Abstract
The effects of the interaction between arbuscular mycorrhizal and phosphate-solubilizing (P-solubilizing) fungi on phosphorous availability, acid phosphatase activity, and the growth and development of coffee plants (Coffea arabica L.) var. garnica were evaluated. The experiment was performed under controlled conditions and was based on a randomized factorial design with two factors. Coffee plants were inoculated with a consortium of arbuscular mycorrhizal fungi (CAMF), two strains of P-solubilizing fungi (PSF) (Aspergillus niger [An] and Penicillium brevicompactum [Pb]), the possible combinations of the latter fungi, and an uninoculated control. After 8 months, the results demonstrated the effectiveness of mycorrhizal and P-solubilizing fungal inoculations in increasing available soil phosphorous. The greatest concentration of available soil phosphorous was detected in the consortium of P-solubilizing fungi (CPSF) treatment at 3.8 mg/kg. The total foliar phosphorous concentration of plants was higher in the CAMF, An + CAMF, CPSF + CAMF, Pb + CAMF, and CPSF treatments in comparison to the control treatment. The growth of coffee plants was also favored by the consortium treatments (P-solubilizing fungi and arbuscular mycorrhizal fungi). The acid phosphatase activity in the rhizosphere significantly increased under the CPSF treatment and also increased in the roots of coffee plants under the An, An + CAMF, and CPSF + CAMF treatments. Given the importance of fungal groups for processes of phosphorous transformation and absorption in coffee plants, it is imperative to continue the search for native fungal strains with high potential for use as biofertilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coffee is one of the most important products of agricultural origin that is commercialized at the international level. During the 2015–2016 agricultural cycle, 72.1% of worldwide coffee production was concentrated in five countries: Brazil (32.2%), Vietnam (19.1%), Colombia (8.9%), Indonesia (7.7%), and Ethiopia (4.2%). Additional producers include Honduras (3.7%), India (3.5%), and Peru (2.3%). Mexico is located in tenth position and contributes 1.6% of production (FIRA 2016). The coffee-growing industry of Mexico represents an important activity for the agricultural sector because of its production value and its capacity to generate income for local farmers. In addition, coffee cultivation provides environmental benefits given that 99% of coffee plantations in Mexico are grown under shade trees or forest canopies.
Shaded coffee plantations are agroecosystems that host an important diversity of native plants and animals (Manson et al. 2008). Various studies have shown that, in contrast to other agricultural uses, the cultivation of shaded coffee, especially under rustic management systems, conserves elements of the vegetal structure of cloud forests where coffee in grown. Therefore, shaded coffee plantations serve as corridors or shelters for various organisms, such as birds (Tejeda-Cruz and Gordon 2008), ants (Valenzuela-González et al. 2008), bats (Sosa et al. 2008), amphibians, reptiles (González-Romero and Murrieta-Galindo 2008), ferns (Mehltreter 2008), vascular epiphytes (García-Franco and Toledo 2008), and saprobic and mycorrhizal fungi (Heredia and Arias 2008; Arias et al. 2012; Arias and Heredia 2014).
However, despite its economic relevance for many countries, the coffee sector has been immersed in recurrent crises mainly as a result of the fall in coffee prices in the international market. One of the most severe crisis in the last century occurred from 1998 to 2004 (Escamilla et al. 2015). Several production alternatives have been developed and promoted in order to overcome this challenge and to promote coffee cultivation as a central axis of community and regional development. Among these alternatives, the most successful have been the production and commercialization of organic coffee and certified fair trade coffee (Roozen and VanderHoff 2002; Pohlan 2002; Sosa et al. 2004), as these products command the best prices and buying conditions in the conventional market (Moguel and Toledo 2004).
In this context, the use of environmentally friendly technologies to fulfill the nutrient requirements of coffee plants is important for the development and the promotion of organic production systems to decrease or eliminate the application of chemical fertilizers. In this context, phosphate-solubilizing (P-solubilizing) fungi (PSF) can contribute to a greater availability of P in soil, and this P can then be directly absorbed by the roots of coffee plants or the hyphae of arbuscular mycorrhizal fungi (AMF) (Van Der Heijden et al. 2008). The interaction of AMF and PSF is particularly important for plants and soil fertility (Jeffries and Barea 2001). Several studies have found these interactions to positively affect different plant systems, for example, those of clover (Souchie et al. 2006), bamboo (Babu and Reddy 2011), green gram (Khan and Zaidi 2006), cashew (Rodrigues-Cabral et al. 2012), avocado (Serna 2013), and chili pepper (Castillo et al. 2013). However, other studies have reported negative or neutral effects on plant systems following inoculations of fungal consortia (McAllister et al. 1995; Gryndler et al. 2002).
The objective of this study was to evaluate the effects of the interaction of AMF and PSF (individually and in consortia) on P availability for coffee plants and on the growth of coffee plants under controlled conditions.
Materials and methods
Coffee plants (Coffea arabica L.) var. garnica in the seedling stage were supplied by Amsa Group (Mexico). Seedlings were transplanted to pots with a 400-g capacity containing a soil-perlite (1:1 ratio) substrate. Each component was sterilized separately in an autoclave (Yamato SM510) at 120 °C using three cycles (1 h/cycle). Soil was obtained from the Conecalli coffee plantation in the municipality of Xalapa, Veracruz (lat.: 19°30′41.09″N, long.: 96°56′25.93″W). Soil P content was estimated at 1.98 mg/kg. Soil chemical analyses were carried out by the Laboratory of Soil, Water, and Plant Chemical Analysis (Laboratorio de Análisis Químico de Suelos, Agua, and Planta [LAQSAP]) of the Ecology Institute (Instituto de Ecología, A.C. [INECOL]); the results are detailed in Table 1.
