Abstract
The rhizosphere is the unique hotspot that is highly influenced by plant roots and characterized by higher microbial activity and nutrient availability. Land uses modify the rhizospheric soil properties through the stimulatory effects of various root exudates and soil nutrients. The present work was aimed to study rhizosphere soil properties under different land use systems at Ballowal Saunkhri watershed in Punjab state, India. For this study, soil samples were collected from three land use systems (horticulture, farm forest and cropland) at four depths, viz. 0–15, 15–30, 30–60 and 60–90 cm during pre-rainy and post-rainy seasons. The results indicated that farm forestry system had significantly higher soil organic carbon (SOC), cation exchange capacity, micronutrient cations (Zn, Fe, Cu, Mn) and microbial properties (total microbial count, microbial biomass carbon, basal soil respiration, dehydrogenase activity, alkaline phosphatase activity and microbial quotient) compared with other land use systems. However, bulk density, available phosphorus, available potassium and metabolic quotient were observed higher under cropland system. The principle component analysis identified that SOC and available potassium were the most contributing and reliable variables for assessing soil quality for different land use systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plant roots, soil and microbial interactions alter the soil physico-chemical properties, which in turn affect the microbiological properties in the rhizosphere region (Nihorimbere et al. 2011). The term “rhizosphere” was first given by Hiltner (1904)—as an area that is under the influence of plant roots. Plants can directly modify the rhizosphere microenvironment through the secretions of various molecules like polysaccharides, carbohydrates, sugars, vitamins, etc., through their roots leading to stimulate microbial activities in that region (Prescott et al. 1999). Therefore, rhizosphere is the hotspot of intense microbial activity that is highly influenced by the plant root secretions. The diversity of rhizosphere microbial activity depends upon the quality and quantity of compounds being released through plant roots (Marschner and Baumann 2003).
Land use change has a direct impact on soil nutrient supply and distribution and stimulates the biological changes in the rooting zone. Grayston et al. (1998) studied the selective influence of plant species on diversity of rhizosphere microbiome. The microbial diversity present near root zone is species specific, i.e. the differences in utilization of carbon compounds secreted by the plant roots recruit the microbial diversity. The root secretions vary with different plant species, which serves as a substrate for microorganisms. Nutrients demand of each plant species is different, and it produces different quantity and quality of litter, which in turn affect the diversity and composition of microorganisms (Bezemer et al. 2006). It has been reported that the same soil type with different land uses affects the rhizosphere environment (Miethling et al. 2000). Hilton et al. (2018) stated that crop type, soil type (either rhizosphere or bulk soil) and sampling time are the major drivers of microbial community composition. The beneficial rhizosphere microorganisms can help in maintenance of ecosystem balance through organic matter decomposition and cycling of nutrients that serves as an indicator of land use changes and ecosystem sustainability (Ros et al. 2006; Balser et al. 2010). Hence, it is important to study the effect of different land uses on the rhizosphere communities for maintaining the soil health and quality in order to regenerate the soil’s ability to provide ecosystem services (Van Leeuwen et al. 2017).
Indian Punjab’s lower Shivalik foothills also known as sub-montane region, covers approximately 0.5 million hectares, which is about 10% of total geographical area of Punjab state. This area adjoins undulating piedmont plains that are situated in the south of Shivalik hills commonly known as Kandi area (rainfed). A seasonal stream of water called choe (rivulets) passes through the watersheds resulting in flooded conditions during rainy season (July–September), causes significant land degradation but remains dry for rest of the period. The Shivalik foothills are represented as the most fragile ecosystem of Himalayan range due to their peculiar geological features and exposure to greater biometrical treatments because of proximity to plains (Panwar et al. 2017). Various other studies have also been carried out in this region, but information related to rhizosphere soil properties in relation to different land use systems is limited. This study was carried out with the hypothesis that different land uses have a direct influence on rhizospheric soil properties due to variation in root exudates and litter fall inputs. The pre- and post-rainy seasons observations under different land use systems would also have its impact on several rhizosphere soil properties at Shivalik foothills of Punjab.
Materials and methods
Site characterization and climate
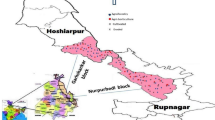
This study was conducted at Ballowal Saunkhri watershed (30° 44′ to 32° 32′ N, 75° 52′ to 76° 43′ E) located at lower Shivalik foothills of Punjab, India, at an altitude of 300–500 m above mean sea level, with semi-arid to sub-humid climate, and this area receives bimodal rainfall pattern (Fig. 1). The normal rainfall of this region is 1055 mm. During 2018, the actual annual rainfall at Ballowal Saunkhri was 1357.7 mm. The mean monthly maximum rainfall was received in September (383.8 mm) and minimum in December (0.6 mm). The mean monthly maximum temperature varied from 18.6 °C (January) to 38.6 °C (May), and mean monthly minimum temperature varied from 5.1 °C (January) to 25.2 °C (July).This area consists of alluvial detritus derived from sub-aerial wastes of inner mountain ranges swept down by numerous rivers and streams deposited at the Himalayan foothills.
