is imperative to encourage oasis owners to grow fruit trees in association with date palms, alternating rows of date palms with those of other fruit trees, such as pomegranates, olives, figs, and apricots. This heterogeneity would help attract more breeding birds in this southern part of Tunisia and contribute to the well-being of local farmers. The same approach should be considered in oases dominated by fruit trees other than date palm trees. Establishing date palms on the outskirts of these oases would encourage date palm breeding birds to use them more.
The same interest should be granted to the herbaceous layer. Indeed, establishing alfalfa crops in one part of the oases while encouraging the presence of the natural herbaceous layer in another would be beneficial for breeding birds.
This study constitutes the first step toward conservation-oriented oases management. To address these issues, the involvement of different stakeholders such as owners, farmers, local people, managers and researchers is necessary to achieve multifunctional outcomes and improve both the conservation of bird diversity and the agricultural system. For example, the involvement of different stakeholders is crucial to determine the thresholds of (i) fruit trees, (ii) date palms and (iii) alfalfa crops to be established in each oasis type in order to increase the structural complexity of oases. Additional research is also needed to reveal how covers of habitats surrounding oases (landscape context) shape breeding bird species occurrence. It is also central to extend our work to cover wintering and spring migrant species to know how oases architectures shape the distribution and richness of non-breeding passerine species.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Abbes K, Zouba A, Harbi A, Ghrissi N, Ksantini M, Chermiti B (2020) The pomegranate butterfly Deudorixlivia (Lepidoptera: Lycaenidae): an emerging pest on dates in Tunisia. EPPO Bull 50(1):191–196. https://doi.org/10.1111/epp.12645
Ait-El-Mokhtar M, Boutasknit A, Ben-Laouane R, Anli M, El Amerany F, Toubali S, Lahbouki S, Meddich A (2022). Vulnerability of oasis agriculture to climate change in Morocco. In: Khosrow-Pour M (ed), Impacts of climate change on agriculture and aquaculture, IGI Global, Hershey, pp 76–106
Alaya-Ltifi L, Selmi S (2014) Passerine abundance and diversity in a polluted oasis habitat in South-Eastern Tunisia. Eur J Wildl 60:535–541. https://doi.org/10.1007/s10344-014-0817-0
Anjos L, Collins CD, Holt RD, Volpato GH, Mendonça LB, Lopes EV, Carvalho J (2011) Bird species abundance–occupancy patterns and sensitivity to forest fragmentation: implications for conservation in the Brazilian Atlantic forest. Biol Conserv 144(9):2213–2222. https://doi.org/10.1016/j.biocon.2011.05.013
Barbaro L, Couzi L, Bretagnolle V, Nezan J, Vetillard F (2009) Multi-scale habitat selection and foraging ecology of the eurasian hoopoe (Upupa epops) in pine plantations. Biodivers Conserv 17:1073–1087. https://doi.org/10.1007/s10531-007-9241-z
Bartoń K (2015) MuMIn: multi-model inference. R Package Version1.9.13. http://CRAN.R-project.org/package=MuMIn. Accessed 25 Jan 2024
Bates D, Maechler M, Bolker B, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R Package Version 1.1–7. http://cran.rproject.org/package=lme4
Ben Hassine J, Nouira S (2012) Répartition géographique et affinité sécologiques des Amphibiens de Tunisie. Rev Écol (Terre Vie) 67:437–457
Ben Salah M (2011) La palmeraie de Gabès. Phoenix Project. http://www.listephoenix.com/wp-content/uploads/2011/12/BENSALAH-oasis-Gabès-fr.pdf/. Accessed 29 Jan 2024