The strains used for the PSF of Aspergillus niger Tiegh (HS165) and Penicillium brevicompactum Dierckx (HS42) were deposited in the Collection of the Mycromycetes Laboratory of the Ecology Institute. These strains originated from coffee plantation soil in the central region of the state of Veracruz, Mexico, and were selected for their high capacity to solubilize tricalcium phosphate in vitro. The AMF consortium was obtained from the collection of the Laboratory of Beneficial Organisms (Laboratorio de Organismos Benéficos) of the Faculty of Agricultural Sciences (Facultad de Ciencias Agrícolas) of the University of Veracruz (Universidad Veracruzana). This consortium was also isolated from coffee plantation soil in the central region of the state of Veracruz. The AMF inoculum contained 60 spores/g of substrate, had a colonization rate of 79%, and was composed of the species Acaulospora morrowiae Spain and N.C. Schenck, A. spinosa C. Walker and Trappe, A. scrobiculata Trappe, Gigaspora rosea T.H. Nicolson and N.C. Schenck, Scutellospora pellucida (T.H. Nicolson and N.C. Schenck) C. Walker and F.E. Sanders, Glomus macrocarpum Tul. and C. Tul., Funneliformis mosseae (T.H. Nicolson and Gerd.) C. Walker and A. Schüßler, F. geosporum (T.H. Nicolson and Gerd.) C. Walker and A. Schüßler, and Rhizophagus aggregatus (N.C. Schenck and G.S. Sm.) C. Walker.
A completely randomized design was established in 4 × 2 factors schemes (four inoculation treatments and presence or absence of AMF), and three repetitions were used. The inoculation treatments were as follows: (1) control non-inoculated; (2) CAMF consortium of arbuscular mycorrhizal fungi; (3) Pb P. brevicompactum; (4) Pb+CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi; (5) An A. niger; (6) An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi; (7) CPSF consortium of P-solubilizing fungi; (8) CPSF + CAMF consortium of P-solubilizing fungi + consortium of arbuscular mycorrhizal fungi.
Fungi were directly inoculated in the substrate on the root system using 10 g of the AMF consortium and 1 × 108 spores of PSF according to the treatments. Coffee plants were grown for 8 months in a bioclimatic chamber (TOP RTOP-430D) with a photoperiod of 12 h, medium light intensity, and 50% relative humidity. Sterile water was supplied by capillary irrigation and was supplemented weekly with 25 ml of Hewitt nutrient solution without P. Several variables were evaluated after the incubation time concluded and are described at following.
Mycorrhizal colonization
Roots were cleared and stained following the method of Phillips and Hayman (1970). Mycorrhizal colonization was quantified according to the method of Trouvelot (1986).
Determination of available soil phosphorus
Soil samples were taken from the rhizosphere of each treatment. The available P concentrations were quantified using the ascorbic acid reduction method (Bray and Kurtz 1945). Colorimetric reactions were measured in a spectrophotometer at 882 nm (Thermo Scientific GENESYS 10S UV–Vis).
Quantification of available foliar phosphorus
Samples of dry foliar material (0.25 g) were calcined in a furnace at 500 °C for 2 h, (Mckean 1993). Then, the P content of the samples was analyzed using the blue molybdenum method (Murphy and Riley 1962); the absorbance was measured at 660 nm.
Coffee plant growth variables
The height (cm) of the plants was measured from the root crown to the shoot apical meristem. The length of the roots (cm) was measured from the root crown to the root apical meristem. The foliar area was assessed using Image J 1.50 i® software. The dry weight of the aerial part of plants was determined with an analytical balance (RADWAG AS 220/C/2).
Determination of acid phosphatase activity
The acid phosphatase activity in the roots and the rhizosphere was assayed using p-nitrophenyl phosphate as the substrate (Tabatabai and Bremner 1969). To extract phosphatases from the roots, 50 mM Tris–HCl extraction buffer (pH 7.2) was used (Rodríguez et al. 2012). Spectrophotometer (Thermo Scientific GENESYS 10S UV Vis) readings were recorded at 412 nm.
Characterization of isoenzymatic patterns
The electrophoretic profiles were analyzed using polyacrylamide gels with the following modifications: 4% polyacrylamide stacking gel and 10% separating gel (Davis 1964). Electrophoresis was run with 19 mM Tris–Glycine buffer (pH 8.3) at 25 mA and 4 °C for 2 h in a vertical electrophoresis chamber (Coler-Parmer 28575-00). Each well was filled with 20 μg of protein (Rodríguez et al. 2012). The acid phosphatase activity was measured in 500 mM Citrate buffer (pH 5.5) with β naphthyl sodium phosphate and fast black K salt as a substrate. The gel was incubated at 37 °C in darkness with agitation (125 rpm) for 4 h.