Soil sampling, preparation and analysis
For the study, three land use systems, namely horticulture, farm forest and cropland from Ballowal Saunkhri watershed in Punjab, were selected for collection of soil samples. Further, three sub-land use systems were selected from these land uses. The horticulture-based land use systems comprised of mango (Mangifera indica), guava (Psidium guajava) and aonla (Phyllanthus emblica) were more than 15 years old; the farm forestry systems included dek (Melia azedarach), poplar (Populus deltoides) and bamboo (Bambusa vulgaris) were also more than 15 years old. No intercrops as well as cultivation were taken in horticulture and farm forestry systems; and the sub-land use systems of cropland comprised of cereals, i.e. maize (Zea mays) and wheat (Triticum aestivum), pulses, i.e. chickpea (Cicer arietinum) and mung bean (Vigna radiata) and oilseeds, i.e. taramira (Eruca vesiaria ssp. sativa) and sesame (Sesamum indicum) were continuously cultivated for more than 10 years. The spacing of mango, guava and aonla was 10 × 10 m, 6 × 5 m and 7.5 × 7.5 m, respectively. The dek and poplar were spaced at 3 × 3 m, and bamboo was planted in 4 × 4 m spacing. Soil sampling was done twice in the year 2018, i.e. post-rainy season in October–November and pre rainy season in April–May. The soil samples were collected with the help of auger from 0–15, 15–30, 30–60 and 60–90 cm depths. Three samples and three replications were taken from each land use. For trees, it is often considered that rhizosphere soil lies within the circular area with trunk at the center, tantamount to canopy area (Debnath et al. 2015). Therefore, soil sampling was performed within this area (just below plant canopy approximately 15 cm away from the trunk of the tree) for forest and horticulture crops. For cropland or agricultural crops, samples were also collected by carefully uprooting of plants and shaking the soil adhere to the roots into polyethylene bags following the method of Zuberer (1994) and Dongmo and Oyeyiola (2006). One half of soil samples were kept field moist, sieved from 0.5-mm-mesh sieve and stored in deep freezer at 4 °C for soil microbiological analysis; the other half was completely air-dried, grounded and passed through a 2-mm-mesh sieve for the analysis of physical and chemical properties. Core samples were taken from four depths for the determination of bulk density.
Soil analysis
Soil bulk density was measured by core sampling method (Blake 1965), and soil particle density was analysed using pycnometer method (Blake 1965). The soil porosity was calculated by using the formula given as: total soil porosity = (1 − Db/Dp) × 100, where, Db = bulk density (gm cm−3) and Dp = particle density (gm cm−3). Soil organic carbon (SOC) was determined according to Walkley and Black’s rapid titration method (1934). Available phosphorus and available potassium were analysed using Olsen et al. (1954) and Merwin and Peech (1950), respectively. The micronutrient cations were extracted by using diethylenetriaminepentaacetic acid (DTPA) and estimated by an atomic absorption spectrophotometer (Lindsay and Norvell 1978). Cation exchange capacity (CEC) was measured by using 1 N ammonium acetate solution described by Jackson (1967). Serial dilution pour plate method (Dhingra and Sinclair 1993) was used to isolate and enumerate bacterial, fungal and actinomycetes count. The basal soil respiration was determined by taking known volume, and strength of alkali (NaOH) was used for absorption of CO2. Excess NaOH was then titrated against standard HCl, and the amount of carbon dioxide released was calculated as given by Anderson (1982). Microbial biomass carbon (MBC) was assayed by chloroform fumigation extraction method (Vance et al. 1987). Dehydrogenase activity (DHA), and alkaline phosphatase activity was measured as described by Tabatabai (1982) and Tabatabai and Bremner (1969), respectively. Microbial quotient was defined as the ratio of microbial biomass C to soil organic C (Xue et al. 2006). The metabolic quotient was calculated as the ratio of basal respiration to microbial biomass; expressed as the amount of CO2-C produced per unit of microbial biomass carbon.
Statistical analysis
The data were statistically analysed using analysis of variance (ANOVA) technique in split plot design for chemical and microbiological properties using locally developed software (Cheema and Singh 1990). Means for treatment effects were separated based on least significant difference (LSD) values. The LSD values were tested at (p ≤ 0.05) level of probability. The principle component analysis for each soil property under different land use systems was carried out by using XLSTAT (2019 Version).
Results
Rhizosphere effect on physical properties
All the soil physical properties using analysis of variance (ANOVA) technique in randomized block design varied significantly among different land use systems and depths except particle density. At surface soil, the bulk density was 7.6% lower under farm forest and 2.1% under horticulture system compared with cropland. Depth-wise bulk density was observed higher at 60–90 cm than 0–15 cm by 7.6, 4.3 and 9.2% under farm forest, horticulture and cropland, respectively. No significant differences were recorded in particle density under all land use systems and depths. The surface layer of farm forest system observed higher total soil porosity by 6.4 and 3.6% compared with cropland and horticulture system, respectively. However, the porosity percentage decreased by 6.6, 14.6 and 6.4% under farm forest, horticulture and cropland, respectively, from 0–15 to 60–90 cm depth (Table 1).