Bibby CJ, Burgess ND, Hill DA, Mustoe S (2000) Bird census techniques. Academic Press, London
BirdLife International (2024) Species factsheet: European Turtle-dove Streptopelia turtur. http://www.birdlife.org. Accessed 25 Jan 2024
Boualem R, Bachir A, Rabah K (2014) The foggara: a traditional system of irrigation in arid regions. J Eng Geol 60(2):30
Brambilla M, Gustin M, Fulco E, Sorace A, Celada C (2017) Coarse landscape features predict occurrence, but habitat selection is driven by specific habitat traits: implications for the conservation of the threatened Woodchat Shrike Lanius senator. Bird Conserv Int 27(1):58–70. https://doi.org/10.1017/S0959270916000034
Breheny P, Burchett W (2012) Visualizing regression models using visreg. http://web.as.uky.edu/statistics/users/pbreheny/publications/visreg.pdf. Accessed 1 Jan 2024
Burnham KP, Anderson DR (Ed) (2002) Model selection and inference: a practical information theoretical approach, New York
Cambardella CA, Moorman TB, Novak JM, Parkin TB, Karlen DL, Turco RF, Konopka AE (1994) Field-scale variability of soil properties in central Iowa soils. Soil Sci Soc Am J 58(5):1501–1511. https://doi.org/10.2136/sssaj1994.03615995005800050033x
Chiatante G (2018) Heterospecific social attraction in migrant birds: habitat niche overlap between two threatened shrikes. Wild Res 46(1):25–36. https://doi.org/10.1071/WR18031
Chiatante G (2022) Habitat use and niche overlap of ground-nesting steppic birds. Avian Biol Res 15(4):180–193. https://doi.org/10.1177/17581559221132189
De Grenade R (2013) Date palm as a keystone species in Baja California peninsula, Mexico oases. J Arid Environ 94:59–67. https://doi.org/10.1016/j.jaridenv.2013.02.008
del Portillo DG, Arroyo B, Morales MB (2022) The adequacy of alfalfa crops as an agri-environmental scheme: a review of agronomic benefits and effects on biodiversity. J Nat Conserv 69:126253. https://doi.org/10.1016/j.jnc.2022.126253
Dhiab O, Selmi S (2021) Patterns of vertebrate road-kills in a pre-Saharan Tunisian area. J Arid Environ 193:104595. https://doi.org/10.1016/j.jaridenv.2021.104595
Djellouli-Tabet Y (2010) Common scarcity, diverse responses in the Maghreb Region. In: Schneier-Madanes G, MF Courel (eds) Water and sustainability in arid regions: An interdisciplinary exploration of human and environmental interactions. Springer science? Business Media, Dordrecht, pp 87–102
Dormann CF (2007) Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob Ecol Biogeogr 16:129–138. https://doi.org/10.1111/j.1466-8238.2006.00279.x
Dray S, Legendre P, Peres-Neto PR (2006) Spatial modelling: a com-prehensive framework for principal coordinate analysis of neigh-bour matrices (PCNM). Ecol Model 196:483–493. https://doi.org/10.1016/j.ecolmodel.2006.02.015
Eddajjani A, Hanane S, Kandry AE, Qninba A (2022) An unexpected presence in urban environment: factors governing occurrence of the vulnerable European turtle-dove (Streptopeliaturtur) in the city of Rabat, Morocco. Urban Ecosyst 25(4):1339–1351. https://doi.org/10.1007/s11252-022-01234-7
El-Fahem T (2003) Salinization of Groundwater in the Nefzaoua Oasis-South Tunisia. Geology 121. https://doi.org/10.1007/s10040-007-0185-x
El-Shafie HAF, Abdel-Banat BMA (2018) Non-arthropod pests of date palm and their management. CABI Rev. https://doi.org/10.1079/PAVSNNR201813020
Faivre-Dupaigre JP (1957) L’irrigation traditionnelle dans les oasis de Gabès (Tunisie). Les Cahiers De Tunisie: Revue De Sciences Humaines 5(17):23–38
Fenech N (2012) Some food sources of the Zitting Cisticola (Cisticola juncidis) in Malta. Bull Ent Soc Malta 5:161–163