Statistical analysis
A one-way ANOVA was performed to detect significant differences in the rate of mycorrhizal colonization among the different treatments. A two-way factorial ANOVA was also calculated to determine the effects of the interactions between the PSF and AMF factors on P availability in the rhizosphere, total foliar P, plant growth variables, and acid phosphatase activity. Post-hoc Tukey HSD tests were calculated when significant differences were found in the rate of colonization or the interaction effects. The data were analyzed in the STATISTICA 7.0 software.
Results
Mycorrhizal colonization
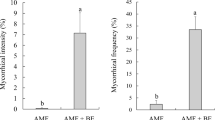
Significant differences were detected between the treatments (F = 19.99, p = 0.000449). The rate of mycorrhizal colonization ranged from 35 to 51.33%. The highest mycorrhization rate was detected in the CAMF and the Pb + CAMF treatments, while the An + CAMF and the CPSF + CAMF treatments had a significantly lower percentage of mycorrhizal colonization (t > 0.05). Mycorrhizal colonization in the plants of the control treatment was 0% (Fig. 1).
Mycorrhizal colonization of coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF) fungi. Control non-inoculated, CAMF consortium of arbuscular mycorrhizal fungi, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi. The data are the average of three repetitions ± standard deviation, shared letters in a column indicate no significant differences
Available soil phosphorus
The interaction between the AMF and PSF factors significantly affected available soil P (F = 224.50, p = 0.0001). All inoculated treatments (CAMF, Pb, Pb + CAMF, An, An + CAMF, CPSF, and CPSF + CAMF) presented significantly higher P values than the control treatment (t < 0.05); however, the available P in the CPSF treatment was significantly greater than in the rest of the treatments (CAMF, Pb, Pb + CAMF, An, An + CAMF, and CPSF + CAMF) (t < 0.05) (Fig. 2a).
a Available soil phosphorous and b Total foliar phosphorus in the rhizosphere of coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF) fungi. Control non inoculated, CAMF consortium of arbuscular mycorrhizal fungi, Pb P. brevicompactum, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An A. niger, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF consortium of phosphate-solubilizing fungi; CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi. The data are the average of three repetitions ± standard deviation, shared letters in a column indicate no significant differences
Total foliar phosphorus
The AMF-PSF interaction effect was significant for total foliar P (F = 51.51, p = 0.0001). The total foliar P concentrations of the treatments, with the exception of the An treatment, were significantly higher than the control treatment. Meanwhile, the consortium treatments (CAMF, An + CAMF, CPSF + CAMF, Pb + CAMF, and CPSF) resulted in significantly higher foliar P values in comparison with the individually inoculated treatments (Pb or An) (t < 0.05) (Fig. 2b).
Coffee plant growth
The evaluated plant growth variables were also significantly affected by the interaction between the AMF and PSF factors (height: F = 6.180, p = 0.0054; root length: F = 15.074, p = 0.000064; foliar area: F = 412.72, p = 0.000; dry weight: F = 5.21, p = 0.01). Significant increases were detected in plant height, root length, foliar area, and dry weight in the consortium treatments (CAMF, Pb + CAMF, An + CAMF, CPSF, and CPSF + CAMF) in comparison with the individually inoculated treatments (Pb or An) and the control treatment (t < 0.05) (Figs. 3, 4).
Pots with coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF) fungi. Control non inoculated, CAMF consortium of arbuscular mycorrhizal fungi, Pb P. brevicompactum, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An A. niger, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF consortium of phosphate-solubilizing fungi; CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi
Growth variables of coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF). a height, b root length, c foliar area and d dry weight. Control non inoculated, CAMF consortium of arbuscular mycorrhizal fungi, Pb P. brevicompactum, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An A. niger, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF consortium of phosphate-solubilizing fungi; CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi. The data are the average of three repetitions ± standard deviation, shared letters in a column indicate no significant differences
Acid phosphatase activity in the rhizosphere
A significant interaction effect between the AMF and PSF factors was observed for acid phosphatase activity (F = 51.63, p = 0.000000). All the inoculated treatments (CAMF, Pb, Pb + CAMF, An, An + CAMF, CPSF, and CPSF + CAMF) presented significantly higher phosphatase activity than the control treatment (t < 0.05). In particular, the acid phosphatase activity of the CPSF treatment was significantly higher than the rest of the treatments (CAMF, Pb, Pb + CAMF, An, An + CAMF, and CPSF + CAMF) (t < 0.05) (Fig. 5a).
a Acid phosphatase activity in the rhizosphere and b acid phosphatase activity in the roots of coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF) fungi. Control non inoculated, CAMF consortium of arbuscular mycorrhizal fungi, Pb P. brevicompactum, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An A. niger, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF consortium of phosphate-solubilizing fungi; CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi. The data are the average of three repetitions ± standard deviation, shared letters in a column indicate no significant differences
Acid phosphatase activity of roots
The acid phosphatase activity of the roots of coffee plants was also significantly affected by the interaction between the AMF and PSF factors (F = 41.56, p = 0.0001). The acid phosphatase activity of the roots was significantly higher in the An, An + CAMF, and CPSF + CAMF treatments in comparison with the CAMF, Pb, Pb + CAMF, CPSF, and control treatments (t < 0.05) (Fig. 5b).
