Abstract
Cacao (Theobroma cacao) cultivation maintaining a high proportion of shade trees in a diverse composition (agroforestry) is currently being viewed as a sustainable land use practice. Our research hypothesis was that cacao agroforests (AF) can support relatively high tree diversity, as compared to surrounding primary and/or secondary forests. The objective of this study was to assess the impact of forest conversion on tree communities by comparing tree composition, community characteristics (richness and diversity) and spatial structure (density, canopy height, basal area) among primary forest, secondary forest, and cacao AF. In total, we collected data from 30 25 × 25 m plots on three land use systems (20 in cacao AF, five in secondary, and five in primary forests) in San Alejandro, Peruvian Amazon. All trees with DBH ≥ 10 cm were counted, identified to species, and their height and DBH were recorded. Our results support the hypothesis that cacao AF present a relatively high tree species richness and diversity, although they are no substitute for natural habitats. We identified most common species used for shading cacao. Tree species composition similarity was highest between cacao AF and secondary forest. Vegetation structure (density, height, DBH) was significantly lower compared to primary and secondary forest. Species richness and diversity were found to be highest in the primary forest, but cacao AF and secondary forests were fairly comparable. The tree species cultivated in cacao AF are very different from those found in primary forest, so we question whether the relatively high tree diversity and richness is able to support much of the diversity of original flora and fauna.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forests contain some of the highest biodiversity of flora and fauna in the world. However, biodiversity at all scales is increasingly threatened by a variety of human-induced structural impacts (Peres et al. 2010). Disappearance of native forests through clearing for agriculture continues to be a major issue worldwide (Lindenmayer 2010). Land use change, including expansion of intensive agriculture, is one of the most cited explanations for biodiversity loss worldwide (Sala et al. 2006). These changes lead to habitat loss for some species and can even drive species decline and extinction.
Biodiversity research in tropical landscapes has been usually conducted in intact-forested areas, with far less emphasis on modified environments (Fazey et al. 2005). Various studies from all over the tropics deal with the question of how much biodiversity can be found in agricultural landscapes. In response, researchers in conservation biology seek to promote less intensive agriculture such as multistrata agroforestry systems that provide farmers with income and also products for household consumption, while protecting biodiversity (Schroth et al. 2004). Agroforestry practices have often been shown to increase levels of natural biodiversity on farmland, and it is hypothesised that they are also able to play a supporting role in the conservation of biodiversity in remnants of natural habitats that are interspersed with farmland in tropical land use mosaics (McNeely and Schroth 2006).
In the tropics, forests are very often cleared to grow plantation crops such as cacao (Theobroma cacao) or coffee (Coffea sp.). As compared to clearcutting or monocrop agriculture, these plantations are very diverse, due to original forest trees being left and/or the eventual planting of shade trees. These so-called agroforests (AF), renowned for their high tree species richness and complex vegetation structure, stand out as promising biodiversity conservation tools (Somarriba et al. 2004). Cacao AF can retain a floristically diverse and structurally complex shade canopy and thus have the potential to harbour significant levels of biodiversity (Schroth and Harvey 2007). Trees help to diversify farm production (e.g., fruit, fodder, timber, fuelwood) and may represent an added benefit for both farmers and the environment. Shade tree systems can contribute to biodiversity conservation by providing a habitat for plant and animal species that are not strictly dependent on a natural forest, and also by connecting otherwise disjunctive fragments of remaining forest patches in the landscape. These systems have at least some structural characteristics of natural forests and may help reduce edge effects between the natural forest and open agricultural fields. Cacao fields grown on the edge of a forest may decrease evapotranspiration and thus the mortality of forest trees that are not well adapted to drier microclimates. This could prevent the ultimate collapse of isolated forest fragments and forest reserves in agricultural landscapes (Gascon et al. 2000).
In many ways, these diverse agroforestry systems may have value in conserving the original biodiversity of an area (e.g., McNeely and Schroth 2006; Harvey et al. 2006; Harvey and González 2007; Asase and Tetteh 2010) and may play a largely undocumented role in providing ecosystem services (Deheuvels et al. 2012).
The overall objective of this study was to assess the impacts of forest conversion on tree communities by comparing tree species composition, community structure (abundances, species richness and diversity) and spatial structure (tree density, basal area and canopy height) between natural primary and secondary forests and cacao AF in the Peruvian Amazon. Our basic hypothesis was that cacao AF can support relatively high tree richness and diversity, albeit lower than primary and secondary forests. We also expected that tree species composition and spatial structure would change along the habitat gradient with forest species being gradually replaced by species of more open habitats, and to evaluate species-specific patterns of abundance along the habitat gradient. This was a baseline study that would serve us for follow-up studies evaluating how the vegetation structure of these AF could influence the composition of other taxa (e.g., arthropods, amphibians, mammals) and help as a conservation tool for local biodiversity.
