Abstract
Vascular growth factor (VEGF) is an important mediator of angiogenesis. PI3K plays essential roles in angiogenesis; however, the mechanisms and specific functions of individual isoforms of PI3K members in tumor angiogenesis regulation are still not fully understood. In this study, we evaluate the role of p55PIK, a PI3K regulatory subunit encoded by PIK3R3 gene, in tumor angiogenesis. We reported that overexpression of p55PIK in cancer cells up-regulated HIF-1α expression and increased VEGF expression. Furthermore, overexpression of p55PIK increased tumor angiogenesis in vivo and in vitro. Moreover, data indicated enhanced HIF-1α expression by p55PIK-PI3K depended on its ability to activate NF-кB signaling pathways, especially to increase the phosphorylation of p65 subunits of NF-κB. Our study suggested that p55PIK-PI3K was essential in regulating cancer cell-mediated angiogenesis and contributed to tumor growth and that the p55PIK provides a potential and specific target for new anti-angiogenesis drug development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiogenesis is an important process in the growth, local invasion, and metastasis of tumor cells that has become an attractive target in the therapeutic management of cancer [7, 24]. Vascular growth factor (VEGF) is an important mediator of angiogenesis. VEGF expression increases in many cancers and contributes to the growth of many tumors by increasing angiogenesis [11, 15]. Anti-VEGF monoclonal antibody bevacizumab (Avastin) is proven beneficial in the treatment for metastatic colorectal cancers either as single agents or combined with chemotherapy [20, 30, 46]. Therefore, the mechanisms regulating VEGF expression in cancer cells, which may have important implications for the identification of new therapeutic targets and agents, should be further understood [7].
Vascular growth factor-A is a primary transcription target of hypoxia-inducible factor-1 (HIF-1), a heterodimeric basic helix-loop-helix transcription factor composed of HIF-1α and HIF-1β subunits [33, 40]. HIF-1α is up-regulated by hypoxia and various growth factors and oncogenes, whereas HIF-1β is stably expressed in cells [28, 33].
Class IA phosphatidylinositol-3-kinase (PI3K)-mediated signaling is critical for tumor growth and metastasis. PI3K regulates HIF-1α expression in many cell systems in response to hypoxia and growth factors [13, 22]. Reconstitution of PTEN, the molecular inhibitor of PI3K, or inhibition of PI3K activity by LY294002 decreases angiogenesis and tumor growth [22, 23, 32].
PI3K consists of a p110 catalytic subunit (α, β, or δ) complexed to one of the regulatory subunits p85α, p85β, p55α, p55γ, or p50α [5, 8]. p85α, p55α, and p50α are encoded byPIK3R1 gene, p85β is encoded by PIK3R2, and p55γ, also known as p55PIK, is encoded by PIK3R3 [8]. Regulatory subunits are needed to recruit the catalytic unit p110 to specific cellular locations to regulate its catalytic activity [42]. The different regulatory subunits of different N-terminals suggest their specificity in mediating different PI3K signaling [42]. However, the mechanisms by which these regulatory subunits regulate specific pathway signaling are not fully understood.
In our previous study, the p55PIK subunit of PI3K promoted cell proliferation in gastric cancer and colorectal cancer. Moreover, the inhibition of the function of p55PIK decreased cell cycle progression and tumor growth [18, 19, 43]. Recent studies showed that knockdown of p55PIK proteins by siRNA decreased cell proliferation in ovarian cancer cell lines [49]. In this study, we aim to determine the role of p55PIK in tumor angiogenesis. We further investigated the possible mechanism by which p55PIK regulates angiogenesis. The results showed that enhancement or inhibition of p55PIK by overexpressing p55PIK or p55PIK shRNA could regulate the expression level of VEGF-A in cancer cells. Moreover, p55PIK simulated VEGF-A expression by activating NF-κB pathway and increasing the HIF-1α expression.