Gabriel D, Sait SM, Hodgson JA, Schmutz U, Kunin WE, Benton TG (2010) Scale matters: the impact of organic farming on biodiversity at different spatial scales. Ecol Lett 13(7):858–869. https://doi.org/10.1111/j.1461-0248.2010.01481.x
Godinho C, Rabaça JE (2011) Birds like it Corky: the influence of habitat features and management of ‘montados’ in breeding bird communities. Agrofor Syst 82:183–195. https://doi.org/10.1007/s10457-010-9345-4
Gregory RD et al (2007) Population trends of widespread woodland birds in Europe. Ibis 149:78–97. https://doi.org/10.1111/j.1474-919X.2007.00698.x
Hamza F, Hanane S (2021) The effect of microhabitat features, anthropogenic pressure and spatial structure on bird diversity in southern Tunisian agroecosystems. Ann Appl Biol 179(2):195–206. https://doi.org/10.1111/aab.12690
Hamza H, Jemni M, Benabderrahim MA, Mrabet A, Touil S, Othmani A, Ben Salah M (2015) Date palm status and perspective in Tunisia. In: Al-Khayri J, Jain S, Johnson D (eds) Date Palm Genetic Resources and Utilization. Springer, Dordrecht, pp 193–221
Hamza F, Kahli A, Chokri MA, Almalki M, Hanane S (2021) Urban and industrial landscapes interact with microhabitat to predict occurrence of European Turtle Dove (Streptopelia turtur) in Mediterranean oases: implications for conservation. Landsc Urban Plann 215:104219. https://doi.org/10.1016/j.landurbplan.2021.104219
Hamza F, Kahli A, Almalki M, Chokri MA (2022) Distance from industrial complex, urban area cover, and habitat structure combine to predict richness of breeding birds in southeastern Tunisian oases. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-18051-8
Hamza F, Hanane S, Almalki M, Chokri MA (2023) How urbanization and industrialization shape breeding bird species occurrence in coastal Mediterranean oasis system. Urban Ecosyst 26(1):185–196. https://doi.org/10.1007/s11252-022-01271-2
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multilevel/mixed) regression models. R package version 0.3.1. https://CRAN.R-project.org/package=DHARMa
Heatwole H, Muir R (1982) Population densities, biomass and trophic relations of birds in the pre-Saharan steppe of Tunisia. J Arid Environ 5(2):145–167. https://doi.org/10.1016/S0140-1963(18)31546-5
Hódar JA (2006) Diet composition and prey choice of the southern grey shrike Lanius meridionalis L. in south-eastern Spain: the importance of vertebrates in the diet. Ardeola 53(2):237–249
Holland JM, Hutchison MAS, Smith B, Aebischer NJ (2006) A review of invertebrates and seed-bearing plants as food for farmland birds in Europe. Ann Appl Boil 148(1):49–71. https://doi.org/10.1111/j.1744-7348.2006.00039.x
Isenmann P, Gaultier T, El Hili A, Azafzaf H, Dlensi H, Smart M (2005) Oiseaux de Tunisie/Birds of Tunisia. SEOF, Paris
Ismail HB, Hassine DB (2021) Tunisian date cultivars: economical aspect, physicochemical properties, sensory characterization and potential valorization. In: Khebour Allouche F, Abu-hashim M, Negm AM (eds) Agriculture productivity in Tunisia under stressed environment. Springer, Cham, pp 57–71
Jayathunga K, Diyabalanage S, Frank AH, Chandrajith R, Barth JA (2020) Influences of seawater intrusion and anthropogenic activities on shallow coastal aquifers in Sri Lanka: evidence from hydrogeochemical and stable isotope data. Environ Sci Pollut Res 27(18):23002–23014. https://doi.org/10.1007/s11356-020-08759-4
Jeliazkov A, Mimet A, Chargé R, Jiguet F, Devictor V, Chiron F (2016) Impacts of agricultural intensification on bird communities: new insights from a multi-level and multi-facet approach of biodiversity. Agric Ecosyst Environ 216:9–22. https://doi.org/10.1016/j.agee.2015.09.017