Materials and methods
Study site
The area in Peru dedicated to cacao cultivation covers around 84,000 ha (FAO 2011) and ~45 % of that area bears native cacao varieties (García Carrion 2010), with average yield of cocoa beans 670 kg ha−1 (FAO 2011). It is estimated that there were around 30,000 Peruvian families cultivating cacao (Anduaga 2009). In the lowland Amazon of the Ucayali region, the area dedicated to cacao production covers about 1,900 ha (García Carrion 2010). Most of the production is concentrated around San Alejandro town.
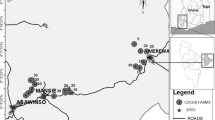
San Alejandro (8°49.584′ S, 75°13.923′ W, 257 masl) is located in the Department of Ucayali, Province of Padre Abad (Fig. 1). Settlement in the area began in the 1940s after construction of a road linking the Ucayali River, a major Amazon tributary, and the capital city of Lima. San Alejandro, with ~20,000 inhabitants (8.1 inhabit/km2), is the capital of the Irazola district. It lies on the main road, 110 km from the regional capital Pucallpa and is situated on the bank of the San Alejandro River, a tributary of the Aguaytía River. The area was originally covered by lowland humid tropical forest, at an altitude of between 250 and 350 masl. The climate is characterized by high temperatures throughout the year; mean annual temperature is 25 °C with relative humidity averaging 85 %. Annual rainfall ranges from 2,500 to 3,500 mm, with concentrated heavy rains from November to March and lower rainfall during the rest of the year. This zone corresponds to a climate that can be considered very wet and warm, and is characterized by low hills, moderately to highly dissected, with dominant slopes varying between 20 and 70 % and a moderate-to-high susceptibility to water erosion.
This area is considered an old settlement with the majority of colonists having arrived more than 40 years ago. The rural residents practice agriculture (slash-and-burn farming with cultivation of cash crops such as cacao), livestock husbandry, forestry, and other land-based production (Gonzales 2008). Farmers cultivate their traditional staple crops such as rice (Oryza sativa), maize (Zea mays) and cassava (Manihot esculenta) and fruits like citrus (Citrus spp.), papaya (Carica papaya), and banana (Musa spp.) and in the last decades also cacao. Smallholders in the area do not plant cacao trees in monoculture (full sun), but intercrop cacao with various other crops in the initial years (mainly banana) and with multipurpose native shade trees in the following years.
The remaining forest is a source of timber and various non-timber products. Major environmental problems faced by the inhabitants of this district are torrential rains and flooding, deforestation as a result of inadequate forest management, and the degradation of habitat with the concomitant loss of biodiversity.
Data collection
Data were collected from June to September 2012. Sampling was done following the modified methodology of Kessler et al. (2005) and Asase and Tetteh (2010). This methodology has been used successfully in Indonesia and Ghana to study vegetation structure in cacao AF. The method basically involves sampling forest trees using square quadrates. We compared tree botanical composition, population and spatial structure in three different land use systems: cocoa AF, secondary forest (SF), and primary forest (PF).
The study was conducted on 20 cacao farms around San Alejandro. The farms were selected randomly from the list of the cacao-growing farmers association, ACATPA (La Asociación de Cacaoteros Tecnificados de Padre Abad), which is comprised of 65 families, all dedicated to cultivation of cacao in agroforestry systems. On each farm, one square 25 × 25 m plot was laid out in the estimated center of the cacao AF. The age of cacao AF ranged between 4 and 12 years (median of 7.5 years). For each cacao AF, the plot location was selected based on visual observations to better assure homogeneity over the plot. For comparison, five plots of the same size were randomly laid out in the nearby (1–5 km) secondary forest (10–15 years old) and five plots in the primary forest. The nearest well-preserved natural primary forest (only moderately logged a few decades ago) was found at the experimental forest station of The National University of Ucayali (UNU), which is located about 30 km from the study site (near the settlement Alexander von Humboldt).
Within each plot, the trees with a diameter at breast height (DBH) ≥ 10 cm were recorded and identified (unknown trees were given a unique morphospecies number); DBH was measured using a diameter tape (±0.1 cm), and total tree height was measured using a clinometer. Because of difficulties due to the dense vegetation in the primary forest, the clinometer could not be used and tree heights were estimated by experienced workers from UNU (these estimates were validated on each plot by measuring several nearby trees with a clinometer). Cacao trees were not measured, but their total number (usually at distance 3 × 3 m) was counted for each plot. All farmers were interviewed about the cacao management and yields, and the use of and reason for planting or retaining each tree species found on inventoried plots in their AF.