Materials and methods
Reagents and antibodies
Antibodies for p55PIK, HIF-1α, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), NF-κB p65, p-p65 (ser536), Akt, p-Akt (ser473), ERK, p-ERK, CD31, and VEGF-A were purchased from Santa Cruz Biotechnology. Antibody for HIF-1β was from Cell Signaling. The enzyme-linked immunosorbent assay (ELISA) kit for VEGF-A was purchased from R&D Systems. Cycloheximide (Chx), dimethyl sulfoxide (DMSO), SH-6, and BAY11-7082 were from Sigma. Growth factor-reduced Matrigel was purchased from BD Bioscience. VEGF-A was purchased from PeproTech. Primers used in this study were synthesized by integrated DNA technology.
Cell culture and treatment
All cells were obtained from American Type Culture Collection. LoVo, SW480 cell lines, were cultured at 37 °C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum (Life Technologies). Human umbilical vein endothelial cells (HUVECs) were cultured at 37 °C in EBM-2 endothelial cell basal medium using the SingleQuot kit (Lonza/Cambrex, Walkersville, MD) in accordance with the manufacturer’s instructions. Hypoxic conditions were induced by culturing cells for 2 h in a sealed hypoxia chamber (Billups Rothenberg) after flushing with a mixture of 1 % O2, 5 % CO2, and 94 % N2. When appropriate, cells were treated with indicated concentrations of Chx, SH-6, and BAY11-7082 under normoxia or hypoxia for indicated time.
Construction of lentiviral-infected cell lines
A cDNA encoding human p55PIK (DF/HCC DNA Resource Core, NM_003629.3) was cloned into the lentivirus vector plasmid pCDF1 (System Biosciences, SBI). A p55PIK-expressing lentivirus (Lenti-p55PIK) and a control lentivirus (Lenti-con) were then constructed and packaged following the manufacturer’s instructions (System Biosciences, SBI). Virus particles were titered with 293FT cells using SBI Lentivector expression systems (Version 5).
Short hairpin RNAs (shRNA, 5′-GGACUUGCUUUAUGGGAAA-3′), targeting the p55PIK, and a non-targeting RNA sequence, serving as negative control (Scr-shRNA), were cloned into the pENTR/U6 entry vector (Invitrogen). A shRNA-expressing lentivirus (Lenti-p55PIKi) and a control lentivirus (Lenti-sc) were then constructed and packaged following the manufacturer’s instructions (Invitrogen). Virus particles were titered using 293FT cells following the manufacturer’s instructions (Invitrogen).
Tissue samples
All colorectal tumors and normal tissues were collected with an informed consent from the patients and under an institutionally approved protocol at the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, PR China. All samples were examined by pathologists. Fresh tumor tissues or normal tissues were divided into three parts: one applied in Trizol for RNA isolation, one used to prepare lysate for Western blotting, and one fixed with formalin for histologic analysis.
cDNA microarray analysis
The expression of p55PIK and VEGF-A mRNA in colorectal cancer samples was examined using the Oncomine database and gene microarray analysis tool, a repository for published cDNA microarray data (www.oncomine.org) [34, 35]. The Oncomine algorithms, which enable multiple comparisons among different studies, were used to statistically analyze the differences in mRNA expression between the aforementioned comparisons [35].
Preparation of conditioned medium (CM) and analysis of VEGF-A expression
Conditioned medium was prepared as described previously with some modifications [39]. Briefly, the LoVo cells were seeded at a density of 1 × 105 cells/ml in 6-well plates. Lenti-p55PIK-, Lenti-con-, Lenti-sc-, or Lenti-p55PIKi-infected LoVo cells were added after 12 h, and the cells were washed three times in phosphate-buffered saline and incubated in serum-free medium for 6 h. CM was collected and stored at −80 °C.
The VEGF-A levels in CM were measured by VEGF-A-specific ELISA kits (R&D Company) according to the manufacturer’s instructions.