Isoenzyme electrophoretic patterns of acid phosphatase in the rhizosphere of coffee plants
Three bands were detected: one monomorphic (1) (appeared in all treatments) and two polymorphic bands (2 and 3) (appeared in only one treatment). Three distinct isoenzymatic patterns were observed. In the first pattern, one band was present; this pattern was observed in the majority of the Pb, An, CPSF, Pb + CAMF, An + CAMF, and CPSF + CAMF treatments. In the second pattern, three bands formed; this pattern corresponded with the CAMF treatment. No bands were observed in the third pattern corresponding with the control treatment (Fig. 6a).
Isoenzymatic patterns of a acid phosphatase in the rhizosphere and b roots of coffee plants inoculated with arbuscular mycorrhizal (AMF) and P-solubilizing (PSF) fungi. Pb P. brevicompactum, An A. niger, CPSF consortium of phosphate-solubilizing fungi, Pb + CAMF P. brevicompactum + consortium of arbuscular mycorrhizal fungi, An + CAMF A. niger + consortium of arbuscular mycorrhizal fungi, CPSF + CAMF consortium of phosphate-solubilizing fungi + consortium of arbuscular mycorrhizal fungi, CAMF consortium of arbuscular mycorrhizal fungi, Control non inoculated
Isoenzyme electrophoretic patterns of acid phosphatases in the roots of coffee plants
Three bands were once again detected: one monomorphic (1) (appeared in all treatments) and two polymorphic bands (2 and 3) (that did not appear in the control treatment). Two distinct isoenzymatic patterns were observed. The first pattern presented three bands and was detected in all inoculated treatments. The second pattern presented one band and was also detected in the control treatment (Fig. 6b).
Discussion
Few studies have documented the benefits of co-inoculation with AMF and PSF for plants (Khan and Zaidi 2006; Souchie et al. 2006; Babu and Reddy 2011; Rodrigues-Cabral et al. 2012; Serna 2013; Castillo et al. 2013; Saxena et al. 2015). Furthermore, no studies have addressed the use of AMF and PSF inoculations in the cultivation of coffee plants. This study represents the first report of the AMF-PSF interaction effects on P availability, acid phosphatase activity, and the growth and development of coffee plants.
In the present study, 8 months after the inoculation of coffee plants with AMF and PSF, a rate of mycorrhizal colonization of 35–51.33% was found. In the control treatment (non-inoculated), mycorrhizal colonization was not observed. Kormanik and McGraw (1982) established five categories or rates of mycorrhizal colonization: null (0%), low (1–25%), moderate (26–50%), high (51–75%), and very high (76–100%). The rate of mycorrhizal colonization observed in the present study was moderate to high. These colonization rates are higher than those reported in other studies evaluating the mycorrhizal colonization of coffee plants inoculated with AMF; for example, the studies of Escalona (2002) and Rivera et al. (1997) detected mycorrhizal colonization rates of 42.9 and 21–54%, respectively. However, Mariscal et al. (1997) found an even higher mycorrhizal colonization rate of 54–84%. The studies of Rodríguez (2001) and González and Rodriguez (2004) also reported mycorrhizal colonization rates of 67 and 43–69% respectively.
In the obtained results, the mycorrhizal colonization values for plants under the CHMA and Pb + CHMA treatments were greater than the rest of the treatments. The colonization value of the Pb + CAMF treatment was smaller than that of the CHMA treatment, although the difference was not significant. Therefore, it can be inferred that the presence of P. brevicompactum in the Pb + CAMF treatment did not promote the mycorrhization of coffee plants.
Other studies have confirmed that saprophytic fungi favor AMF colonization in crops/plants such as chili when co-inoculated with P. albidum and P. frequentans (Castillo et al. 2013), tomato when co-inoculated with A. niger (Velázquez et al. 2005), bamboo when co-inoculated with A. tubingensis (Babu and Reddy 2011), and Leucaena leucocephala when co-inoculated with Mortierella sp. (Londoño 2010). For this reason, more strains of P-solubilizing fungi should be explored in order to discover additional potential synergistic relationships that could favor the mycorrhizal colonization of coffee plants.
The experimental coffee plants were grown in a soil substrate from a coffee plantation. This soil had a low content of available P (1.97 mg/kg) according to the classification of Carvajal (1984); for coffee plantation soils, intervals of < 10 mg/kg correspond with low available P content, 10–30 mg/kg with medium P content, and > 40 mg/kg with high P content.
The available soil P content at the end of the experiment ranged from 3.39 to 5.77 mg/kg in the fungal treatments and 0.0879 mg/kg in the treatment without fungi (control); thus, an increase of 1.41–3.8 mg/kg was observed with respect to initial P in the fungi-inoculated treatments. Meanwhile, available P in the control treatment reduced; this possibly resulted from the absorption of soil P by coffee plants for the production of organic compounds such as nucleic acids (DNA and RNA), phosphoproteins, phospholipids, enzymes, and energy-rich phosphate compounds such as adenosine triphosphate (ATP) (Domínguez 1997).
An increase in the concentration of soluble P was evident in the soil of plants inoculated with the fungal treatments. The consortium treatment of P-solubilizing fungi (CPSF) was the most effective treatment, promoting an increase in the P concentration of 3.8 mg/kg. These values are superior in comparison with other studies; for example, Rodrigues-Cabral et al. (2012) found a P increase of 2.9 mg/kg in the soil of cashew plants after co-inoculations with three strains of bacteria, two strains of PSF, and the species Glomus etunicatum. Meanwhile, Babu and Reddy (2011) observed a P increase of 3.1 mg/kg in bamboo plants using co-inoculations of A. tubingensis and an AMF consortium.
