Abstract
Mycorrhizal and non-mycorrhizal holm oak (Quercus ilex L.) seedlings inoculated with black truffle (Tuber melanosporum) were grown under nursery conditions and subjected to drought hardening for 4 months in autumn and winter followed by irrigation for 10 days. Leaf water potential and stomatal conductance were monitored during the 4 months of drought. When the test was completed (March), measurements were made for each treatment (inoculated or non-inoculated), and watering regime (watered and water-stressed). Pressure–volume curves, osmotic potential at full turgor, osmotic potential at zero turgor and the tissue modulus of elasticity near full turgor were calculated. Mycorrhizal colonization and growth, and the content of the main mineral nutrients N, P, K, Ca and Mg were measured. Water stress affected plant growth, caused an elastic adjustment of the plant tissues, and decreased the P and K content, and inoculation improved the nitrogen content. Drought acclimation apparently achieved the goal of improving the drought tolerance of holm oak seedlings, without depressing ectomycorrhizal root colonization by T. melanosporum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The beneficial effect of mycorrhizal symbiosis on plant nutrition is well known, but its role in water-related factors has been studied less frequently. Drought is one of the main factors that impede successful stand establishment. Some species tolerate dehydration without loss of viability of the cell membrane (Turner 1986), maintaining tissue turgidity by osmotic adjustments, and by a rise in elasticity in the cell walls or an increase in cellular tolerance to desiccation. Ectomycorrhizal symbiosis has both direct and indirect effects on the water status of trees, but there is a different specific capacity of ectomycorrhizae to survive drought (Parke et al. 1983; Garbaye 2000; di Pietro et al. 2007). In ectomycorrhizal plants, stomatal conductance and leaf water potential are usually elevated under drought conditions, since water uptake is increased (Duddrige et al. 1980; Walker et al. 2004; Lamhamedi et al. 1992a; Morte et al. 2001; Parke et al. 1983), and hydraulic conductance is increased (Nardini et al. 2000). Some authors have suggested that ectomycorrhizae can cause an increase in water stress by stimulating transpiration, thereby leading to a lower leaf water potential (Pallardy et al. 1995) but the effect of ectotrophic mycorrhizal symbiosis is poorly understood in all these mechanisms. All studies concerning water-related factors in mycorrhizal plants should consider factors related to the plant, such as the nutrient status of the tissue, stomatal conductance and age (Smith and Read 1997).
The black truffle (Tuber melanosporum Vitt.) is the most important edible mycorrhizal fungus, from an economic, social and ecological point of view, in the Spanish Mediterranean rural area (Reyna 1999). Holm oak (Quercus ilex L.), an evergreen tree that dominates many forest communities in the mid and western Mediterranean basin, is a symbiotic organism associated with T. melanosporum. The ecological value of this symbiosis in the recuperation of the Mediterranean ecosystem has rarely been tested, although there have been a few specific studies of the contribution of T. melanosporum to the growth and physiology of a tree (Domínguez 2002). An improvement of growth and P content as an effect of this inoculation and, in some cases, the modification of some water parameters of oak seedlings were observed in the nursery in an earlier study (Domínguez et al. 2008). Field tests of Quercus faginea and Q. ilex reforestation during the first year after planting showed that T. melanosporum mycorrhization improved the survival rate, growth and the N and P content of plants, as well as water absorption in the summer drought period (Domínguez et al. 2006).
Winter drought hardening of forest tree seedlings, before planting in spring, is common practice in nurseries in the Mediterranean area. This study investigated the interaction between winter drought-hardening and inoculation with T. melanosporum in nursery Q. ilex seedlings just before planting in the field. We asked if this common practice can change the mycorrhizal colonization of seedlings that will be established in black truffle plantations. To answer this, seedlings inoculated with T. melanosporum and subjected to water stress were assessed for water relations, mineral nutrient content, and growth attributes.