Real-time PCR assay for p55PIK, HIF-1α, and VEGF-A
Real-time PCR was performed to determine the messenger RNA (mRNA) levels of p55PIK, HIF-1α, and VEGF-A. Total mRNA was extracted using the TRIzol reagent (Invitrogen). Reverse transcription was performed using an RT–PCR kit (TaKaRa). The PCR primer sequences of p55PIK, HIF-1α, VEGF-A, and GAPDH were as follows: p55PIK (forward primer: 5′-GAGTATGGACCGCGATGACG and reverse primer: 5′-GCTTAGGTGGCTTTGGTGGAA), HIF-1α (forward primer: GGCGCGAACGACAAGAAAAAG and reverse primer: CCTTATCAAGATGCGAACTCACA), VEGF-A (forward primer: CGCAGCTACTGCCATCCAAT and reverse primer: GTGAGGTTTGATCCGCATAATCT), and GAPDH (forward primer: 5′-GGTGTGAACCATGAGAAGTATGACAAC and reverse primer: 5′-CCAGTAGAGGCAGGGATGATGTTC). The comparative CT method was used to quantitate the expression of p55PIK, HIF-1a, and VEGF-A using GAPDH as control [37].
Cell proliferation, scratch-wound assay, and in vitro angiogenesis assay
Cell proliferation was determined using MTT reduction method. Briefly, 103 HUVECs were cultured in 96-well plates for 24 h, and various CMs were added to the wells. After 48 h, cell numbers were quantified via MTT assay.
When the density of HUVEC in 24-well plates reached about 100 %, scratch-wound assay was carried out using a pipette tip. After scratch wound, 1 μM of 5-fluorouracil (Sigma) was added to block cell proliferation. HUVEC migration was scored 24 h after wound scratch. The distance of each scratch closure was obtained based on the distances measured by software.
In vitro angiogenesis assay was conducted by tube formation. Briefly, growth factor-reduced Matrigel was placed in 96-well tissue culture plates and allowed to form a gel at 37 °C for 30 min. HUVECs (2 × 104 cells) were added into each well and incubated in CM at 37 °C for 24 h. Endothelial tubes were examined under a light microscope every 4 h by inspecting the branch points.
Western blot assay
Cells were lysed in 1 % NP40. Equal amount of proteins in lysates was separated on 10 or 12 % SDS–PAGE and transferred to PVDF membrane (Bio-Rad). Proteins were detected by immunoblotting using specific antibodies.
Animal samples
Male BALB/c nude mice (Wuhan Laboratory Animal Center) were housed under specific pathogen-free conditions in a temperature- and humidity-controlled environment and given unlimited access to water and food. All animal experiments were conducted in accordance with the institutional animal research guidelines approved by the local ethics committee.
Cells were harvested after 24 h and resuspended in 50 % mixture of Matrigel and DMEM (5 × 107/ml). A total of 5x106 cells were injected subcutaneously into the flank of each mouse. Each mouse was implanted with two xenografts of Lenti-con-infected cells in the right flank and Lenti-p55PIK-infected cells in the left flank. Tumor volumes were calculated by recording tumor dimensions every 7 days, starting from the first day of implantation. Animals were killed 35 days after injection. The tumor xenografts were removed from the mice, immediately weighed, and then bisected. Part of each tumor was fixed in 4 % paraformaldehyde overnight and analyzed by immunohistochemistry, while the other half was homogenized to prepare lysates for Western blot analysis.
Microvascular density (MVD) was determined according to the method described by Weidner [45]. Briefly, the stained sections were screened under a light microscope at 40× magnification to identify the areas of highest CD31-positive vessel density. These areas were then counted at 200× magnification in ten random fields. Data were collected by two independent observers without knowledge of which tumors were viewed. The number of microvessels in each field was determined and expressed as the MVD.
Statistical analysis
All data were analyzed using Student’s t test. Comparison between multiple experimental groups was accomplished by a Bonferroni test with SPSS 13.0 for Windows. Values of p < 0.05 were considered statistically significant.