Scientific and vernacular name identification was done with the help of an experienced botanist, Maria Elena Chuspe Zans, from the Universidad Nacional Intercultural de la Amazonia (UNIA). Specimens that could not be identified directly in the field were collected and verified with voucher specimens at the regional herbarium in Pucallpa (Instituto Vetenarion de Investigaciones Tropicales de Altura—IVITA) and deposited at the herbarium at the UNIA.
Data evaluation and analysis
First, we documented the botanical composition of tree species in the sample, their abundance found in each of the three land use systems, and their main use by the local population. For the most numerous tree species found in cacao AF we calculated the importance value (IV) (the sum of relative values of frequency, density, and basal area) (Sambuichi et al. 2012). Similarity among the land use systems was evaluated by counting shared species and calculating the Jaccard coefficient (Krebs 1999; Chao et al. 2005). The coefficient uses species presence/absence data for two sample sets (in this case, land use types), and weight matches and mismatches in species composition between the two samples (Krebs 1999).
For the analysis of tree population structure, species richness and diversity were calculated for non-cacao trees in each system using data from all sample plots. Species richness was expressed as (i) observed species richness—the number of non-cacao tree species per plot/land use system by combining all the species recorded in sample plots and (ii) estimated total species richness, which was calculated by a nonparametric first-order Jacknife estimate based on the observed frequency of rare species (Heltshe and Forrester, 1983; Magurran 2004). Species heterogenity was estimated using Shannon (H′ base e logs) (Krebs 1999; Magurran 2004) and Simpson’s reciprocal (1/D) diversity index (Krebs 1999) at the level of each plot and land use system. The Shannon index tends to be weighted slightly towards less abundant or rare species, while Simpson’s index favors the more abundant or dominant species (Krebs 1999). To evaluate and compare spatial structure, tree density, canopy height, and basal area were calculated for each plot and averaged for each land use system.
To assess statistical differences among the above-mentioned indices and variables of the three land use systems, we compared them with an analysis of variance (ANOVA using Tukey’s SD test) for parametric data distribution, and the Kruskal–Wallis test (KW-ANOVA) for non-parametric data using STATISTICA 9.0 software [StatSoft].
To account for differences in sample areas (20 plots in cacao AF compared to five plots each in primary and secondary forests), the rarefaction method of Gotelli and Colwell (2001) allowed us to construct the species accumulation curves in two ways: (i) based on the number of sampled plots; and (ii) based on the number of sampled individuals, using EstimateS software (Colwell 2009). The randomization process used in EstimateS also allowed us to recalculate the species richness and diversity indices based on a similar sample size (n = 5).
Results
Tree species’ botanical composition
A total of 538 individual trees (DBH ≥ 10 cm) belonging to 105 species in 34 families were found on 18,750 m2 surveyed (Table 1). Out of the total number of the trees inventoried, 58 (10.8 %) were identified only to the genus level (1.5 % in AF, 10.2 % in SF, and 26.1 % in PF); whereas, we were able to identify 17 trees (3.2 %) only to morphospecies level but without scientific name (1.2 % in AF and 8.9 % in PF). The tree families with the highest variety of species and number of individuals were: Fabaceae (18 species/112 individuals), Palmae (7/84), Cecropiaceae (1/67), Rubiaceae (7/53), and Sterculiaceae (2/43). With the exception of one genus (Citrus—5 trees), all tree species encountered were native to the Amazon.
In addition to shading the cacao, the most highly reported uses of trees in AF were fruit, timber, firewood, and thatching material; most of the trees were planted by farmers during plantation establishment. The most common tree species found in AF was Inga edulis (Fabaceae; locally called guaba), a native fruit tree with highest importance value (IV = 153.7), largest number of individuals (78), highest frequency of occurrence (100 %), as well as, the second highest basal area (5.27 m2, 23 % of total BA) (Table 2). Other species of significant importance were: timber trees, Calycophyllum spruceanum (Rubiaceae; capirona) (IV = 87.1) and Guazuma crinita (Sterculiaceae, bolaina blanca) (IV = 44.9); and three palm species, Phytelephas macrocarpa, Attalea phalerata, and Bactris gasipaes, (Palmae; yarina, shapaja and pijuayo) (IV = 78.1, 70.2, 29.0, respectively), which were all used for fruit and thatching material. Twenty-three tree species (almost 70 %) found in AF were reported to be commonly planted there.
The average yield of cacao beans on surveyed area was 745 kg ha−1 with the range between 400 and 1,000 kg ha−1. The yields were slightly positively correlated with the number of shade trees (r = 0.56), but not significantly (t test at p = 0.073). Neither did we find any significant correlation of cocoa yields with other variables (e.g., age, number of cacao trees, tree species richness and diversity).