Materials and methods
Plant production and mycorrhizal inoculation
Holm oak seedlings were grown in containers at the Escuela Técnica Superior de Ingenieros de Montes (ETSI Montes) in Madrid (central Spain) in the spring of 2000.
Quercus ilex seeds from the Espadan mountain range in the Valencia area (eastern Spain) were received in Madrid during the winter of 1999/2000. They were soaked in water and any that floated were discarded because of probable non-viability. The seeds that did not float were placed in closed polyethylene bags at 4°C until they were sown. Before sowing, all seeds were sterilized by immersion in 30% (v/v) H2O2 for 15 min and then rinsed several times with distilled water. Conical plastic cells in ForestPot® containers (300 ml) were used for growing the seedlings. One acorn was placed into each tube at the beginning of March 2000. The growing medium was a (2:1) mixture of white peat (98% MO)/dark peat (30% MO) and sphagnum-type moss (Floragard©) pH 6, combined with vermiculite in the peat/vermiculite ratio of 3:1 (v/v), plus 3% (w/v) CaCO3. The peat had been sterilized in an autoclave at 120°C for 3 h. From March (sowing) to May (inoculation) in 2000, 240 seedlings were cultivated in a greenhouse at 20–30°C and watered daily to saturation.
Tuber melanosporum inoculum was prepared from underground fungal fruiting bodies (sporocarps) that had been collected in Molina de Aragón, in Guadalajara Province (northeast of Madrid), in January 2000. The truffles were cleaned and sterilized by brief superficial flaming, and the inoculum was prepared by suspending the ascospores in distilled water (375 × 105 spores/l) and stored at 4°C.
At the end of May 2000, half of the seedlings, 120 plants, were chosen at random and inoculated by manual injection of approximately 7.5 × 105 spores at 3–8 cm depth in each tube. The 120 plants that were not inoculated served as controls. Immediately after inoculation, all 240 plants were transferred to an outdoor nursery, with daily watering to saturation. No fertilizer was applied, in order to observe the effect of mycorrhization in host plants growing in poorly fertilized soils (Marx et al. 1977; Castellano and Molina 1989).
Water stress conditions
On 6 November 2000, 60 inoculated and 60 control plants were chosen at random and watered to saturation daily. The other 60 inoculated and 60 control plants were subjected to water stress (water deficit), with no watering during the autumn and winter of 2000 and 2001 until leaf wilting was observed and the weight of the containers was reduced to 40–45% of maximum weight at saturation. A two-factorial (ECM × water) design was used with the 60 plants in each treatment group distributed randomly in three blocks with 20 plants per experimental unit. All plants were grown outdoors in the same nursery, and a transparent plastic cover shielded the water-stressed plants to prevent them receiving any water from rainfall. Plants under water stress remained without water until 26 February 2001, when general foliar chlorosis was observed in 25% of the plants (loss of 158 g of water/plant). At that point, watering to saturation was re-established and the experiment finished 10 days afterwards, on 7 March 2001.
Mean daily air temperature during the hardening period varied between 4 and 13°C, there were few days with frost (T > −2°C), and relative humidity ranged from 51 to 95%.
Measurements
Water potential and stomatal conductance
Six inoculated and six non-inoculated plants under water stress were chosen at random during both water stress and drought recovery (autumn to winter), on 6 November and 16 November 2000, and 12 January, 26 February and 7 March 2001 For the watered plants, measurements were made at the beginning of the treatment, on 26 February, and at the end of the experiment. At dawn, the leaf water potential of six plants per treatment group (three leaves/plant) was determined with a pressure chamber (Scholander et al. 1965). The stomatal conductance rate was measured around midday under natural conditions with an open gas analyser working in differential mode (LCA-4, Analytical Development Co., Hoddesdon, UK).