Results
p55PIK and VEGF-A expression levels in colorectal cancer
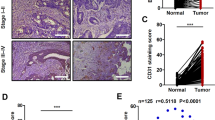
The Oncomine database and gene microarray data analysis tool enabled the meta-analysis of PIK3R3 (p55PIK) and VEGF-A expressions in the colorectal cancer microarray studies [38]. Based on the analysis, we considered the expression levels of p55PIK and VEGF-A as positively correlated (Fig. 1a). To confirm these data, we detected the expression levels of p55PIK and VEGF-A in colorectal cancer samples by Western blot. The data showed that the expression levels of p55PIK and VEGF-A were positively correlated (Fig. 1b). Moreover, we compared p55PIK expression level in LoVo cells, a colon cancer cell line, and normal colorectal epithelial cells, which showed that expression of p55PIK in LoVo cells is higher than in normal colorectal epithelial cells (Supplement Figure. 1).
p55PIK and VEGF-A expression levels in colorectal cancer samples. a p55PIK and VEGF-A expressions in colorectal cancers. Box plots show differences in mRNA expression between cancer tissues (blue) and normal control (white). The statistical significance is shown for comparisons of tumor versus normal. b p55PIK and VEGF-A protein levels in normal tissues (N) and tumor tissues (T). Nine patients are analyzed in Western blot. (Color figure online)
p55PIK promotes tumor xenograft growth and tumor-induced angiogenesis in nude mice
To determine whether p55PIK promoted tumor growth and tumor angiogenesis in vivo, nude mice were subcutaneously injected with LoVo cells infected by Lenti-p55PIK and control cells infected by Lenti-con. We found that the tumors derived from p55PIK-overexpressing LoVo cells were heavier and larger than those of the control group (Fig. 2a,b). The MVD of tumors derived from p55PIK-overexpressing LoVo cells was more than that of the control group (Fig. 2c). We then paraffin stained the sections from tumor xenografts of p55PIK, CD31, HIF-1α, and VEGF-A. The expression of CD31, HIF-1α, and VEGF-A in tumors derived from p55PIK-overexpressing LoVo cells was obviously higher than those of the control group (Fig. 2d). The expression of CD31 and VEGF-A in tumors derived from p55PIK-overexpressing LoVo cells was obviously more than those of control group (Fig. 2d). In addition, we also showed the protein levels of p55PIK and HIF-1α in the tumor xenografts (Fig. 2e). Then, down-regulation of p55PIK showed reduction in tumor growth and angiogenesis (Supplement Figure 2). All these data further illustrated that p55PIK could promote tumor angiogenesis and VEGF-A expression in vivo.
p55PIK promotes tumor xenograft growth and tumor-induced angiogenesis in nude mice. a Tumor xenograft growth from LoVo cells. Shown are representative tumor xenografts and final mean tumor weights (mean ± SD, n = 6, p = 9.31111E-06; Lenti-p55PIK group compared with Lenti-con group). b Time course comparing LoVo tumor xenograft growth after intra-tumoral injection of Lenti-p55PIK and Lenti-con. The tumor volumes and the general health of mice are monitored before they are killed. The length, width, and depth of tumors are measured twice a week to determine tumor volume (mean ± SD, n = 6). c Microvascular density (MVD) is determined according to the method described by Weidner [45] (Lenti-p55PIK group compared with Lenti-con group). d Immunohistochemical analyses of tumor xenografts. CD31, p55PIK, and VEGF-A protein expressions are examined in each tumor group and carrier. e The expression level of VEGF-A, p55PIK, and HIF-1α in xenograft tumors
p55PIK regulates the expression of VEGF-A
Lenti-p55PIK-, Lenti-con-, Lenti-sc-, and Lenti-p55PIKi-infected LoVo cells. After 12 h, the cell number showed no distinction between each of the groups (data not shown). The overexpression of p55PIK by Lenti-p55PIK promoted the mRNA expression and protein levels of VEGF-A in both normoxia and hypoxia (Fig. 3a, b), whereas the knockdown of p55PIK by Lenti-p55PIKi reduced the mRNA and protein levels of VEGF-A in normoxia and hypoxia (Fig. 3c, d). These results indicated that p55PIK can positively regulate the expression of VEGF-A at both mRNA and protein levels.