Although the PSF consortium treatment (CPSF) resulted in a greater available P concentration in soils than the Pb + CAMF consortium treatment, the combination of AMF with P. brevicompactum appeared to be associated with an increase in P in comparison to the rest of the treatments. For this reason, the utilized P. brevicompactum strain and the AMF consortium may act synergistically. This finding supports the use of consortium inoculations of AMF and PSF; in some cases, these may result in greater beneficial effects on plants than individual inoculations. Londoño (2010) indicated that the inoculation of AMF-PSF consortia favors an increase in the rhizodeposition of carbon, thereby providing a greater quantity of carbon compounds for use by fungi (Sharma and Johri 2002). Phosphate-solubilizing fungi use carbon compounds in several ways, for example, in the production of different organic acids or phosphatase enzymes responsible for P transformation processes (Relwani et al. 2008).
Plants can also solubilize P by producing radical exudates (Dakora and Phillips 2002). In addition, Grimal et al. (2001) confirmed the solubilization of P in plants by means of radical mucilage production and by root-associated bacteria. These mechanisms can explain the presence of P in the soils and plant tissues of the CAMF treatment that were not inoculated with P-solubilizing microorganisms.
Overall, in the present study, the concentration of total foliar P in treated plants increased by 110% with respect to the control plants. These results showed a greater increase in total foliar P than the similar study of Velázquez et al. (2005), who found an increase of 78% in total foliar P in tomato plants inoculated with Glomus mosseae and A. niger. Rodríguez-Cabral et al. (2012) found a 20% increase in foliar P in cashew trees. Souchie et al. (2006) did not find any increase in foliar P in Trifolium plants following their inoculation with G. mossae and A. niger. Meanwhile, Zhang et al. (2014) obtained similar results as the present study and found a 100% increase in foliar P in a herbaceous plant (Kosteletzkya virginica) inoculated with G. mosseae and Mortierella sp. Babu and Reddy (2011) detected a 115% increase in foliar P in bamboo plants after performing co-inoculations with a consortium of HMA and A. tubingensis. However, Zhang et al. (2014) found an even greater increase (300%) in foliar P in castor plants inoculated with G. mosseae and Mortierella sp.
In addition, an increase in the total P in plant leaves was observed in the treatments inoculated with fungal consortia (CPSF + CAMF, An + CAMF, Pb + CAMF, and CAMF). Mycorrhizae were present in the majority of the treatments; thus, it can be inferred that mycorrhizal consortia favored greater concentration of P in leaves. The mycelia of AMF possibly explore a greater area in the soil and thus transport a greater quantity of P to plants (Barea et al. 2008), which is reflected in the total P content of foliage. For the plants inoculated with a consortium of P-solubilizing fungi (CPSF), a smaller concentration of P was found in the leaves; for these plants, it can be inferred that the majority of P is found in the soil (Fig. 2a).
The benefits of using AMF and PSF consortia were also evident in the results for coffee plant growth (height, foliar area, dry weight of aerial parts, and root length). As this is the first study on co-inoculations of both AMF and PSF in coffee plants, we can only compare our results to studies examining the effect of mycorrhizal fungal consortia on coffee plants. In testing the effects of fungal consortia on plants, Rodríguez (2001) and Trejo et al. (2011) detected positive effects on height, Aguirre et al. (2011) on dry weight, and Rodrigues-Cabral et al. (2012) on root length and number of leaves. Likewise, Adriano et al. (2011) discovered an increase in the growth of coffee plants when these were co-inoculated with nitrogen-fixing bacteria and AMF. According to these results, we may conclude that the interaction between AMF and PSF promotes physiological changes that involve an increase in the photosynthetic rate; as a result, greater P acquisition stimulates growth at the apical meristems and results in more vigorous plants (Aguirre et al. 2011).
A possible synergism exists between the strains of P. brevicompactum and A. niger of the PSF consortium (CPSF), as coffee plants inoculated with the consortium treatments exhibited better development in comparison with plants inoculated with individual fungi. The growth benefits resulting from CPSF inoculation could be due to the greater contribution of soluble P from the rhizosphere as well as the increased production of phytohormones, vitamins, or amino acids (Barea et al. 2008).
An increase in the production of phosphatase enzymes in the rhizosphere of plants inoculated with the strains of P-solubilizing fungi was also detected. This indicated that the utilized strains of P. brevicompactum and A. niger actively participated in the mineralization process of organic P in the soil of the coffee plants. Agnihotri (1970), Tarafdar and Chhonkar (1978), and Tarafdar and Classen (1988) also demonstrated the active participation of species of the genera Aspergillus and Penicillium in the mineralization of organic P in the soil. The consortium treatment of P-solubilizing fungi (CPSF) favored a greater acid phosphatase activity; thereby, it can be inferred that the use of consortia strengthens the production of this enzyme. Different fungal strains have been shown to produce distinct forms of acid phosphatases (Kropp 1990) or increase their activity when used in combination (Kroehler 1988).
Considering the activity of acid phosphatases in the roots, plants inoculated with the strain A. niger (individually or in consortium) presented a significantly higher concentration of acid phosphatase. This could be a consequence of the plant response to A. niger inoculation; microbial inoculation reportedly stimulates the enzymes associated with plant defense mechanisms against biotic and symbiotic stress (Pérez 2010). The findings of our study agree with the reports of Adriano et al. (2011) and Collados (2006) in which root phosphatase activity of coffee plants and wheat, respectively, increased after inoculation with microorganisms.