The most common tree species found in secondary forests was Cecropia polystachya (Cecropiaceae; cetico), a pioneer species with largest number of individuals (63), the highest frequency of occurrence (100 %), as well as, the largest basal area (1.43 m2, 24 %). Other species of significant importance were: G. crinita, Trema micrantha (Ulmaceae; atadijo), and Ochroma pyramidale (Malvaceae; topa, balsa). All of these fast-growing species are harvested for their light wood. In primary forests, trees were locally used for timber, fruit, and medicinal products. All inventoried tree species in PF had a low abundance and we did not find any particularly dominant species.
Tree species similarity among land use systems
Astrocaryum murumuru was the only tree species that could be found in all three land use systems (Fig. 2). Several Inga sp. also were found in all land use systems, but the domesticated species, I. edulis, was found only in AF. Eleven tree species were recorded both in AF and SF, only two in AF and PF, and four tree species were found to occur both in SF and PF. Thus, tree species composition showed significant responses to land use change. If we account for the larger sample size in AF (four times as many), the abundance of tree species, namely, P. macrocarpa, A. phalerata, Croton draconoides, and Cedrela odorata, were comparable in AF and SF. The abundance of common pioneer species G. crinita, C. polystachya, O. pyramidale, and T. micrantha, was much higher in SF than in AF.
At the family level, the taxonomic composition of the three habitat types showed major differences. In order of numbers, cacao AF were dominated by Fabaceae, Arecaceae, and Rubiaceae; primary forests were dominated by Fabaceae, Nyctaginaceae, and Moraceae; secondary forests by Cecropiaceae, Sterculiaceae, and Fabaceae.
When comparing tree species similarity among land use systems using Jaccard similarity coefficients, the highest similarity was observed between AF and SF (0.256), and the lowest between AF and PF (0.019). However, the similarity between SF and PF was also relatively low (0.048).
Community and spatial structure among the land use systems
To compare community and spatial structure among the land use systems, we compared the samples on three levels: in total, means per plot and rarefaction for the same number of plots (Table 3). In total, we found 254 trees belonging to 33 species in AF, 127 trees belonging to 16 species in SF and 157 trees belonging to 71 species in PF. The highest estimated species richness (Jackknife) was found in PF (108.8), followed by AF (45.4), with the lowest in SF (23.2). The Shannon index of species diversity was shown to be highest for PF (4.02), intermediate for AF (2.47), and the lowest in SF (1.83); the same order was found using Simpson’s index.
Analysis of spatial structure revealed considerable variability in tree densities, canopy heights, and tree dimensions among the land use systems (Table 3—means per plot). Mean tree density, canopy height, and basal area in AF was significantly lower than in SF and PF. Looking at community structure, mean family and species richness was comparable between AF and SF, but significantly higher in PF. The diversity indices (Shannon and Simpson) were found to be comparable between AF and SF, but significantly higher for PF.
To standardize all samples from the three land use systems, we compared the systems according to their species accumulation curves (Figs. 3, 4). The curve for PF based on the number of plots sampled is far from asymptotic (Fig. 3), indicating that the area sampled was too small to estimate the total number of species in this land use type. However, with an increased sampling size, tree species richness is likely to be significantly higher in PF compared to that of SF and AF, but comparable between AF and SF. Individual-based accumulation curves again showed the highest tree species richness in PF; but, in this case, tree species richness in AF was higher than in SF (Fig. 4). We also compared species richness and diversity for the same number of sample plots (n = 5) (Table 3—rarefaction). Tree abundance was highest in PF, followed by SF and AF. Observed species richness again was found to be highest in PF, but comparable between SF and AF. In PF, there was the highest number of unique species and species that were found only in one plot. Considering all of the species richness estimators, we found the highest values in PF and lower but comparable numbers in SF and AF. Comparing the various diversity indices, the highest diversity occurred in PF, followed by AF, with the lowest in SF.