p55PIK regulates the expression of VEGF-A. a VEGF-A mRNA level in cells treated with Lenti-con or Lenti-p55PIK. All experiments are detected and analyzed in triplicates (normoxic and hypoxic conditions, Lenti-p55PIK group compared with Lenti-con group). b VEGF-A protein level in LoVo cells treated with Lenti-con or Lenti-p55PIK. All experiments are detected and analyzed in triplicates (normoxic and hypoxic conditions, Lenti-p55PIK group compared with Lenti-con group). c VEGF-A mRNA level in cells treated with Lenti-sc or Lenti-p55PIKi. All experiments are detected and analyzed in triplicates (normoxic and hypoxic conditions, Lenti-p55PIKi group compared with Lenti-sc group). d VEGF-A protein level in LoVo cells treated with Lenti-con or Lenti-p55PIK. All experiments are detected and analyzed in triplicates (normoxic and hypoxic conditions, Lenti-p55PIKi group compared with Lenti-sc group)
p55PIK regulates the expression of HIF-1α at transcription level
HIF-1α is one of the most important genes that regulate tumor angiogenesis. To further indicate the correlation of p55PIK and HIF-1α in vitro, we infected LoVo cells with Lenti-p55PIK, Lenti-con, Lenti-sc, and Lenti-p55PIKi and detected the mRNA and protein levels of HIF-1α in LoVo cells treated under normoxia and hypoxia. Compared with the control group, the mRNA and protein levels of HIF-1α were augmented when p55PIK was overexpressed under normoxia, whereas the mRNA and protein levels of HIF-1α were reduced when p55PIK was knocked down by Lenti-p55PIKi, but with no effect on HIF-1β expression level. Similar results were obtained under hypoxia (Fig. 4a, b). The same result was extended to SW480 cells in which p55PIK overexpression enhanced the mRNA level and protein level of HIF-1α (data not shown).
p55PIK regulates the expression of HIF-1α at transcription level. a (UP) HIF-1α mRNA level in cells treated with Lenti-con or Lenti-p55PIK. (Down) p55PIK and HIF-1α expression levels in LoVo cells treated with Lenti-con or Lenti-p55PIK. Glyceraldehyde phosphate dehydrogenase expression is also analyzed with an appropriate antibody to show the equal loading of proteins in every well (normoxic and hypoxic conditions). b HIF-1α mRNA level in cells treated with Lenti-sc or Lenti-p55PIKi. (Down) p55PIK and HIF-1α expression levels in LoVo cells treated with Lenti-sc or Lenti-p55PIKi (normoxic and hypoxic conditions). c HIF-1α expression level in LoVo cells treated with Lenti-p55PIK, Lenti-con, and Chx. Comparison of the protein level at 0, 1, 2, and 3 h. d HIF-1α expression level in LoVo cells treated with Lenti-p55PIK, Lenti-con, and MG132 (normoxic and hypoxic conditions)
HIF-1α expression level was regulated by transcription activation and its protein stability [41]. Since p55PIK can change the HIF-1α expression level, we wanted to determine whether p55PIK could increase HIF-1α transcription activation or affect the stability of HIF-1α protein. LoVo cells infected with Lenti-p55PIK and Lenti-con cells were treated with the protein translation inhibitor Chx. We determined the HIF-1α expression level at 0, 1, 2, and 3 h in LoVo cells. The relative HIF-1α protein level after Chx treatment for 1, 2, and 3 h was similar for both Lenti-p55PIK and Lenti-con groups (Fig. 4c). This result suggests that p55PIK cannot affect the degradation of HIF-1α. To further confirm this result, LoVo cells infected with Lenti-p55PIK and Lenti-con cells were treated with the proteasome inhibitor MG132 under normoxic or hypoxic condition. We found that overexpression of p55PIK increased the HIF-1α level (Fig. 4d), which confirmed that p55PIK was not involved in HIF-1α stabilization. These results showed that p55PIK was involved in HIF-1α transcription but not in stabilization.