Pressure–volume (P–V) curves
Pressure–volume (P–V) curves were determined 5–10 days after the end of the test. Shoot xylem pressure potentials were measured with a pressure chamber (Scholander et al. 1965). Six seedlings watered the afternoon before were chosen at random from each treatment group and maintained in the dark until shoot sampling the following morning. Shoots with 8–10 leaves were used. From each curve, the osmotic potential at full turgor (Ψπ full), the osmotic potential at zero turgor (Ψπ 0), and the modulus of elasticity near full turgor (E max) were calculated as described by Koide et al. (1989). Some P–V curves had a plateau and in these cases the shoot weight at full saturation was calculated as described by Kubiske and Abrams (1990).
Growth, mycorrhizal infection and mineral nutrition
Before the construction of P–V curves, growth parameters were measured for the same seedlings (six plants per treatment group) in March 2001. Shoot height and stem basal diameter were recorded. Mycorrhizal infection was confirmed after the P–V curves were constructed. The samples were dried at 70°C for 48 h, and dry weights of shoots, total roots and total short roots were measured.
The rooted “soil ball” of each of six plants chosen at random from each treatment group was immersed in water several times, so that the seedling roots could be carefully freed from most of the medium in which they had been grown. The whole root system of each seedling was cut in half longitudinally (one half being discarded each time) and only one half were used as samples. Mycorrhizal short roots were washed and separated by passage through two sieves (2.5 and 0.5 mm). All short roots and root tips were cut into pieces up to 2–3 cm long and divided into ectomycorrhizal and non-mycorrhizal tips under a stereomicroscope. All roots showing any of the characters (blunt tips, altered branching pattern, pigmented mantle, emanating hyphae) indicating ectomycorrhizal infection were removed for morphotyping. Different features of mycorrhizae were the basis for the identification of ectomycorrhizal morphotypes (Agerer 1987–1998). The presence of emanating hyphae branched at a right-angle and a mantle with a puzzle-like cell layer suggested the presence of T. melanosporum. The results are given as a percentage of mycorrhizal root tips, root tip number (mycorrhizal and total) per dry weight of short roots (No. g−1 dw) and root tip number (mycorrhizal and total) per plant (No./plant).
Fifteen plants chosen at random from each treatment group were divided into three groups of five plants each for mineral analysis (N, P, K, Ca, and Mg). The total dry weight was calculated for each group, and the nutrient contents per whole plant were determined. Nitrogen was measured with a LECO CHN-600 total analyser. After digestion by HNO3 in a microwave oven, P, K, Ca, and Mg were measured by vacuum inductively coupled plasma emission spectroscopy (Optima 2000, Perkin Elmer Inc., MA, USA).
Statistical analysis
All statistical analysis was done with Statgraphics Plus 5.0. An analysis of variance (ANOVA) was used to detect differences and interactions between inoculation treatment and watering regime. Temperature and radiation (PAR) were introduced as co-variables in the analysis of variance of stomatal conductance. Comparisons among treatment means were done with Tukey’s multiple range test. Effects of treatments on measured variables were tested for significance at the 0.05 level of confidence, and the non-parametric Kruskal–Wallis test was used in cases of non-homogenous variance.
Results
Drought tolerance and recovery from water stress
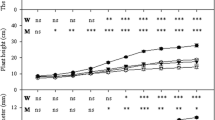
The stomatal conductance (Fig. 1) in stressed inoculated plants was higher (0.03 mol m−2 s−1) than that in non-inoculated plants (0.01 mol m−2 s−1), on 16 November 2000 but by the middle of January 2001 no significant difference was observed and the stomatal conductance did not change when watering was resumed. Leaf water potential decreased slightly in stressed plants (Fig. 1). Leaf water potential was fairly stable from the beginning of water stress, with no major difference between treatments, until leaf wilting was observed in plants. At that time, although the leaf water potential was higher in inoculated plants (−2.5 MPa) than that in non-inoculated controls (−3.3 MPa), this difference was not significant. When plants were irrigated again, water potential recovered progressively until a similar level was reached in all plants. In non-stressed plants, the stomatal conductance and leaf water potential were similar in the inoculated and in the non-inoculated plants and the leaf water potential was fairly constant at −0.5 to −1 MPa.