The electrophoresis technique allowed for the characterization of the differential induction of the isoenzymes produced by the studied fungal-plant interactions, which are the result of polygenetically controlled traits (Kropp 1990). In our results, distinct isoenzymatic patterns were observed in the evaluated treatments of both the rhizosphere and the plant roots. Some studies (Kroehler 1988; Dighton 1991; Joner and Johansen 2000) have affirmed that mineralizing fungi in the soil can produce different forms of phosphatases (isoenzymes); in these cases, certain factors may be regulating the induction of enzymes and their interactions. Enzyme expression could also be regulated by interactions among microorganisms present in the rhizosphere.
In the characterization of the rhizosphere, three electrophoretic bands were detected for the AMF consortium treatment (CAMF), while only one band was observed in the treatments with P-solubilizing fungi (alone or in consortium); in the control treatment (without inoculation), no bands were detected. Thus, the detected enzymes possibly result from the interaction between the AMF and the PSF present in the rhizosphere. On the other hand, in the characterization of the coffee roots, three bands were detected in all treatments, and only one band was observed in the control treatment (without inoculation). In this case, the expression of the enzymes could be regulated by the symbiotic relationship of these organisms with their host, thereby stimulating the production of acid phosphatase (Ridge and Rovira 1971; Shaykh and Roberts 1974; Juma and Tabatabai 1988; Tadano and Sakai 1991). Another study demonstrated that conditions of nutrient deficit induce the production of phosphatases in plants (Tarafdar and Classen 1988), possibly leading to the appearance of the band that was observed in the control treatment.
We conclude that a consortium of arbuscular mycorrhizal and P-solubilizing fungi (whether in co-inoculations or independent consortia) may act synergistically on coffee plants to increase growth and phosphorus uptake. Therefore, the use of such consortia may represent a promising strategy for organic coffee cultivation. Given the importance of arbuscular mycorrhizal and P-solubilizing fungi in the processes of P transformation and absorption, further research should be performed on their presence in the rhizosphere in both greenhouse and field settings in order to evaluate their effects on the growth and development of plants. Furthermore, it is important to continue the exploration and identification of other native fungal strains with high potential for use as biofertilizers. Additional molecular tools may be used to monitor changes in plant P and to determine the influence of inoculated microorganism populations on native fungal communities.
References
Adriano AML, Hernández RC, Figueroa MS, Jarquin Gálvez R (2011) Actividad biológica y enzimas de estrés en plántulas de café Coffea arabica L. In: Aguilar JCE, Galdámez J, Bahena JF, Vázquez GM, López BW, Pinto RR (eds) Agricultura Sostenible Vol. V. Sociedad Mexicana de Agricultura Sostenible A.C., Mexico, pp 15–20
Agnihotri VP (1970) Solubilization of insoluble phosphates by some soil fungi isolated from nursery seedbeds. Can J Microbiol 16(9):877–880
Aguirre MJF, Moroyoqui ODM, Mendoza LA, Cadena IJ, Avendaño ACH, Aguirre CJF (2011) Hongo endomicorrízico y bacteria fijadora de nitrógeno inoculadas a Coffea arabica en vivero. Agron Mesoam 22(1):71–80
Arias RM, Heredia G (2014) Fungal diversity in coffee plantation systems and in a tropical montane cloud forest in Veracruz, Mexico. Agrofor Syst 88(5):921–933
Arias RM, Heredia G, Sosa V, Fuentes-Ramírez LE (2012) Diversity and abundance of arbuscular mycorrhizal fungi spores under different coffee production systems and in a tropical montane cloud forest patch in Veracruz, Mexico. Agrofor Syst 85(1):179–193
Babu A, Reddy M (2011) Dual inoculation of arbuscular mycorrhizal and phosphate solubilizing fungi contributes in sustainable maintenance of plant health in fly ash ponds. Water Air Soil Pollut 219(1–4):3–10
Barea JM, Ferrol N, Azcón C, Azcón R (2008) Mycorrhizal symbioses. In: White PJ, Hammond JP (eds) The ecophysiology of plant–phosphorus interactions. Springer, Dordrecht, pp 143–163
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soil. Soil Sci 59:39–45
Carvajal JF (1984) Cafeto—cultivo y fertilización. Instituto Internacional de la Potassa, Berna
Castillo C, Morales A, Rubio R, Barea JM, Borie F (2013) Interactions between native arbuscular mycorrhizal fungi and phosphate solubilizing fungi and thier effect to improve plant development and fruit production by Capsicum annuum L. Afr J Microbiol Res 7(26):3331–3340
Collados C (2006) Impacto de Azospirillum modificado genéticamente sobre la diversidad y actividad de los hongos de la micorriza arbuscular en la rizósfera de trigo y maíz. Dissertation, Universidad de Granada
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245(1):35–47
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Annu N Y Acad Sci 121:404–427