p55PIK regulates HIF-1α expression independent of the AKT and ERK pathways
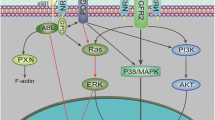
PI3K/AKT/mTOR pathway is one of the most important regulators of HIF-1α protein synthesis [9, 23]. Since p55PIK is a regulatory subunit of PI3K, it may influence the phosphorylation of AKT and induce changes in the expression level of HIF-1α. We detected the phosphorylation level of AKT in LoVo cells after overexpression or knockdown of p55PIK by Lenti-p55PIK or Lenti-p55PIKi, respectively. The result showed that p55PIK had no effect on the phosphorylation of AKT (Fig. 5a). We also found that p55PIK had no effect on the phosphorylation of ERK (Fig. 5a). To further confirm whether p55PIK regulates HIF-1α through AKT-independent pathway, SH6, an AKT phosphorylation inhibitor, was used. The inhibition of AKT phosphorylation did affect the regulation of p55PIK on HIF-1α either in normoxia or in hypoxia (Fig. 5b). These results showed that p55PIK regulates HIF-1α independent of the PI3K/AKT and ERK pathways.
p55PIK regulates HIF-1α independent of AKT and ERK pathways. a (Left) LoVo cells treated with Lenti-p55PIK and Lenti-con. AKT, p-AKT, ERK, and p-ERK are detected by Western blot analysis. (Right) LoVo cells treated with Lenti-p55PIKi and Lenti-sc. AKT, p-AKT, ERK, and p-ERK are detected by Western blot analysis. Representative histograms from an individual experiment and similar results obtained in three independent experiments. b LoVo cells treated with Lenti-p55PIK, Lenti-con, and SH-6. HIF-1α expression level is detected by Western blot analysis (normoxic and hypoxic conditions). Representative histograms from an individual experiment and similar results obtained in three independent experiments
p55PIK regulates HIF-1α dependent on NF-κB pathway
Several studies demonstrated that NF-κB pathway could regulate the synthesis of HIF-1α protein in both normoxic and hypoxic conditions [12, 41]. In our previous study, we found that p55PIK activates the NF-κB pathway. In this study, we attempted to determine whether p55PIK regulates HIF-1α through the NF-κB pathway. We found that overexpression of p55PIK could promote the phosphorylation of NF-κB p65 (ser536) and low expression of p55PIK could decrease the phosphorylation of p65 (ser36) in LoVo cells. Similar results were obtained either in normoxic or in hypoxic condition (Fig. 6a, b). We also detected that the activation of p55PIK in the NF-κB pathway was AKT-dependent or AKT-independent. Although the AKT phosphorylation was inhibited, the p65 (ser536) phosphorylation was still promoted by the overexpression of p55PIK, which suggests that p55PIK regulates the activation of NF-κB pathway in an AKT-independent manner (Fig. 6c).
p55PIK regulates HIF-1α dependent on NF-κB pathway. a p55PIK, NF-кB p65, and p-p65 expression levels in LoVo cells treated with Lenti-p55PIK and Lenti-con under normoxic or hypoxic condition. b p55PIK, NF-кB p65, and p-p65 expression levels in LoVo cells treated with Lenti-p55PIKi and Lenti-sc under normoxic or hypoxic condition. c LoVo cells treated with Lenti-p55PIK, Lenti-con, and AKT inhibitor SH-6. p55PIK, AKT, p-AKT, NF-кB p65, and p-p65 are detected by Western blot analysis under normoxic and hypoxic conditions. d LoVo cells treated with Lenti-p55PIK, Lenti-con, and NF-κB pathway inhibitor BAY. HIF-1α, NF-кB p65, and p-p65 are detected by Western blot analysis under normoxic and hypoxic conditions
The phosphorylation of p65 (serine 536) is necessary for its translocation into the nucleus, which activates NF-κB [36]. To further confirm whether the p55PIK-enhanced HIF-1α expression depends on the NF-κB pathway, the inhibitor of NF-κB pathway BAY-117082 was used. The phosphorylation of p65 (ser536) was used as the sign of the activation of NF-κB pathway [26]. We found that BAY-117082 could suppress the regulatory effects of p55PIK on HIF-1α. Similar results were obtained both in normoxic and in hypoxic conditions (Fig. 6d). The results showed that the regulation of HIF-1α expression by p55PIK may depend on the NF-κB pathway activation. Overall, we concluded that p55PIK regulated HIF-1α through the NF-κB pathway in an AKT-independent manner.