Rhizospheric effect on land uses, seasons and chemical properties
All the three land uses (farm forest, horticulture and cropland) had significant effect on soil chemical properties in their rhizosphere region (Table 2). During pre-rainy season, the SOC (0–15 cm) was observed 33.5 and 34.1% higher under farm forest compared with horticulture and cropland, respectively. Similarly, during post-rainy season, the SOC was higher under farm forest by 29.3 and 38.3% compared with horticulture and cropland, respectively. The relative trend of CEC under different land use systems was recorded in the order of farm forest > horticulture > cropland. The CEC of cropland and horticulture declined by 20.5 and 12.5% during post-rainy season and 27.3 and 18.9% during pre-rainy season compared with farm forestry system, respectively. In comparison with farm forestry system, the cropland and horticulture had higher available phosphorus by 3.3 and 5.3%, respectively, during pre-rainy season. While during post-rainy season, available phosphorus showed non-significant results. The available potassium content of cropland and horticulture system increased by 7 and 2.2% during pre-rainy season and 4.6 and 1.5% during post-rainy season, respectively, compared with farm forestry system. The trend of DTPA extractable micronutrients (Fe, Mn and Cu) expect Zinc was recorded in the order of farm forest > horticulture > cropland. During pre-rainy season, the farm forestry system at surface layer showed the higher content of DTPA extractable Fe, Mn and Cu by 38.7, 22.7 and 51.6% under farm forest land use and 12.2, 15.2 and 28.3% under horticulture in comparison with cropland. However, the DTPA extractable Fe, Mn and Cu was reduced by 39.3, 26.2 and 56.1% under cropland and 31, 11 and 24.09% under horticulture system compared with farm forest system, respectively. The DTPA extractable Zn was declined by 9.7 and 11.4% under horticulture and 2.6 and 1.03% under cropland as compared to farm forest system during post- and pre-rainy seasons, respectively. Seasonally, during post-rainy season, the SOC, CEC, available P and available K were higher by 10, 7.14, 21.4 and 5.9% under farm forest, 14, 13.2, 14.7 and 5.3% under horticulture and 7.1, 13.1, 18.2 and 3.5% under cropland, respectively, than to the pre-rainy season. Similarly, the post-rainy season had higher DTPA extractable Fe, Mn, Zn and Cu by 9.7, 7.5, 24.1 and 13.18% under farm forest, 3.2, 2.8, 22.2 and 7.8% under horticulture and 9.2, 4.6, 25 and 10% under cropland than pre-rainy season, respectively. Depth-wise, all the SOC, CEC, available phosphorus, available potassium and DTPA extractable Fe, Mn, Zn and Cu decreased gradually with increase in soil depth (Tables 2, 3, 4, 5).
Rhizospheric effect in different land uses, seasons and microbiological properties
Soil microbiological properties significantly (p < 0.05) varied under different land use systems and seasons. The bacteria, fungi and actinomycetes count were observed highest under farm forest followed by horticulture and cropland (Fig. 2). During pre-rainy season, the farm forest system had highest mean microbial count, i.e. 10.26 × 104, 4.57 × 104 and 3.09 × 103 cfu g−1 of bacteria, fungi and actinomycetes, respectively. Similar trend was followed during post-rainy season. However, higher microbial count was observed during post-rainy season than pre-rainy season due to high moisture content. The basal soil respiration (BSR) (Table 6) significantly varied under different land uses and seasons. The mean BSR was found highest in farm forest followed by horticultural and cropland systems in both the seasons. The observed mean values in pre-rainy season were 32.54 > 23.26 > 20.70 μg CO2-C g−1 day−1 in farm forest, horticulture and cropland, respectively. The BSR values recorded during post-rainy season were 40.78 (farm forest) > 29.36 (horticulture) > 23.91 (cropland) μg CO2-C g−1 day−1. The MBC was significantly higher in farm forest land use (184.2 μg g−1 soil) followed by horticulture (126.2 μg g−1 soil) and cropland (123.3 μg g−1 soil) during pre-rainy season. However, during post-rainy season, relatively higher MBC was observed and trend followed a decreasing order, i.e. farm forest (252 μg g−1 soil) > horticulture (158.5 μg g−1 soil) > cropland (142 μg g−1 soil). The DHA activity (Fig. 3) varied significantly among different land uses. During pre-rainy season, the highest DHA was recorded in farm forest (26.1 μg TPF g−1 h−1) > cropland (14.7 μg TPF g−1 h−1) > horticulture (13.4 μg TPF g−1 h−1). During post-rainy season, similar trend was followed among land uses. However, seasonally DHA values were found higher in post-rainy season than pre-rainy season. The alkaline phosphatase activity significantly varied among three land uses and in both the seasons. The highest alkaline phosphatase activity was recorded in post-rainy season as compared to pre-rainy season. The observed mean values in pre-rainy season were 59.1, 28.7 and 25.9 μg pNP g−1 h−1 in farm forest, horticulture and cropland, respectively. While in post-rainy season, mean values were 71.2 > 49.1 > 35.2 μg pNP g−1 h−1 in farm forest, horticulture and cropland system, respectively. The microbial quotient was recorded higher in farm forest land use system (0.026) followed by horticulture (0.024) and least in cropland (0.023) during pre-rainy season. Higher values were recorded in post-rainy season, and similar trend was followed as in pre-rainy season, i.e. farm forest (0.032) > horticulture (0.028) > cropland (0.025). On the other hand, the metabolic quotient trend observed during both seasons was horticulture > cropland > farm forest. The values observed under horticulture, cropland and farm forest were 0.184, 0.170 and 0.165 (μg CO2-C day−1 μg biomass C−1), respectively, during pre-rainy season and 0.173, 0.170 and 0.162 (μg CO2-C day−1 μg biomass C−1), respectively, during post-rainy season.