Jemai S, Ellouze M, Abida H (2017) Variability of precipitation in arid climates using the wavelet approach: case study of watershed of Gabes in South-East Tunisia. Atmosphere 8(9):178. https://doi.org/10.3390/atmos8090178
Katayama N, Mashiko M, Koshida C, Yamaura Y (2021) Effects of rice-field abandonment rates on bird communities in mixed farmland–woodland landscapes in Japan. Agric Ecosyst Environ 319:107539. https://doi.org/10.1016/j.agee.2021.107539
Kočí J, Krištín A (2020) On the occurrence and diet of a migrating Woodchat Shrike (Lanius senator) in Slovakia. Tichodroma 32:47–50. https://doi.org/10.31577/tichodroma.2020.32.3
Laity JJ (2009) Deserts and desert environments. Wiley, New York, p 357
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Amsterdam, The Netherlands
Mahmoudi S, Sheykhi Ilanloo S, Keyvanloo Shahrestanaki A, Valizadegan N, Yousefi M (2016) Effect of human-induced forest edges on the understory bird community in Hyrcanian forests in Iran: implication for conservation and management. For Ecol Manage 382:120–128. https://doi.org/10.1016/j.foreco.2016.10.011
Mamou A (1989) Caractéristiques et évaluation et gestion des resources en eau du Sud tunisien. Thése Doctorat. Univ de Paris Sud, France
MEDD (2015) Elaboration d’une monographie complète des oasis en Tunisie, first ed. Tunis, Tunisie
Nakagawa S, Schielzeth H (2013) A general and simple method forobtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Nikolov BP, Kodzhabashev ND, Popov VV (2004) Diet composition and spatial patterns of food caching in wintering Great Grey Shrikes (Lanius excubitor) in Bulgaria. Biol Lett 41(2):119–133
Nuhlíčková S, Svetlík J, Eckenfellner M, Knauer F, Hoi H (2021) Interaction between nestling behaviour and nest-space use. Ethol Ecol Evol 33(5):496–514. https://doi.org/10.1080/03949370.2020.1858173
Paczuska M, Jaskuła R, Golawski A (2021) Diet composition and prey choice by the Great Grey Shrike Lanius excubitor during the non-breeding period: comparing two methods of diet analysis. Bird Study 68(2):183–189. https://doi.org/10.1080/00063657.2021.1976103
Prodon R, Lebreton JD (1981) Breeding avifauna of a Mediterranean succession: the holm oak and cork oak series in the eastern Pyrenees. 1. Analysis and modelling of the structure gradient. Oikos 37:21–38. https://doi.org/10.2307/3544069
Quantum GIS Development Team (2020) Quantum GIS Geographic Information System. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
Rhouma A (1996) Le palmier dattier en Tunisie: un secteur en pleine expansion. Cah Options Mediterr 28:85–104
Rhouma A, Mougou I, Rhouma H (2020) Determining the pressures on and risks to the natural and human resources in the Chott Sidi Abdel Salam oasis, southeastern Tunisia. Euro-Mediterr J Environ Integr 5:1–11. https://doi.org/10.1007/s41207-020-00176-w
Rutledge CE, O’Neil RJ (2005) Orius insidiosus (say) as a predator of the soybean aphid, Aphis glycines Matsumura. Biol Conserv 33:56–64. https://doi.org/10.1016/j.biocontrol.2005.01.001
Saâd N, Hanane S, Farhi K, Khemis MDEH (2020) Nest age as predictor of nest survival in three sympatric dove species breeding in a Mediterranean arid agroecosystem. Ardea 108(2):171–182. https://doi.org/10.5253/arde.v108i2.a5
Saâd N, Hanane S, El Hak Khemis MD, Farhi K (2021) Landscape composition governs the abundance patterns of native and invasive Columbidae species along an urban–rural gradient and contribute to their partitioning. Biol Invasions 23(7):2077–2091. https://doi.org/10.1007/s10530-021-02489-5