Discussion
Tree species management and preferences in cacao agroforests
Nearly all tree species occurring in the surveyed cacao AF were trees used for their productive value (e.g., timber, thatching material, fruit, medicines) or service (e.g., shade, control of erosion, soil improvement) role. The most abundant tree species in cacao AF, I. edulis (guaba), is commonly used mainly for shading cacao trees and soil protection in all of Latin America. Its leaf litter protects the soil surface and roots of other plants, helps retain nutrients in the topsoil, and controls weeds. It is also important for its fruit and timber and is a source of fuelwood (Reynel et al. 2003). The second most abundant species C. spruceanum (capirona) is used mainly for timber production and also provides valuable fuelwood. It is a fast-growing tree that can reach 1.4–1.6 m in 6 months and 3.5–4.7 m in 1 year (Sotelo et al. 2000). Another fast-growing tree species, G. crinita, is commonly planted in cacao plantations for its rapid timber production (harvested after 6–10 years). It can reach 2–2.3 m in 6 months and 4.9–5.7 m in 1 year (Sotelo et al. 2000). Both species are commonly found in the regeneration of secondary forests; farmers usually use this regeneration as a source of seedlings. Several palm species are also commonly found in cacao plantations in the study area. P. macrocarpa (yarina) and A. phalerata (shapaja) are usually not planted, but left by farmers when establishing a cacao plantation: their leaves are used as a long-lasting roofing material. The fruit of B. gasipaes (pijuayo), another domesticated and widely planted species, is consumed throughout the Amazon region. The apex of the stem is edible; it has a sweet and pleasant flavor, with this species growing in demand in domestic and international markets. The trunk wood is locally used in construction. One of the most cultivated medicinal tree species is C. draconoides (sangre de grado). The reddish sap is medicinal and is used also for for healing wounds and ulcers. The sap contains active antibacterial substances and is valued by pharmaceutical companies (Reynel et al. 2003). The remaining patches of primary and secondary forests serve farmers mainly as a source of softwood (SF) and hardwood (PF) timber, firewood, and various fruits. Species occurring in secondary forests are fast growing pioneer taxa typical of early successional stages throughout the tropics (Turner 2001). These pioneer species also were found in our study: C. polystachya, G. crinita, T. micrantha, O. pyramidale. Secondary forests are developed on previously clear-felled and subsequently cropped areas that were allowed to regrow by the natural processes of community change (for about 10 years in our study). As a result, large hardwood tree species were almost completely missing.
In several studies of traditional cacao-based agroforestry (e.g., Oke and Odebiyi 2007; Atkins and Eastin 2012; Sambuichi et al. 2012; Daghela Bisseleua et al. 2013), it was found that many of the trees retained in cacao AF were native multipurpose fruit and timber trees. The selection and/or active planting of such useful tree species may lead to a significant increase in their density in cacao AF, compared with elsewhere in the landscape. For example, in southern Cameroon, the density of Dacryodes edulis is ten times higher and that of Milicia excelsa is three times higher in cacao plantations than elsewhere in the landscape (Van Dijk 1999). A similar phenomenon was evidenced in San Alejandro, where we observed a high abundance of multipurpose I. edulis, and of the fast-growing timber species G. crinita and C. spruceanum, as compared to an adjacent secondary forest.
In accordance with (Anglaaere et al. 2011), we found that trees are of enormous importance in farming systems in the Peruvian Amazon. Farmers in our study area have a strong belief that the presence of trees on their farms greatly enhances soil fertility. Many tree species in the agroforestry systems in San Alejandro were identified by farmers as improvers of soil fertility. Farmers prefer species that are fast-growing, produce marketable fruit or timber and are propagated easily (by seeds or collecting plantlets). Further tree preference is focused on a service role: either for their soil nutrients and moisture-enhancing qualities, or purely for the quality of shade they provide. The decision to classify a tree as a good shade tree appeared, however, to be greatly influenced by the value of their products. Our results, together with those of Duguma et al. (2001), confirm that the trees occurring in AF are mainly grown for a well-defined product and, secondly, provide desired shade for cacao trees, improve soil fertility, and reduce soil erosion.
The average yield of cacao beans in surveyed area was 745 kg ha−1which was close to average yield in Peru (670 kg ha−1 as reported by FAO 2011). The yields positively correlated with shade tree density, but not significantly. Contrary to our results, in the study made in Cameroon, (Daghela Bisseleua et al. 2013) found that increase of shade (thus tree density) was negatively related to cocoa yield, with yield significantly higher at shade <50 %. This study did, however, show the importance of a diverse shade canopy in reducing damage caused by cocoa pests. In Côte d’Ivoire, Koko et al. (2013) discovered a negative effect of fruit tree intercropping on cocoa yield. Yields per plant strongly decreased with increasing shade. Deheuvels et al. (2012), during the study in Costa Rica, did not find any significant influence of tree density, diversity, or vegetation structure on cocoa yields, however, all selected cocoa-based systems could be considered as low-yielding in terms of cocoa productivity. Somarriba and Beer (2011) in their study of productivity of cacao AF with timber or legume trees in Costa Rica, also found no influence of shade tree species on dry cocoa bean yield or pod counts.
Agroforests and forests comparison
The cacao AF surveyed showed relatively high diversity of shade trees for an agroforestry system. Sambuichi and Haridasan (2007) assessed 15 ha in five traditional cacao growing farms (cabruca) in Southern Bahia, Brazil, with different ages and degrees of abandonment of management practices, and found 293 species (DBH ≥ 10 cm), with Shannon diversity ranging from 3.31 to 4.22. Rolim and Chiarello (2004) found 105 species in cabrucas of the Espirito Santo state, Brazil, by sampling trees with DBH ≥ 10 cm in 4.8 ha of 20 farms. Sonwa et al. (2007) studying the dense and complex AF of Southeast Cameroon sampled trees and pseudo-trees (e.g., banana) with DBH ≥ 2.5 cm and found 206 species in 9.1 ha surveyed in 60 cocoa farms, with Shannon diversity indices ranging between 3.1 and 4.2 per AF. In our study, we found 33 tree species on 1.25 ha, with Shannon diversity of 2.47. The results of these studies, however, are not directly comparable with ours due to differences in the survey methodologies employed.