Dighton J (1991) Acquisition of nutrients from organic sources by mycorrhizal autotrophics plants. Experientia 47:362–369
Domínguez VA (1997) Tratado de fertilización. Mundi-Prensa, Madrid
Escalona MA (2002) Interacción de plantas de café fertilizadas con fósforo e inoculadas con hongos micorrízico arbusculares y Phoma costarricencis Echandi. Dissertation, Universidad de Colima
Escamilla PE, Ruiz RO, Zamarripa CA, González HVA (2015) Calidad en variedades de café orgánico en tres regiones de México. Rev Geog Agric 55:45
Fira (2016) Panorama Agroalimentario. Café 2016. Dirección de Investigación y Evaluación Económica y Sectorial
García-Franco J, Toledo T (2008) Epífitas vasculares: bromelias y orquídeas. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 69–77
González ME, Rodriguez Y (2004) Respuesta de plantas de Coffea canephora a la inoculación con hongos micorrizógenos arbusculares durante la fase de aclimatización. Cultiv Trop 25(1):13–16
González-Romero A, Murrieta-Galindo R (2008) Anfibios y Reptiles. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 135–143
Grimal JY, Frossard E, Morel JL (2001) Maize root mucilage decreased adsorption of phosphate on goethite. Biol Fertilil Soils 33:226–230
Gryndler M, Vosatka M, Hrselova H, Chvatalova I, Jansa J (2002) Interaction between arbuscular mycorrhizal fungi and cellulose in growth substrate. Appl Soil Ecol 19(3):279–288
Heredia G, Arias R (2008) Hongos saprobios y endomicorrizógenos en suelos. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 193–203
Jeffries P, Barea JM (2001) Arbuscular Mycorrhiza—a key component of sustainable plant-soil ecosystems. In: Hock B (ed) The Mycota, vol IX. Fungal associations. Springer, Berlin, pp 95–113
Joner EJ, Johansen A (2000) Phosphatase activity of external hyphae of two arbuscular mycorrhizal fungi. Mycol Res 104:81–86
Juma NG, Tabatabai MA (1988) Phosphatase activity in corn and soybean roots: conditions for assay and effects of metals. Plant Soil 107:39–47
Khan MS, Zaidi A (2006) Influence of composite inoculations of phosphate solubilizing organisms and an arbuscular mycorrhizal fungus on yield, grain protein and phosphorus and nitrogen uptake by greengram. Arch Agron Soil Sci 52(5):579–590
Kormanik PP, McGraw AC (1982) Quantification of vesicular-arbuscular mycorrhizae in plant roots. In: Schenck NC (ed) Methods and principles of mycorrhizal research. American Phytopathological Society, Minnesota, pp 37–45
Kroehler C (1988) The effects of organic and inorganic phosphorus concentration on the acid phosphatase activity of ectomycorrhizal fungi. Can J Bot 66:750–756
Kropp BR (1990) Variation in acid phosphatase activity among progeny from controlled crosses in the ectomycorrhizal fungus Lacaria bicolor. Can J Bot 68:864–866
Londoño A (2010) Efecto de la inoculación con un hongo micorrizal y un hongo solubilizador de fósforo en la absorción de fosfato y crecimiento de Leucaena leucocephala en un oxisol. Dissertation, Universidad Nacional de Colombia
Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (2008) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico
Mariscal E, Anzueto F, García A, Molina A (1997) Evaluación del efecto de las micorrizas en almácigos de café. Memorias del XVIII Simposio Latinoamericano de Café (IICA, ICAFE). http://www.anacafe.org/glifos/index.php?title=Efecto_micorrizas_almacigos. Accessed 17 March 2016
McAllister C, Garcia-Romera I, Martín J, Godeas A, Ocampo J (1995) Interaction between Aspergillus niger van Tiegh. and Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe. New Phytol 129:309–316
McKean SJ (1993) Manual de análisis de suelos y tejido vegetal: una guía teórica y práctica de metodologías. Cent Int Agric Trop 129:1–99
Mehltreter K (2008) Helechos. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: Biodiversidad, Manejo y Conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 83–93
Moguel P, Toledo VM (2004) Conservar produciendo: biodiversidad, café orgánico y jardines productivos. Biodiversitas 55:2–7
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Pérez E (2010) Hongos micorrízicos arbusculares (HMA) para la bioprotección de patógenos en el cultivo del tomate (Solanum lycopersicum L.). Dissertation, Universidad de la Habana
Phillips JM, Hayman DS (1970) Improved procedures for cleaning roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment to infection. Trans Br Mycol Soc 55:158–161
Pohlan J (2002) México y la cafeticultura chiapaneca. Reflexiones y alternativas para los caficultores. Shaker Verlag, Alemania
Relwani L, Krishna P, Reddy MS (2008) Effect of carbon and nitrogen sources on phosphate solubilization by a wild-type strain and UV-induced mutants of Aspergillus tubingensis. Curr Microbiol 57:401–440
Ridge EH, Rovira AD (1971) Phosphatase activity of intact young wheat roots under sterile and non-sterile conditions. New Phytol 70:1017–1026
Rivera R, Fernández F, Sánchez C, Bustamante C, Herrera R, Ochoa M (1997) Efecto de la inoculación con hongos micorrizógenos VA y bacterias rizosféricas sobre el crecimiento de las posturas de cafeto. Cultiv Trop 18(3):15–23