Stomatal conductance (a) and leaf water potential at dawn (b) in inoculated (I) and noninoculated (NI) plants subjected to full watering (NS) and water-stress (S) regime. Arrow indicate the moment when watering is re-established. Bars represent the standard error, N = 6. * Represent significantly different values according to Tukey’s test with P < 0.05, respectively
Water relations
No interaction between inoculation treatment and water regime was observed for the three water parameters measured (Table 1). In plants subjected to water stress, there was a significant rise in cell wall elasticity, owing to a lower E max (modulus of elasticity at full turgor). In water-stressed inoculated and non-inoculated plants, E max was 27 and 26 MPa, respectively, whereas in well watered inoculated and non-inoculated plants E max was 36 and 33 MPa, respectively. The differences of the osmotic potential parameters Ψπ full and Ψπ 0 between the water-stress regime and the inoculation treatment were not significant.
Seedling growth and mycorrhizal infection
The inoculation treatment and water regime affected most of the growth parameters significantly (Table 2). Inoculated plants had greater growth than control plants in terms of shoot height and stem basal diameter, and dry weights of shoots and roots. Water-stressed plants showed less growth than non-water-stressed plants according to root and shoot dry weights. There was an interaction among factors in short root dry weight: in water-stressed inoculated plants the short root dry weight was significantly higher than that in water-stressed non-inoculated plants. However, these differences were not seen in the non-stressed plants. Only T. melanosporum mycorrhizae were found in the inoculated plants (Table 3). The water regime did not affect any of the mycorrhizal parameters. The inoculation treatment increased the extent of T. melanosporum infection and the percentage was acceptable (~30%). Neither inoculation treatment nor watering regime affected the total number of root tips significantly.
Mineral nutrition
Interactions between inoculation treatment and watering regime were observed only for Ca and Mg (Table 4). The N content was increased significantly by inoculation but was unaffected by watering regime; the maximum value (21.03 mg plant−1) was observed in the watered, inoculated plants. The P and K contents were significantly lower in water-stressed plants than those in the watered plants, but were not affected by inoculation treatment: The content of P was highest (2.04 mg plant−1) in well watered, inoculated plants; and the K content was highest (18.13 mg plant−1) in the watered, non-inoculated plants. The Ca content was lower in water-stressed plants, but there was an interaction between inoculation treatment and watering regime. Finally, the Mg content was not affected by either of these factors.
Discussion
In this study, there was no significant difference of leaf water potential (Ψ) between inoculated and non-inoculated treatments in either watered or water-stressed plants. Generally, mycorrhizae cause increased water uptake by the plant as a result of an increase in the effective rhizosphere and the presence of fungal hyphae and rhizomorphs, which act as extra-fine searching and absorption elements that diminish the resistance to water flow from the soil to the roots (Brownlee et al. 1983; Garbaye and Guehl 1997), but low temperature can obstruct water absorption by the plant roots in certain circumstances (Burdett 1990). In this study, perhaps because of low temperature or the small size of the containers (300 ml), no effect of inoculation was observed, and inoculation did not significantly improve the water status of plants.
At the beginning of wilting, inoculated plants under water-stress had slightly higher leaf water potential than control plants, and leaf water potential was 0.8 MPa higher in inoculated than in non-inoculated plants. However, these differences were not significant and had disappeared 10 days after watering was re-established. Studies of water uptake have shown positive as well as negative effects of ectomycorrhization on root hydraulic properties (Coleman et al. 1990; Lamhamedi et al. 1992b; Nardini et al. 2000; Landhäusser et al. 2002). An improvement of water status in Q. ilex + T. melanosporum plants has been reported (Domínguez 2002) but earlier studies suggested that ectomycorrhizal infection per se does not necessarily increase the water uptake by plants in space-limited conditions. In this study, the stomatal conductance decreased in the water stress regime in both inoculated and non-inoculated plants. The stomatal conductance rate was significantly higher in inoculated plants than in control plants only at the start of the test. This could be due to modification of the level of hormonal abscisic acid (ABA) by the effect of mycorrhization (Augé et al. 1986), or a higher P and/or K uptake (Harley and Smith 1983), or an increased efficiency in soil water uptake (Muhsin and Zwiazek 2002) but these last two causes were not reflected in the results of this study.