The relatively high tree diversity in cacao AF in study area is a reflection of the high natural tree diversity in that region. However, the rich floristic diversity of native forest trees reminiscent of a natural forest was found to have decreased substantially in cacao AF, but that this diversity was comparable between AF and secondary forests. Also, the studies of Daghela Bisseleua et al. (2007) and Asase et al. (2009) show that cacao AF support relatively high tree species richness.
Besides harbouring far fewer tree species than intact forests, AF also demonstrate different species compositions, with relatively higher proportions of early successional trees. These results differ from those in several previous studies. For example, Bobo et al. (2006) and Parthasarathy (1999) report that tree species richness and diversity found in natural forests decreases in secondary forest and is lowest in cacao agroforestry. Also in a study by Kessler et al. (2005), cacao AF had by far the lowest tree species richness. Differences in tree species richness within cacao AF are commonly a function of management intensity, dominant crop, and farm history (Schroth and Harvey 2007). High levels of tree species richness in cacao AF observed in San Alejandro could be due to extensive farmers’ knowledge of the management and use of various trees, along with relatively high species richness remaining in the surrounding environment.
Field observations of Turner et al. (1997) have shown that, in common with other tropical forests, even 50-year-old secondary forests, despite their tree canopy attaining a height comparable to that of primary forests, have a conspicuously different composition of taxa, a fact that we also found in our study. We found that the species richness in secondary forest was substantially lower than in primary forest, whereas vegetation structure (canopy height, density and basal area) was quite comparable; however, taxonomic composition was very different.
Primary forest data from our study can be compared with the large number of similar forest plots inventoried elsewhere in the tropics. The estimated species richness in the primary forest in our study (109 tree species) is within the range of 100–160 species this is considered to be typical in tropical rain forests (Whitmore and Sayer 1992).
Compared to the results of Asase and Tetteh (2010), we found significant differences in tree spatial structure between cocoa AF and primary/secondary forests. We observed significantly lower tree density, canopy height and basal area in cacao AF. The obvious reason is that cacao trees were not included in our analysis (DBH ≤ 10 cm), as well as, the fact that farmers manage lower tree densities because cacao trees need space to grow and the competition between cacao and non-cacao trees could likely occur.
Conservation value of cacao agroforests
From the data that we presented, it is clear that cacao AF are a poor substitute for the natural forests, both in terms of botanical composition, tree community, and spatial structure. However, they contribute to heterogeneity at the landscape level and thus can favor biodiversity conservation. Additionally, due to the high diversity of their shade tree component, can function as ecological corridors, ameliorating the isolation of plant and animal species in forest fragments. These AF also can provide additional habitat for some forest tree species and reduce anthropogenic pressure on forests remnants by providing firewood and timber to meet the needs of rural families.
We believe that cacao agroforestry, when compared to open field or pasture, has potential for biodiversity conservation as its structure, even though lower than in primary forest, provides resources and niches for a variety of native species of fauna and flora. Also in our study area, the cacao AF are intespersed in the mosaic of young and old secondary forest and farm fields and thus they can make an important contribution to the conservation of regional biodiversity by enhancing landscape connectivity and reducing edge effect (Schroth et al. 2004). Cacao AF also can be employed as a buffer zone around protected areas of primary forests. Farmers must therefore be encouraged to retain trees in farmlands or replant native trees in cacao AF that commonly occur in surrounding primary or secondary forests.
As there is an increasing demand for land and food production leading to agricultural intensification, the heterogeneous mosaic landscape, of which the cacao agroforestry systems form a part, could be strategically managed to maximize the benefits of both sustainable agricultural production and biodiversity conservation.
Conclusion
In our study, tree species richness and diversity were found to be significantly higher in the surveyed primary forest as compared to the secondary forests and cacao AF, but that cacao AF and secondary forests were fairly comparable. Farmers retained only few trees from original vegetation; most of the trees were planted when establishing the cacao plantation and were most highly valued for their products and secondarily for their service role. We observed a very low similarity between primary forests and cacao AF. Tree species cultivated by farmers in cacao AF were very different from those found in primary forests, thus these AF could be a relatively poor substitute for the conservation of tree species found naturally in primary forests. This also raises the question as to how well these agroforestry systems are able to support the native diversity of fauna found in a natural forest, a focus of our future research. It may be that agroforestry systems could play important conservation role in agricultural landscapes where forests are highly fragmented, a typical situation in large areas of humid tropics. Our results represent a scientific baseline for further monitoring of ecological changes as the landscape in the Amazon region as it becomes progressively more human-modified.