Effect of different land use systems on dehydrogenase activity and alkaline phosphatase activity in rhizosphere soil at 0–15 and 15–30 cm depths during pre-rainy and post-rainy season. Line bars indicate standard error of mean. For a given depth, the values indicated by same small letters are not significantly different (p = 0.05) by DMRT
Principle component analysis
The principle component analysis (PCA) was conducted to simplify the complexity of high dimensional data while maintaining trends and patterns. In our study, we used PCA to analyse the degree of correlation between various soil properties under different land use systems and seasons. The PCA acquiring higher eigenvalues and higher factor loading variables was claimed as the best variable that represent system attributes (Brejda et al. 2000). The biplot shows the position of soil variables (Fig. 4), different sub-land use systems and seasons, in orthogonal space determined by two PCs, which explained 86.55% of total variability. The distribution of PC1 and PC2 demonstrated variability of 69.10 and 17.44%, respectively. The PC1 indicates that all the soil variables were positively correlated except K and showed 69.10% variability with eigenvalue 10.37. Under PC1, SOC (9.01%) was recorded as the highest factor loading and contributing variable. However, PC2 demonstrated 17.44% variability with eigenvalue 2.62 and SOC, Fe, Mn, Cu, bacteria and fungi were negatively correlated with other soil variables. Under PC2, the available K was the highest factor loading and was the highest (32.05%) contributing variable (Table 7).
Biplot graph of assayed variable in rhizosphere region at surface layer of soil. S1 pre-rainy season, S2 post-rainy season, OC organic carbon, CEC cation exchange capacity, P available phosphorus, K available potassium, Fe DTPA extractable iron, Mn DTPA extractable manganese, Zn DTPA extractable Zinc, Cu DTPA extractable copper, Actino actinomycetes, BSR basal soil respiration, DHA dehydrogenase activity and Alk_P alkaline phosphatase
Discussions
Physical properties
This study showed that farm forest had significantly lower bulk density than horticulture and cropland. However, total porosity was recorded higher under farm forest system. Lower bulk density in farm forest system was due to continuous availability of organic matter input through leaf litter attained from farm forest and horticulture trees, which is more than cropland system. Probably, the loss of soil organic matter combined with greater sand content and poorer aggregation; also trampling by grazing livestock in the surface layer resulted in higher bulk density under the natural forests. On the other hand, increase in bulk density with increase in depth could be explained on the basis of reduction in organic matter content and aggregation stability of soil that might be responsible for soil compaction in lower depths of soil (Stockfisch et al. 1999; Chauhan et al. 2019). Zolfaghari and Hajabbasi (2008) demonstrated that forest conversion into agricultural land was resulted in significant increase of bulk density from 1.19 and 1.3 Mg cm−3. No significant differences were observed in particle density. It could be due to the reason that particle density is independent of different land uses as it is regarded as a function of soil mineralogy (Igwe 2001). The highest porosity in farm forest soils may be attributed to higher organic matter availability in the soil. The root system also has direct influence on porosity percent, i.e. the dense root system of trees is not only responsible for aggregation of soil but also improves soil aeration that provides favourable conditions to soil fauna.
Chemical properties
Soil chemical properties significantly varied among different land uses. The lower SOC content in cropland may be attributed to crop uptake exacerbated due to continuous cropping and soil tillage that lead to breakdown of organic residues that resulted in more accessibility to the microbial attack (Chandel and Hadda 2018). However, decrease in SOC with increase in depth was observed in all land uses, which might be due to deposition of litter fall and residues. The highest SOC content under farm forest as compared to other land uses might be due to luxuriant vegetation. The post-rainy season showed higher SOC content because of a low temperature, lack of soil disturbance that would expose the organic matter to oxidation (Asima et al. 2020). The low-temperature conditions favoured slow rate of decomposition that contributed to the larger quantity of soil organic carbon level (Paul et al. 2011; Dadhwal et al. 1997; Singh and Munth 2013). Although, the decomposition rate, carbon inputs and quality of litter during post-rainy increase the carbon content of soil (Perrott et al. 1990; Zhao et al. 2009).Thus, increase in humus/organic matter content could be held responsible for increase in the negative charge of organic colloid of soil that led to increase in CEC of forest as compared to horticulture and cropland (Dutta et al. 2011). The depletion of SOC under cropland could be due to intensive cropping contributed to the reduction of CEC of soils. The post-rainy season was found to have higher CEC due to higher accumulation of organic matter during this season. Seasonal changes of this kind are probably related to decrease in soil temperature and moisture, which limit an intensity of abiotic mineralization during autumn and winter seasons (Dłużewski et al. 2019). Additionally, we cannot exclude influence of increasing inflow of fresh organic matter in the end of summer and autumn periods (Prusinkiewicz et al. 1974). Guoju et al. (2012) studied the relationship between winter temperature rises and soil fertility properties and results showed that the post-rainy temperature rise led to increased soil organic matter and available P content. Schlecht et al. (2006) suggested that organic matter has been identified as a store house of cations. Relatively higher available phosphorus and potassium content was observed under cropland as compared to farm forest and horticulture, which could be ascribed to the continuous application of fertilisers—P and K. (Chandel et al. 2018). Higher levels of phosphorus and potassium were recorded in rhizosphere region than bulk soil due to more microbial activity and root secretions (Qureshi et al. 2012; Toberman et al. 2011). The reason behind the higher amount of micronutrient in farm forest system may be same as in macronutrients (N, P and K), i.e. more addition of organic matter through litter fall and greater root biomass of farm forest trees. These results revealed that rhizosphere region influences the micronutrient availability in these soils. Moreover, soil organic matter enhances soil aeration and protect micro-nutrients against oxidation and precipitation and supply chelating agents, thereby increasing micro-nutrients’ availability in soils (Singh et al. 2000; Dhaliwal and Dhaliwal 2019).