Studies in natural ecosystems have shown that adult Q. ilex plants undergo important osmotic adjustments during summer periods of water stress (Kyriakopoulos and Richter 1991; Sala and Tenhunen 1994), which maintains a healthy tissue turgor with relatively less water and thereby increases resistance to water deficit. In this study, the application of water stress during autumn and winter, and subsequent re-watering, caused an increase of tissue elasticity (decrease of the maximum modulus of elasticity). Both osmotic adjustment and an increase in cell wall elasticity permit a higher cell turgor at low tissue water potential, which maintains gas exchange and growth of plants under conditions of water deficit (Joly and Zaerr 1987; Villar-Salvador et al. 2004). It is possible to obtain hardening in plants by osmotic adjustment (Kyriakopoulos and Richter 1991) induced by low temperature and a reduced photoperiod during winter (Van den Driessche 1989). In this study, the drought-hardening regime did not affect the osmotic potential, but perhaps the winter conditions influenced the hardening of all the plants (Villar-Salvador et al. 1998). Villar-Salvador et al. (2004) reported osmotic adjustment and reduction of stomatal conductance in Q. ilex seedlings by means of water stress hardening applied during August to November. In this study, the water stress regime was applied between November and the following March and, as in recent studies (Nardini et al. 2000), inoculation with T. melanosporum did not cause an osmotic adjustment in the Q. ilex plants. As was to be expected, a general decrease in seedling growth was caused by water stress, related to lowered plant water availability and to a lower nutrient content, especially of P and K (Table 4).
Ectomycorrhizal fungi are known to be quite sensitive to water stress (Coleman et al. 1989), and many studies have shown that severe drought inhibits ectomycorrhizal colonization (Swaty et al. 1998; Nilsen et al. 1998; Runion et al. 1997; Valdes et al. 2006; Bell and Adams 2004; Kennedy and Peay 2007). In this study, water stress did not affect either mycorrhizal population (acceptable colonization by T. melanosporum of 33–34%) or the total number of root tips. Perhaps the level of drought stress applied was not sufficient to inhibit EM root colonization. However, T. melanosporum is an ECM with a high level of drought tolerance. Earlier studies found an increase of T. melanosporum colonization with summer drought in 7 years old Q. ilex plantations, in different Mediterranean environments (Rodríguez et al. 2005). Also, nocturnal water translocation from host plant to fungus might maintain the integrity of the mycorrhizal mycelium under drought conditions (Querejeta et al. 2003). There was an interaction between inoculation treatment and watering regime only in the case of the dry weight of short roots, which increased with inoculation treatment under water stress; i.e., a combination of water stress and inoculation improved the root system and potential mycorrhization and stimulated the production of short roots in the Q. ilex seedlings.
Tuber melanosporum mycorrhization ameliorates plant nitrogen content, particularly for ammonium ions (NH4+) and protein content (Guttenberger 1995). There was an increase of N uptake in this study and in Q. ilex plants in the field during the same period (Dominguez et al. 2006). The water stress regime had a negative effect on the P and K content. Although other studies (Harley and Smith 1983) have indicated that mycorrhization improves P uptake, the P content in this study was not affected. There are few quantitative data on the amounts of K and Mg absorbed by the external mycelium of ectomycorrhizal fungi and transmitted to host plants; however, mycorrhizae have the potential to supply these mineral nutrients in quantities sufficient to cover a large proportion of the growth requirements of the host plant (Marschner 1995). In the present study, mycorrhization improved Mg absorption from the soil to the plant only in water-stressed plants.