References
Anduaga RS (2009) Situación y Perspectivas de la Cadena del Cacao Chocholate en el Perú. IICA, Lima
Anglaaere LCN, Cobbina J, Sinclair FL, McDonald MA (2011) The effect of land use systems on tree diversity: farmer preference and species composition of cacao-based agroecosystems in Ghana. Agrofor Syst 81:249–265
Asase A, Tetteh DA (2010) The role of complex agroforestry systems in the conservation of forest tree diversity and structure in southeastern Ghana. Agrofor Syst 79:355–368
Asase A, Ofori-Frimpong K, Ekpe PK (2009) Impact of cocoa farming on vegetation in an agricultural landscape in Ghana. J Afr Ecol 48:338–346
Atkins JE, Eastin I (2012) Farmers perceptions of indigenous forest trees within the cultivated cocoa landscape. Forestry Chron 88(5):535–541
Bobo K, Waltert M, Sainge NM, Njokagbo N, Fermon H, Muhlenberg M (2006) From forest to farmland: species richness patterns of trees and understorey plants along a gradient of forest conversion in southwestern Cameroon. Biodiv Conserv 15:4097–4117
Chao A, Chazdon RL, Colwell RK, Shen TJ (2005) A new statistical approach for assessing compositional similarity based on incidence and abundance data. Ecol Lett 8:148–159
Colwell RK (2009) Estimates: statistical estimation of species richness and shared species from samples, version 8.2. Persistent http://purl.oclc.org/estimates
Daghela Bisseleua HB, Herve B, Vidal S (2007) Plant biodiversity and vegetation structure in traditional cocoa forest gardens in southern Cameroon under different management. Biodiv Conserv 17:1821–1835
Daghela Bisseleua HB, Fotio D, Yede Missoup AD, Vidal S (2013) Shade tree diversity, cocoa pest damage, yield compensating inputs and farmers’ net returns in West Africa. PLoS One 8(3):e56115
Deheuvels O, Avelino J, Somarriba E, Malezieux E (2012) Vegetation structure and productivity in cocoa-based agroforestry systems in Talamanca, Costa Rica. Agric Ecosyst Environ 149:181–188
Duguma B, Gockowski J, Bakala J (2001) Smallholder Cacao (Theobroma cacao) cultivation in agroforestry systems of West and Central Africa: challenges and opportunities. Agrofor Syst 51:177–188
FAO (2011) FAOSTAT crop production. (http://faostat3.fao.org) [online]. Available at http://faostat3.fao.org/faostat-gateway/go/to/download/Q/QC/E. Accessed Sept 20, 2013
Fazey I, Fischer J, Lindenmayer DB (2005) What do conservation biologists publish? Biol Conserv 124:63–73
García Carrion LF (2010) Catálogo de Cultivares de Cacao del Perú. Ministerio de Agricultura Dirección General de Competividad Agraria, Lima 112 pp
Gascon G, Williamson GB, da Fonseca GAB (2000) Receding forest edges and vanishing reserves. Science 288:1356–1358
Gonzales TT (2008) Plan vial provincial participativo de Padre Abad. Municipalidad provincial de Padre Abad, Pucallpa
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Harvey CA, González JAV (2007) Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodivers Conserv 16:2257–2292
Harvey CA, González J, Somarriba E (2006) Dung beetle and terrestrial mammal diversity in forests, indigenous agroforestry systems and plantain monocultures in Talamanca, Costa Rica. Biodivers Conserv 15:555–585
Heltshe JF, Forrester NE (1983) Estimating species richness using the jackknife procedure. Biometrics 39:1–11
Kessler M, Kesler PJA, Gradstein SR, Bach K, Schmull M, Pitopang R (2005) Tree diversity in primary forest and different land use systems in Central Sulawesi, Indonesia. Biodiv Conserv 14:547–560
Koko LK, Snoeck D, Lekadou TT, Assiri AA (2013) Cacao-fruit tree intercropping effects on cocoa yield, plant vigour and light interception in Cote d’Ivoire. Agrofor Syst 87:1043–1052
Krebs CJ (1999) Ecological Methodology, vol Second. Addison Wesley Longman, Inc University of British Columbia, Canada
Lindenmayer DB (2010) Landscape change and the science of biodiversity conservation in tropical forests: a view from the temperate world. Biol Conserv 143:2405–2411
Magurran AE (2004) Measuring biological diversity. Blackwell Science Ltd, Malden
McNeely J, Schroth G (2006) Agroforestry and biodiversity conservation—traditional practices, present dynamics, and lessons for the future. Biodiv Conserv 15:549–554
Oke DO, Odebiyi KA (2007) Traditional cocoa-based agroforestry and forest species conservation in Ondo State, Nigeria. Agric Ecosyst Environ 122:305–311
Parthasarathy N (1999) Tree diversity and distribution in undisturbed and human-impacted sites of tropical wet evergreen forest in the southern Western Ghats, India. Biodiv Conserv 8:1365–1381
Peres CA, Gardner TA, Barlow J, Zuanon J, Michalski F, Lees AC, Vieira ICG, Moreira FMS, Feeley KJ (2010) Biodiversity conservation in human-modified Amazonian forest landscapes. Biol Conserv 143:2314–2327
Reynel C, Pennington RT, Pennington TD, Flores C, Daza A (2003) Árboles útiles de la Amazonía Peruana. Tarea Gráfica Educativa, Perú.