Rodrigues-Cabral JS, De Assis KC, Silva FG, Souchie EL, Carneiro MAC (2012) Seedlings of cashew trees of the Brazilian Cerrado inoculated with arbuscular mycorrhizal fungi and phosphate-solubilizing microorganisms. Agrociencia 46(8):809–821
Rodríguez MJL (2001) Efecto del biofertilizante Mycoral® (micorriza arbuscular) en el desarrollo del café (Coffea arabica L.) en vivero en Zamorano, Honduras. Dissertation, Escuela Agrícola Panamericana
Rodríguez Y, Vierheilig H, Mazorra LM (2012) Alterations of the antioxidant enzyme activities are not general characteristics of the colonization process by arbuscular mycorrhizal fungi. Chil J Agric Res 72(3):411
Roozen N, VanderHoff F (2002) La aventura del comercio justo. Una alternativa de globalización por los fundadores de Max Havelaar. El Atajo, México
Saxena J, Saini A, Ravi I, Chandra S, Garg V (2015) Consortium of phosphate solubilizing bacteria and fungi for promotion of growth and yield of chickpea (Cicer arietinum). J Crop Improv 29:353–369
Serna GLS (2013) Efecto de la inoculación conjunta con hongos micorrizales y microorganismos solubilizadores de fósforo en plantas de aguacate. Dissertation, Universidad Nacional de Colombia
Sharma AK, Johri BN (2002) Physiology of nutrient uptake by arbuscular mycorrhizal fungi. In: Sharma AK, Johri BN (eds) Arbuscular mycorrhizae. Interaction in plants, rhizosphere and soils. Science Publishers, Enfield, pp 279–308
Shaykh MN, Robertsm LW (1974) A histochemical study of phosphatase in root apical meristems. Ann Bot 38:165–174
Sosa ML, Escamilla PE, Díaz CS (2004) Organic coffee. In: Wintgens JE (ed) Coffee: growing, processing, sustainable production. Wiley-VCG Verlag GmbH & Co., Weinheim, pp 339–354
Sosa V, Hernández-Salazar, Hernández-Conrique, Castro-Luna A (2008) Murciélagos. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: Biodiversidad, Manejo y Conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 181–191
Souchie EL, Azcón R, Barea JM, Saggin-Júnior OJ, Silva EMRD (2006) Phosphate solubilization and synergism between P-solubilizing and arbuscular mycorrhizal fungi. Pesqui Agropecu Bras 41(9):1405–1411
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tadano T, Sakai H (1991) Secretion of acid phosphatase by the roots of several crop species under phosphorus-deficient conditions. Soil Sci Plant Nutr 37:129–140
Tarafdar JC, Chhonkar PK (1978) Status of phophatases in the root-soil interface of leguminous and non-leguminous crops. Z Pflanzenernäh Bodenkd 141(3):347–351
Tarafdar JC, Classen N (1988) Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatase produced by plant roots and microorganisms. Biol Fertil Soils 5:308–312
Tejeda-Cruz, Gordon C (2008) Aves. In: Manson RH, Hernández-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. instituto de ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INE-SEMARNAT), Mexico, pp 149–160
Trejo D, Ferrera-Cerrato R, García R, Valera L, Lara L, Alarcón A (2011) Efectividad de siete consorcios naticos de hongos micorrízicos arbusculares en plantas de café en condiciones de invernadero y campo. Rev Chil Hist Nat 84:23–31
Trouvelot A (1986) Mesure du taux de mycorhization VA d’un systemeradiculaire. Recherche de methodes d’estimation ayant une signification fonctionnelle. Mycorrhizae: physiology and genetics, pp 217–221
Valenzuela-González, Quiroz-Robledo L, Martínez-Tapia D (2008) Hormigas (Insecta: Hymenoptera: Formicidae). In: Manson RH, Hernandez-Ortiz V, Gallina S, Mehltreter K (eds) Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación. Instituto de Ecología, A.C. (INECOL) e Instituto de Nacional de Ecología (INESEMARNAT), Mexico, pp 107–121
Van Der Heijden MG, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11(3):296–310
Velázquez M, Elíades L, Irrazabal G, Saparrat C, Cabello M (2005) Mycobization with Glomus mosseae and Aspergillus niger in Lycopersicon esculentum plants. J Agric Technol 1(2):315–326
Zhang HS, Qin FF, Qin P, Pan SM (2014) Evidence that arbuscular mycorrhizal and phosphate-solubilizing fungi alleviate NaCl stress in the halophyte Kosteletzkya virginica: nutrient uptake and ion distribution within root tissues. Mycorrhiza 24(5):383–395
Acknowledgements
This study was part of the CONACyT (C01-0194) project, “Aplicación de las interacciones fúngicas en la restauración y fertilización del suelo” (2011/169124) carried out at the Instituto de Ecología, A.C. The first author thanks CONACyT for her master fellowship at the Instituto de Investigaciones Forestales, Universidad Veracruzana. We thank Biol. Miriam Lagunes Reyes, Noemí Orozco Domínguez, and Ing. Abraham Romero Fernández for their valuable support in processing samples. We also thank MGR Ariadna Martínez Virues for assistance with the chemical analyses. Allison Marie Jermain revised the English version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perea Rojas, Y.C., Arias, R.M., Medel Ortiz, R. et al. Effects of native arbuscular mycorrhizal and phosphate-solubilizing fungi on coffee plants. Agroforest Syst 93, 961–972 (2019). https://doi.org/10.1007/s10457-018-0190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-018-0190-1