Water stress did not affect either the T. melanosporum mycorrhizal infection or the total number of root tips. Water stress decreased the P and K content at the end of winter, and inoculation improved the N content. Finally, water stress increased the elasticity of plant cell walls, thereby improving drought tolerance. Therefore, the combined action of drought-hardening during the winter and inoculation with T. melanosporum can improve drought tolerance in Q. ilex seedlings, at least during the first period of plantation, without affecting the T. melanosporum mycorrhizal colonization.
References
Agerer R (1987–1998) Colour atlas of ectomycorrhizae. Einhorn-Verlay, Munich
Augé RM, Schekel KA, Wample RL (1986) Osmotic adjustment in leaves of VA mycorrhizal and non-mycorrhizal rose plants in response to drought stress. Plant Physiol 82:765–770
Bell TL, Adams MA (2004) Ecophysiology of ectomycorrhizal fungi associated with Pinus spp. in low rainfall areas of Western Australia. Plant Ecol 171:35–52. doi:10.1023/B:VEGE.0000029372.78102.9d
Brownlee C, Duddridge JA, Malibari A, Read DJ (1983) The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for asimilate and water transport. Plant Soil 71:433–443. doi:10.1007/BF02182684
Burdett AN (1990) Physiological processes in plantation establishment and the development of specifications for forest planting. Can J For Res 20:415–427. doi:10.1139/x90-059
Castellano MA, Molina R (1989) Mycorrhizae. In: Landis TD et al (eds) The container tree nursery manual, vol 5. Agric. handbook 674. US Department of Agriculture Forest Service, Washington, DC, pp 101–167
Coleman MD, Bledsoe CS, Lopushinsky W (1989) Pure culture responses of ectomycorrhizal fungi to imposed water stress. Can J Bot 67:29–39. doi:10.1139/b89-005
Coleman MD, Bledsoe CS, Smit BA (1990) Root hydraulic conductivity and xylem sap levels of zeatin riboside and abscisic acid in ectomycorrhizal Douglas fir seedlings. New Phytol 115:275–284. doi:10.1111/j.1469-8137.1990.tb00453.x
di Pietro M, Churin JL, Garbaye J (2007) Differential ability of ectomycorrhizas to survive drying. Mycorrhiza 17:547–550. doi:10.1007/s00572-007-0113-x
Domínguez JA (2002) Aportaciones de la micorrización artificial con trufa negra en planta forestal. Tesis Doctoral. Universidad Politécnica de Madrid, 402 pp
Domínguez JA, Selva J, Rodríguez Barreal JA, Saiz de Omeñaca JA (2006) The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For Ecol Manag 231:226–233. doi:10.1016/j.foreco.2006.05.052
Domínguez JA, Planelles R, Rodríguez Barreal JA, Saiz de Omeñaca JA (2008) The effect of Tuber melanosporum Vitt. mycorrhization on growth, nutrition, and water relations of Quercus petraea Liebl., Quercus faginea Lamk., and Pinus halepensis Mill. seedlings. New For 35:159–171. doi:10.1007/s11056-007-9069-0
Duddrige JA, Malibari A, Read JD (1980) Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport. Nature 287:834–836. doi:10.1038/287834a0
Garbaye J (2000) The role of ectomycorrhizal symbiosis in the resistance of forest to water stress. Outlook Agric 29(1):63–69
Garbaye J, Guehl JM (1997) Le rôle des ectomycorrhizes dans l’utilisation de l’eau par les arbres forestiers. Rev For Fr. XLIX. Nº SP. 110–119
Guttenberger M (1995) The protein complement of ectomycorrhizas. In: Varma A, Hoch B (eds) Mycorrhiza. Structure, function, molecular biology and biotechnology. Springer, Berlin, pp 59–78