Microbiological properties
The farm forest system has more ability to develop rhizosphere effect due to variation in root exudates, soil characteristics and presence of specialized rhizoflora than disturbed land use system, therefore showed higher count of bacteria, fungi and actinomycetes under farm forest system as compared to horticulture and cropland. The reason may be the presence of sufficient organic matter in terms of exudates, mucilage, favourable pH and moisture conditions that led to the establishment and colonization of root exudates secretions and prevailing congenial edaphic and climatic conditions (Niemia et al. 2007). The higher microbial activities in autumn as compared to spring could be ascribed to the enrichment of upper horizons with fresh litter (Siles and Margesin 2016), rainfall and temperature regimes (Jha et al.1992). The BSR significantly varied under different land uses and seasons. The mean BSR was higher in farm forest system. Wang et al. (2007) stated that conversion of farm forest into cropland showed 33% decline in soil respiration. Various management practices like tillage, fertilisers, etc., have a great influence in alterations of SOC so as soil respiration (Li et al. 2012; Tian et al. 2013). Lack of fresh input of organic matter may be responsible for lower microbial biomass carbon content in cropland, which resulted in lower substrate availability (C) essential for microorganisms and causes poor microbial growth (Fontaine et al. 2007). It has also been suggested by Piao et al. (2001) that MBC and SOC varied to great extent in pre-rainy and post-rainy season, which indicated that temperature had a great influence on the organic matter decomposition in soils that is directly associated with its effects on microbial biomass carbon. The seasonal fluctuations showed higher microbial biomass carbon during post-rainy, possibly some of the organic carbon released after the death of microbial biomass was converted to CO2 (Piao et al. 2000). The increased DHA content in surface soil of farm forest could be attributed to the greater availability of nutrients, soluble organic carbon and increased microbial activity (Adak et al. 2014). The higher alkaline activity observed under farm forest system may be attributed to the continuous recycling of litter fall and root exudates resulting in higher organic matter content and microbial biomass carbon, which serves as a principle source of enzyme activities in soil (Tabatabai 1994; Chauhan et al 2018). The higher activity of enzymes during post-rainy season may be due to beneficial effect of lower soil temperature and higher soil moisture by the microorganisms that in turn is directly correlated to the dehydrogenase and alkaline phosphatase activities. The gradual decrease in enzyme activities with soil depth may be due to increased soil microbial abundance and organic matter content at shallow soil layers. Skujins (1976) stated that phosphatase enzymes were adaptive enzymes that depend on the intensity of the plant root excretions and microbial population was apparently determined on the basis of their requirement for phosphates. Therefore, it has been suggested that soil with greater root biomass tends to possess the higher activity of phosphatases enzyme by soil microorganisms (Tabatabai 1994). In soil, phosphatase enzyme catalyses the hydrolysis of organic phosphorus to inorganic phosphorus (Nannipieri et al. 2011) and is an index to evaluate the biological conversion direction and intensity for soil (Guoju et al. 2012). Higher enzyme activity was recorded during post-rainy season could possibly be due to the addition of more complex C substrates, which significantly enhanced the enzyme activity. Wallenstein et al. (2009) demonstrated seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils and resulted in higher enzymatic activity during post-rainy. Dinesh et al. (2010) who observed that variation in activities of enzymes among the tree species was due to the relationship between MBC and SOC. Microbial quotient (qMIC) is a useful index for SOM changes as a result of changes in land management practices (Ross et al. 1982; Hart et al 1989). When microbial biomass is under stress, its potential to convert SOC to microbial biomass decreases for lower qMIC level. In contrast, higher values of qMIC signifies more favourable conditions for growth of microorganisms that are facilitated by the supply of good quality organic matter (Souza et al. 2015). The lower qMIC values indicate reduced microbial activity and inhibition of soil microbes (Cunha et al. 2012). The cropland system had comparatively lower values for microbial quotient due to continuous tillage practices, which leads to accelerated microbial decomposition of organic matter (Sharma et al. 2014). In these systems, low levels of fresh organic matter and labile C substrates result in poor growth of microbes (Fontaine et al. 2007). Geraei et al. (2016) found the highest values of microbial quotient in pasture land (3.3%), followed by native forests (2.9%), pastures converted to arable land (2.7%), and least in forests converted to arable land (2.0%), respectively. Metabolic quotient (qCO2) is the respiration per unit of microbial biomass; low values indicate stable and mature systems, because energy optimization occurs as the systems mature (Wang et al. 2005). A study conducted by Novak et al. (2017) demonstrated that native vegetation areas showed lower values of qCO2, whereas sugarcane fields had higher qCO2 due to higher consumption of carbon by the microbial community. The higher values of qCO2 may be associated with the modified soil structure by the applied management system. Higher qCO2 values suggested that imbalance of microbial community, change in metabolism of microorganisms and ecosystems subjected to stress conditions (Anderson and Domsch 1993; Santos et al. 2015).