Salah MB (2015) Food value of soft dates cultivated in Tunisian coastal oases. J Life Sci 9:234–241. https://www.davidpublisher.com/Public/uploads/Contribute/55fb74b478ac1.pdf
Santoro A (2023) Traditional oases in Northern Africa as multifunctional agroforestry systems: a systematic literature review of the provided Ecosystem Services and of the main vulnerabilities. Agrofor Syst 97(1):81–96. https://doi.org/10.1007/s10457-022-00789-w
Selmi S, Boulinier T (2003) Breeding bird communities in southern Tunisian oases: the importance of traditional agricultural practices for bird diversity in a semi-natural system. Biol Conserv 110(2):285–294. https://doi.org/10.1016/S0006-3207(02)00231-8
Sikka DR (1997) Desert climate and its dynamics. Curr Sci 72:35–46
Snow DW, Perrins CM (1998) The birds of the Western Palearctic. Oxford University Press, Oxford
Sol D, Santos DM, Feria E, Clavell J (1997) Habitat selection by the Monk Parakeet during colonization of a new area in Spain. The Condor 99(1):39–46. https://doi.org/10.2307/1370222
Steinmetz M, Coppes J, Schneider S (2019) Hunting habitat selection of breeding great grey shrikes Lanius excubitor Linnaeus, 1758 in Luxembourg. Bull Soc Nat Luxemb 121:291
Tengberg M (2012) Beginnings and early history of date palm garden cultivation in the Middle East. J Arid Environ 86:139–147. https://doi.org/10.1016/j.jaridenv.2011.11.022
Twiti R, Haddad M, Ferchichi A (2009) The importance of vegetables crops in the Oases of Gabès. J Arid Land 19(1):217–219
Valera F, Wagner RH, Romero-Pujante M, Gutiérrez JE, Rey PJ (2005) Dietary specialization on high protein seeds by adult and nestling serins. The Condor 107(1):29–40. https://doi.org/10.1093/condor/107.1.29
Vetter D, Hansbauer MM, Vegvari Z, Storch I (2011) Predictors of forest fragmentation sensitivity in Neotropical vertebrates: a quantitative review. Ecography 34:1–8. https://doi.org/10.1111/j.1600-0587.2010.06453.x
Veyrac-Ben Ahmed B, Abdedayem S (2017) Oases in Southern Tunisia: The end or the renewal of a clever human invention? In: Lavie E, Marshall A (eds) Oases and globalization. Springer Geography. Springer, Cham. https://doi.org/10.1007/978-3-319-50749-1_1
Zammouri M, Siegfried T, El-Fahem T, Kriâa S, Kinzelbach W (2007) Salinization of groundwater in the Nefzawa oases region, Tunisia: results of a regional-scale hydrogeologic approach. Hydrogeol J 15:1357–1375. https://doi.org/10.1007/s10040-007-0185-x
Zwarts L, Bijlsma RG, van der Kamp J (2023) Granivorous birds in the Sahel: is seed supply limiting bird numbers? Ardea 111(1):283–304. https://doi.org/10.5253/arde.2022
Acknowledgements
We thank the Editor-in-chief of Agroforestry Systems, as well as the anonymous reviewers for their comments and advice. This study was supported by grants from The Ministry of Higher Education of Carthage (Tunisia), Laboratory of Environment Biomonitoring, Faculty of Sciences of Bizerte 7021 Jarzouna (Tunisia).
Author information
Authors and Affiliations
Contributions
ME: Data collection, Writing – original draft. SH: Data analysis, Methodology, Supervision, Writing – review & editing. FH: Data collection, Data analysis, Writing – original draft. MAC: Data collection, Writing – original draft. HB: Conceptualization, Data curation, Methodology, Supervision, Writing – review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elghoul, M., Hanane, S., Hamza, F. et al. Occurrence of breeding birds and habitat composition in oasis systems: assessment in Tunisia with implications for management planning. Agroforest Syst (2024). https://doi.org/10.1007/s10457-024-01069-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10457-024-01069-5