Rolim SG, Chiarello AG (2004) Slow death of Atlantic forest trees in cocoa agroforestry in southeastern Brazil. Biodivers Conserv 13:2679–2694
Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, Huenneke LF, Jackson RB, Kinzig A, Leemans R, Lodge DM, Mooney HA, Oesterhel M, Poff NL, Sykes M, Walker BH, Walker M, Wall DH (2006) Global biodiversity scenarios for the year 2100. Science 287:1770–1774
Sambuichi RHR, Haridasan M (2007) Recovery of species richness and conservation of native Atlantic forest trees in the cacao plantations of southern Bahia in Brazil. Biodivers Conserv 16:3681–3701
Sambuichi RHR, Widal DB, Piasentin FB, Jardim JG, Viana TG, Menezes AA, Mello DL, Ahnert D, Baligar VC (2012) Caburca agroforests in southern Bahia, Brazil: tree component, management practices and tree species conservation. Biodivers Conserv 21(4):1055–1077
Schroth G, Harvey CA (2007) Biodiversity conservation in cocoa production landscapes: an overview. Biodiv Conserv 16:2237–2244
Schroth G, Da Foneseca AB, Harvey CA, Gascon C, Vasconcelos HL, Izac AMN (2004) The role of agroforestry in biodiversity conservation in tropical landscapes. In: Schroth G et al (eds) Agroforestry and biodiversity conservation in tropical landscapes. Island Press, Washington, pp 1–12
Somarriba E, Beer J (2011) Productivity of Theobroma cacao agroforestry systems with timber or legume service shade trees. Agrofor Syst 81:109–121
Somarriba E, Harvey CA, Semper M, Anthony F, González J, Staver C, Rice RA (2004) Biodiversity conservation in Neotropical coffee (Coffea arabica) plantations. In: Schroth G, da Fonseca GAB, Harvey CA, Gascon C, Vasconcelos HL, Izac AMN (eds) Agroforestry and biodiversity conservation in tropical landscape. Island Press, Washington, pp 198–226
Sonwa DJ, Nkongmeneck BA, Weise SF et al (2007) Diversity of plants in cocoa agroforests in the humid forest zone of Southern Cameroon. Biodivers Conserv 16:2385–2400
Sotelo C, Vidaurre H, Weber J, Simons A, Dawson I (2000) Domesticación participativa de árboles agroforestales en la amazonia peruana. In: Congreso Forestal Latinoamericano 2000. Capítulo de Ingeniería Forestal, Lima, Perú
Turner IM (2001) The Ecology of trees in the tropical rainforest. Cambridge University, Cambridge
Turner IM, Wong YK, Chew PT, bin Ibrahim A (1997) Tree species richness in primary and old secondary tropical forest in Singapore. Biodiv Conserv 6:237–543
Van Dijk JM (1999) Non-timber forest products in the Bipindi-Akom Region, Cameroon: a socio-economic and ecological assessment. The Tropenbos-Cameroon Programme, Kribi
Whitmore TC, Sayer JA (1992) Tropical Deforestation and Species Extinction. Chapman & Hall, London
Acknowledgments
This research was supported by grants from The Czech Foundation for Development of Education; Foundation Nadání Josefa, Marie a Zdeňky Hlávkových; the Fulbright Commission in the Czech Republic and internal grant of the Faculty of Tropical AgriSciences, CULS Prague (N°20135121). We would like to thank to many people from Universidad Nacional Intercultural de la Amazonía, Universidad Nacional de Ucayali and local cacao farmers from the cooperative ACATPA in San Alejandro for their cooperation during our research. We also highly appreciate the work of two anonymous reviewers that substantially improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vebrova, H., Lojka, B., Husband, T.P. et al. Tree diversity in cacao agroforests in San Alejandro, Peruvian Amazon. Agroforest Syst 88, 1101–1115 (2014). https://doi.org/10.1007/s10457-013-9654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-013-9654-5