Principle component analysis
The biplot graph (Fig. 4) of PCA among various soil variables revealed that there was a high correlation between the variables that were proximate to each other. The soil properties were highly influenced by bamboo, dek, poplar, mango, guava and aonla of post-rainy season. The PCA study recorded that SOC and available K were the sensitive indicators towards PC1 and PC2, respectively (Table 7). Chandel et al. (2018) performed PCA for assessing soil quality index under different land uses of sub-montane Punjab and observed that SOC contributed maximum to soil quality. Among different land use systems, forest and grassland had highest values for soil quality index. Therefore, importance of soil organic matter in rainfed condition may be due to changes in weather conditions. Irregular rainfall distribution with time and space, poor socioeconomic status of farmers, lack of awareness, undulating slopes and improper soil conservation practices resulting in maintaining low organic matter levels, which in turn, resulted in declining soil quality and health in this region. Nair (1984) demonstrated that agroforestry, agro-horticulture and grassland systems have more ability to prevent run off and erosion and maintenance of better soil organic matter that leads to improved soil fertility status. Sharma et al. (2004) reported that potassium as key indicator contributed 17% towards soil quality index. It could be attributed to importance of potassium to play a key role in osmotic regulations and provide osmotic pull to draw water into the plant roots. As the deficiency of potassium may cause stomata malfunctioning, it may lead to lower photosynthates and inefficient utilization of water, which is not a required feature for moisture stress conditions in dryland regions.
Conclusion
Farm forestry system (bamboo, dek and poplar) followed by horticulture (mango, guava and aonla) had a significant positive effect on rhizospheric soil properties. Post-rainy season had higher microbial activity and nutrient availability due to increased SOC content during this season. The PCA revealed that SOC and available K are the most sensitive indicators with higher eigen and factor loading variable that provide guidelines to differentiate the most sustainable land use systems (e.g. farm forestry and horticulture system) at sub-montane region of India. Since different land uses play an important role in soil dynamics, proper strategies like the introduction of forest trees in cropland may be more beneficial because nutrients accessed by forest trees from a deeper region would potentially enhance the nutrient availability to crops for enhanced crop yield. Therefore, in order to sustain soil fertility, it is necessary to understand the effects of rhizosphere under different land use systems in terms of nutrient cycling and maintaining soil quality.
References
Adak T, Kumar K, Singha A, Shukla SK, Singh VK (2014) Assessing soil characteristics and guava orchard productivity as influenced by organic and inorganic substrates. J Anim Plant Sci 24:1157–1165
Anderson JPE (1982) Soil respiration. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2. 2nd edn. Chemical and microbiological properties. Agronomy Monograph no. 9, ASA-SSSA Publisher, Madison, Wisconsin, USA, pp 831–871
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Asima M, Sharma V, Sharma S (2020) Assessment of soil carbon and biochemical indicators of soil quality under rainfed land use systems in north eastern region of Punjab India. Carbon Manag 11:169–182
Balser TC, Wixon D, Moritz LK, Lipps L (2010) The microbiology of natural soils. In: Dixon GR, Tilston EL (eds) Soil microbiology and sustainable crop production. Springer, Berlin, pp 27–58
Bezemer TM, Lawson CS, Hedlund K, Edwards AS, Brooks AJ, Lgual JM (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant-soil feedback responses in two grasslands. J Ecol 94:893–904
Blake GR (1965) Bulk density. In: Black CA (ed) Methods of soil analysis. Agronomy part I No. 9. American Society of Agronomy, Madison, Wisconsin, USA, pp 374–390
Brejda JJ, Moorman TB, Karlen DL, Dao TH (2000) Identification of regional soil quality factors and indicators. I. Central and Southern high plains. Soil Sci Soc Am J 64:2115–2124
Chandel S, Hadda MS (2018) Soil loss tolerance assessment under different land uses in submontane Punjab. J Soil Water Conserv 17:303–310
Chandel S, Hadda MS, Mahal AK (2018) Soil quality assessment through minimum data set under different land uses of submontane Punjab. Commun Soil Sci Plant 49:658–674
Chauhan SK, Singh S, Sharma S, Vashist BB, Sharma R, Saralch HS (2018) Soil health (physical, chemical and biological) status under short rotation tree plantations on riverain soils. J Pharmacogn Phytochem 7:1599–1605
Chauhan SK, Sidhu MS, Sharma S (2019) Soil properties under fresh and replanted poplar (Populus deltoides Bartr. ex Marsh.) sites. J Tree Sci 38:52–60
Cheema HS, Singh B (1990) CPCS1—a computer program package for the analysis of commonly used experimental designs. Punjab Agricultural University, Ludhiana, India
Cunha E, Stone LF, Ferreira EPB, Didonet AD, Moreira JAA (2012) Atributosfísicos, químicos e biológicos de solo sob produção orgânica impactada por sistemas de cultivo. Revista Brasileira de Engenharia Agrícola e Ambiental 16:56–63
Dadhwal VK, Shukla N, Vora AB (1997) Forest litterfall in India: a review and an estimate of litterfall carbon flux. Ind For 123:45–52