Harley JL, Smith SE (1983) Mycorrhizal symbiosis. Academic Press, London, 483 pp
Joly RJ, Zaerr JB (1987) Alteration of cell-wall water content and elasticity in Douglas-fir during periods of water deficit. Plant Physiol 83:418–422
Kennedy PG, Peay KG (2007) Different soil moisture conditions change the outcome of the ectomycorrhizal symbiosis between Rhizopogon species and Pinus muricata. Plant Soil 291:155–165. doi:10.1007/s11104-006-9183-3
Koide RT, Robichaux RH, Morse SR, Smith CM (1989) Plant water status, hydraulic resistance and capacitance. In: Pearcy RW, Ehleringer J, Mooney HA, Rundel PW (eds) Plant physiological ecology. Chapman & Hall, London, pp 161–179
Kubiske ME, Abrams MD (1990) Pressure–volume relationships in non-rehydrated tissue at various water deficits. Plant Cell Environ 13:995–1000. doi:10.1111/j.1365-3040.1990.tb01992.x
Kyriakopoulos E, Richter H (1991) Desiccation tolerance and osmotic parameters in detached leaves of Quercus ilex L. Acta Oecol 12:357–367
Lamhamedi MS, Bernier PY, Fortin JA (1992a) Growth, nutrition and response to water stress of Pinus pinaster inoculated with ten dikaryotic strains of Pisolithus sp. Tree Physiol 10:153–167
Lamhamedi MS, Bernier PY, Fortin JA (1992b) Hydraulic conductance and soil water potential at the soil–root interface of Pinus pinaster seedlings inoculated with different dikaryons of Pisolithus sp. Tree Physiol 10:231–244
Landhäusser S, Muhsin T, Zwiazek J (2002) The effect of ectomycorrhizae on water relations in aspen (Populus tremuloides) and white spruce (Picea glauca) at low soil temperatures. Can J Bot 80:684–689. doi:10.1139/b02-047
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Marx DH, Hatch AB, Medicino JF (1977) High soil fertility decreases sucrose content and susceptibility of lobolly pine roots to ectomycorrhizal infection by Pisolithus tinctorius. Can J Bot 55:1569–1574. doi:10.1139/b77-185
Morte A, Diaz G, Rodríguez P, Alarcón JJ, Sánchez-Blanco MJ (2001) Growth and water relations in mycorrhizal and nonmycorrhizal Pinus halepensis plants in response to drought. Biol Plant 44(2):263–267. doi:10.1023/A:1010207610974
Muhsin TM, Zwiazek JJ (2002) Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus Americana seedlings. New Phytol 153:153–158. doi:10.1046/j.0028-646X.2001.00297.x
Nardini A, Salleo S, Tyree M, Vertovec M (2000) Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann For Sci 57:305–312. doi:10.1051/forest:2000121
Nilsen P, Borja I, Knutsen H, Brean R (1998) Nitrogen and drought effects on ectomycorrhizae of Norway spruce [Picea abies L. (Karst.)]. Plant Soil 198:179–184. doi:10.1023/A:1004399303192
Pallardy SG, Cermák J, Ewers FW, Kaufmann MR, Parker WC, Sperry JS (1995) Water transport dynamic in trees and stands. In: Smith MK, Hinckley WK (eds) Resource physiology of conifers: acquisition, allocation and utilization. London, Academic Press, pp 301–389
Parke J, Linderman RG, Black CH (1983) The role of ectomycorrhizas in drought tolerance of Douglas-fir seedlings. New Phytol 95:83–95. doi:10.1111/j.1469-8137.1983.tb03471.x
Querejeta JI, Egerton-Warburton LM, Allen MF (2003) Direct nocturnal water transfer from oaks to their mycorrhizal symbionts during severe soil drying. Oecologia 134:55–64. doi:10.1007/s00442-002-1078-2
Reyna S (1999) Aproximación a una Selvicultura Trufera. Tesis Doctoral. Universidad Politécnica de Madrid, 325 pp