Debnath S, Patra AK, Ahmed N, Kumar S, Dwivedi BS (2015) Assessment of microbial biomass and enzyme activities in soil under temperate fruit crops in north western Himalayan region. J Soil Sci Plant Nutr 15:848–866
Dhaliwal MK, Dhaliwal SS (2019) Impact of manure and fertilizers on chemical fractions of Zn and Cu in soil under rice-wheat cropping system. J Indian Soc Soil Sci 67:85–91
Dhingra OD, Sinclair JB (1993) Basic plant pathology Methods. CBS Publishers and Distributors, Delhi, India. Indian Phytopathol 59:368–369
Dinesh R, Srinivasan V, Hamza S, Parthasarathy VA, Aipe KC (2010) Physico-chemical, biochemical and microbial properties of the rhizospheric soils of tree species used as supports for black pepper cultivation in the humid tropics. Geoderma 158:252–258
Dłużewski P, Wiatrowska K, Kozłowski M (2019) Seasonal changes in organic carbon content in post-arable forest soils. Soil Sci Annu 70:3–12
Dongmo JC, Oyeyiola GP (2006) Fusaria in the rhizosphere and rhizoplane of groundnut (Arachis hypogaea). J Agric Res Dev 5:153–164
Dutta SK, Singh D, Sood A (2011) Effect of soil chemical and physical properties on sorption and desorption behaviour of lead in different soils of India. Soil Sediment Contam 20:249–260
Fontaine S, Barot S, Barre P, Bdioui N, Mary B, Rumpel C (2007) Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 450:277–280
Geraei DS, Hojati S, Landi A, Cano AF (2016) Total and labile forms of soil organic carbon as affected by land use change in southwestern Iran. Geoderma 7:29–37
Grayston SJ, Wang S, Campbell CD, Edwards AC (1998) Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol Biochem 30:369–378
Guoju X, Qiang Z, Jiangtao B, Fengju Z, Chengke L (2012) The relationship between winter temperature rise and soil fertility properties. Air Soil Water Res 5:85–99
Hart PBS, August JA, West AW (1989) Long-term consequences of topsoil mining on select biological and physical characterizes of two New Zealand loessial soils under grazed pasture. Land Degrad Dev 1:77–88
Hiltner L (1904) Uber neuereerfahrungen und probleme auf demgebiet der bodenbakleriologie und unterbcsondererberücksichligung der grúndúngung und brache. Arb Dtsch Landwirl Ces 98:59–78
Hilton S, Bennett AJ, Chandler D, Mill P, Bending GD (2018) Preceding crop and seasonal effects influence fungal, bacterial and nematode diversity in wheat and oilseed rape rhizosphere and soil. Appl Soil Ecol 126:34–46
Igwe CA (2001) Effects of land use on some structural properties of an Ultisol in south-eastern Nigeria. Int Agrophys 15:237–241
Jackson ML (1967) Soil chemical analysis. Prentice Hall of India. Pvt. Ltd., New Delhi, pp 38–56
Jha DK, Sharma GD, Mishra RR (1992) Soil microbial population numbers and enzyme activities in relation to altitude and forest degradation. Soil Biol Biochem 24:761–767
Li CF, Zhou DN, Kou ZK, Zhang ZS, Wang JP, Cai ML (2012) Effects of tillage and nitrogen fertilizers on CH4 and CO2 emissions and soil organic carbon in paddy fields of central China. PLoS ONE 7:1–9
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test method for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Marschner P, Baumann K (2003) Changes in bacterial community structure induced by mycorrhizal colonisation in split-root maize. Plant Soil 251:279–289
Merwin HD, Peech M (1950) Exchangeability of soils potassium in the sand, silt and clay fractions as influenced by the nature of the complementary exchangeable cations. Soil Sci Soc Am Proc 15:125–128
Miethling R, Wieland G, Backhaus H, Tebbe CC (2000) Variation of microbial rhizosphere communities in response to crop species, soil origin and inoculation with Sinorhizo biummeliloti L33. Microbial Ecol 40:43–56
Nair P (1984) Role of trees in soil productivity and conservation. Soil productivity aspect of agroforestry. The International Council for Research in Agroforestry, Nairobi, p 85
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action. Springer, Berlin, Heidelberg, pp 215–243
Niemi RM, Vepsalainen M, Erkomaa K, Llvesniemi H (2007) Microbial activity during summer in humus layers under Pinus silvestris and Alnusincana. For Ecol Manag 242:314–323
Nihorimbere V, Ongena M, Smargiassi M, Thonart P (2011) Beneficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol Agron Soc Environ 15:327–337
Novak E, Carvalho LA, Santiago EF, Portilho IIR (2017) Chemical and microbiological attributes under different soil cover. Cerne 23:19–30
Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus by extraction with sodium bicarbonate. United States Department of Agriculture Circular 939 Washington, DC
Panwar P, Mishra PK, Tiwari AK, Bhatt VK, Pal S (2017) Study on conservation of subsoil water in Shivalik hills of Punjab. Consultancy Funded by Department of Forest and Wildlife, Government of Punjab. Printed by ICAR-Indian Institute of Soil and Water Conservation, Research Center, Chandigarh
Paul EA, Follett RF, Haddix M, Pruessner E (2011) Soil N dynamics related to soil C and microbial changes during long-term incubation. Soil Sci 176:527–536
Perrott K, Sarathchandra S, Waller J (1990) Seasonal storage and release of phosphorus and potassium by organic matter and the microbial biomass in a high producing pastoral soil. Aust J Soil Res 28:593–608
Piao HC, Hong YT, Yuan ZY (2000) Seasonal changes of microbial biomass carbon related to climatic factors in soils from karst areas of southwest China. Biol Fertil Soils 30:294–297