Rodríguez JA, Domínguez JA, Saiz de Omeñaca JA, Mingot D, Placenave GJ (2005) Seguimiento de parcelas micorrizadas con Tuber melanosporum en la comunidad valenciana. Actas del IV Congreso Forestal Español, Zaragoza
Runion GB, Mitchell RJ, Rogers HH, Prior SA, Counts TK (1997) Effects of nitrogen and water limitation and elevated atmospheric CO2 on ectomycorrhiza of longleaf pine. New Phytol 137:681–689. doi:10.1046/j.1469-8137.1997.00865.x
Sala A, Tenhunen JD (1994) Site-specific water relations and stomatal response of Quercus ilex in a Mediterranean watershed. Tree Physiol 14:601–617
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346. doi:10.1126/science.148.3668.339
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. 2nd edn. Academic Press, London, 605 pp
Swaty RL, Gehring CA, Van Ert M, Theimer TC, Keim P, Whitham TG (1998) Temporal variation in temperature and rainfall differentially affects ectomycorrhizal colonization at two contrasting sites. New Phytol 139:733–739. doi:10.1046/j.1469-8137.1998.00234.x
Turner NC (1986) Adaptation to water deficits: a changing perspective. Aust J Plant Physiol 13:175–190
Valdes M, Asbjornsen H, Gomez-Cardenas M, Juarez M, Vogt KA (2006) Drought effects on fine-root and ectomycorrhizal-root biomass in managed Pinus oaxacana Mirov stands in Oaxaca, Mexico. Mycorrhiza 16:117–124. doi:10.1007/s00572-005-0022-9
Van Den Driessche R (1989) Changes in osmotic potential of Douglas-fir (Pseudotsuga menziesii) seedlings in relation to temperature and photoperiod. Can J For Res 19:413–421. doi:10.1139/x89-065
Villar-Salvador P, Planelles González R, Oliet Palá J, González de Chavez Fernández M (1998) Efecto de diferentes niveles de estrés hídrico y su duración en las relaciones hídricas de plántulas de Quercus ilex. Actas del 4º Simposium Hispano—Portugués de Relaciones Hídricas de las plantas. 2–3 noviembre 1998. Murcia, pp 65–68
Villar-Salvador P, Planelles R, Oliet J, Penuelas-Rubira JL, Jacobs DF, Gonzalez M (2004) Drought tolerance and transplanting performance of holm oak (Quercus ilex) seedlings after drought hardening in the nursery. Tree Physiol 24(10):1147–1155
Walker RF, McLaughlin SB, West DC (2004) Establishement of sweet birch on surface mine spoil as influenced by mycorrhizal inoculation and fertility. Restor Ecol 12:8–19. doi:10.1111/j.1061-2971.2004.00255.x
Acknowledgments
This research was supported by the regional government of Valencia, Spain (Programa de Investigación y Desarrollo en Relación con la Restauración de la Cubierta Vegetal, CEAM, 1996–1999) and the regional government of Cantabria, Spain (Desarrollo de procedimientos de producción de setas comestibles de hongos de micorrización como alternativa a la obtención de productos excedentarios de cultivo agrícola, Consejería de Agricultura, 1999–2002). We thank ETSI Montes, EUIT Forestal in Madrid, and INIA (Instituto Nacional de Investigaciones Agrarias) for their support and help.
Author information
Authors and Affiliations
Corresponding author
Additional information
José Antonio Rodríguez Barreal—Deceased
Rights and permissions
About this article
Cite this article
Domínguez Núñez, J.A., Planelles González, R., Rodríguez Barreal, J.A. et al. Influence of water-stress acclimation and Tuber melanosporum mycorrhization on Quercus ilex seedlings. Agroforest Syst 75, 251–259 (2009). https://doi.org/10.1007/s10457-008-9197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-008-9197-3