Piao HC, Liu GS, Wu Y, Xu WB (2001) Relationships of soil microbial biomass carbon and organic carbon with environmental parameters in mountainous soils of southwest China. Biol Fertil Soils 33:347–350
Prescott L, Harley J, Klein DA (1999) Microbiology. Mc Graw-Hill, Boston, pp 962–963
Prusinkiewicz Z, Dziadowiec H, Jakubusek M (1974) Return to soil of elements—biogens with leaf fall in deciduous and mixed forest on loose sand soils. Soil Sci Ann 25:237–245
Qureshi MA, Ahmad ZA, Akhtar N, Iqbal A, Mujeeb F, Shakir MA (2012) Role of phosphate solubilizing bacteria (PSB) in enhancing P availability and promoting cotton growth. J Anim Plant Sci 22:204–210
Ros M, Klammer S, Knapp B, Aichberger K, Insam H (2006) Long term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag 22:209–218
Ross DJK, Tate R, Cairus A, Mayricbt KF, Pursic EA (1982) Restoration of pasture after topsoil removal: effect of soil carbon and nitrogen mineralization, microbial biomass and enzyme activities. Soil Biol Biochem 14:575–581
Santos CA, Krawulski CC, Bini D, Filho TG, Knob A, Medina CC, Filho GA, Nogueira MA (2015) Reclamation status of a degraded pasture based on soil health indicators. Sci Agric 72:195–202
Schlecht E, Hiernaux P, Kadaoure I, Hulsebusch C, Mahler F (2006) A spatiotemporal analysis of forage availability, grazing and excretion behaviour of cattle, sheep and goats in Western Niger. Agric Ecosyst Environ 113:226–242
Sharma P, Rai SC, Sharma R, Sharma E (2004) Effects of land use change on soil microbial C, N and P in a Himalayan watershed. Pedobiologia 48:83–92
Sharma V, Hussain S, Sharma KR, Arya VM (2014) Labile carbon pools and soil organic carbon stocks in the foothill Himalayas under different land use systems. Geoderma 232:81–87
Siles JA, Margesin R (2016) Abundance and diversity of bacterial, archaeal and fungal communities along an altitudinal gradient in alpine forest soils: what are the driving factors? FEMS Microbiol Ecol 72:207–220
Singh PK, Munth H (2013) Comparative study of physico-chemical, nutrients availability and acidic properties of Arunachal Pradesh soil under different land use systems. Asian J Soil Sci 8:457–462
Singh Y, Singh B, Meelu OP, Khind CS (2000) Long-term effects of organic manuring and crop residues on the productivity and sustainability of rice–wheat cropping system in Northwest India. Long-term soil fertility experiments in rice–wheat cropping systems. Consortium Paper Series 6:149–162
Skujins J (1976) Extracellular enzymes in soil. Crit Rev Microbial 4:383–421
Souza RF, Brasil EPF, Figueiredo CC, Leandro WM (2015) Soil microbial biomass and activity in wetlands located in preserved and disturbed environments in the Cerrado biome. Biosci J 31:1049–1061
Stockfisch N, Forstreuter T, Ehlers W (1999) Ploughing effects on soil organic matter after twenty years of conservation tillage in Lower Saxony, Germany. Soil Till Res 52:91–101
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Part 2, 2nd edn. Agron 9. American Society of Agronomy, Madison, WI, pp 903–947
Tabatabai MA (1994) Soil enzymes In: Weaver RW, Angle JS and Bottomley PS (eds) Methods of soil analysis: microbiological and biochemical properties, Part 2: SSSA Book Ser 5, SSSA, Madison, WI, pp 775–883
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tian SZ, Wang Y, Ning TY, Zhao HX, Wang BW, Li N (2013) Greenhouse gas flux and crop productivity after 10 years of reduced and no tillage in a wheat-maize cropping system. PLoS ONE 8:1–10
Toberman H, Chen C, Xu Z (2011) Rhizosphere effects on soil nutrient dynamics and microbial activity in an Australian tropical lowland rainforest. Soil Res 49:652–660
Van Leeuwen JP, Djukic I, Bloem J, Lehtinen T, Hemerik L, de Ruiter PC, Lair GJ (2017) Effects of land use on soil microbial biomass, activity and community structure at different soil depths in the Danube floodplain. Eur J Soil Biol 79:14–20
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem 19:703–707
Walkley A, Black CA (1934) An examination of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci 37:1367–1378
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15:1631–1639
Wang H, Huang Y, Huang H, Wang KM, Zhou SY (2005) Soil properties under young Chinese fir-based agroforestry system in mid-subtropical China. Agrofor Syst 64:131–141
Wang XG, Zhu B, Wang YQ, Zheng XH (2007) Soil respiration and its sensitivity to temperature under different land use conditions. Acta Ecol Sin 27:1960–1968
Xue D, Yao H, Huang C (2006) Microbial biomass, N mineralization and nitrification, enzyme activities and microbial community diversity in tea orchard soils. Plant Soil 288:319–331
Zhao Q, Zeng D, Fan Z, Yu Z, Hu Y, Zhang J (2009) Forest ecology and management seasonal variations in phosphorus fractions in semi-arid sandy soils under different vegetation types. For Ecol Manag 258:1376–1382
Zolfaghari AA, Hajabbas MA (2008) The occurrence of soil water repellency under different vegetation and land uses in central Iran. J Environ Sci Technol 1:175–180
Zuberer D (1994) Recovery and enumeration of viable bacteria. In: Weaver RW, Angle JS, Bottomlyy PJ (eds) Methods of soil analysis part 2: microbial and biochemical properties. Soil Society of American Books, Madison, pp 119–144
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neha, Bhople, B.S. & Sharma, S. Seasonal variation of rhizospheric soil properties under different land use systems at lower Shivalik foothills of Punjab, India. Agroforest Syst 94, 1959–1976 (2020). https://doi.org/10.1007/s10457-020-00512-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-020-00512-7