p55PIK promotes the proliferation, migration, and tube formation of HUVECs induced by CM from LoVo cells
Vascular growth factor-A can promote the proliferation, migration, and angiogenesis of endothelial cells [17]. We detected the impact of various CMs prepared on the growth, migration, and tube formation of HUVECs. After incubating the HUVECs with CM for 48 h, we detected the cell proliferation ability by MTT. We found that either in normoxic or in hypoxic condition, the CM of p55PIK-overexpressing LoVo cells could notably promote cell proliferation of HUVECs compared with the CM of control LoVo cells (Fig. 7a). On the contrary, the low expression of p55PIK could inhibit the cell proliferation of HUVECs both in normoxic and in hypoxic conditions (Fig. 7b). We then detected the migration ability of HUVECs by scratch-wound assay. To rule out the influence of cell proliferation, 1 μM of 5-fluorouracil was added to the cell immediately after scratch wound. After incubating the cells with CMs for 24 h, we found that CM produced by p55PIK-overexpressing LoVo cells can significantly promote the migration ability of HUVECs in normoxic condition. Similar results were obtained in hypoxic condition (Fig. 7c). The CM produced by LoVo cells which knocked down p55PIK decreased the migration ability of HUVECs in normoxic and hypoxic conditions (Fig. 7d). To determine whether p55PIK can promote angiogenesis, we performed tube formation assay in vitro. We found that HUVECs in CMs prepared from p55PIK-overexpressing LoVo cells formed more tubes than those in the control group both in normoxic and in hypoxic conditions (Fig. 7e). The CM produced by LoVo cells which knocked down p55PIK decreased the ability of tube formation by HUVECs in normoxic and hypoxic conditions, which could be rescued by using VEGF-A (Fig. 7f). Similar results were obtained from replicated experiments. These results showed that p55PIK could regulate the tumor cell-mediated proliferation, migration, and tube formation of HUVECs.
p55PIK promotes the proliferation, migration, and tube formation of HUVECs induced by CM from LoVo cells. a MTT assay detected HUVECs proliferation treated with CMs from LoVo cells treated with Lenti-p55PIK or Lenti-con (normoxic and hypoxic conditions; Lenti-p55PIK group compared with Lenti-con group). b MTT assay detected proliferation of HUVECs treated with CMs from LoVo cells treated with Lenti-p55PIKi or Lenti-sc (normoxic and hypoxic conditions; Lenti-p55PIKi group compared with Lenti-sc group). c Scratch-wound assay detected migration of HUVECs treated with CMs from LoVo cells treated with Lenti-p55PIK or Lenti-con (normoxic and hypoxic conditions; Lenti-p55PIK group compared with Lenti-con group). d Scratch-wound assay detected migration of HUVECs treated with CMs from LoVo cells treated with Lenti-p55PIKi or Lenti-sc (normoxic and hypoxic conditions; Lenti-p55PIKi group compared with Lenti-sc group). e Tube formation assay detected formed tubes of HUVECs treated with CMs from LoVo cells treated with Lenti-p55PIK or Lenti-con under normoxic and hypoxic conditions. Calculating branch points per field was used to quantify the ability of tube formation (normoxic and hypoxic conditions; Lenti-p55PIK group compared with Lenti-con group). f Tube formation assay detected formed tubes of HUVECs treated with CMs from LoVo cells treated with Lenti-p55PIKi or Lenti-sc with or without adding VEGF-A under normoxic and hypoxic conditions. * p < 0.05
Discussion
Tumor progression is manifested by the ability of cancer cells to grow in an unrestrained manner and to establish secondary colonies, both of which require a well-developed set of blood vessels, such as angiogenesis [29]. The genetic alterations in oncogenes and tumor suppressors or the reduced availability of oxygen induces tumor cell expression of many pro-angiogenic growth factors, which stimulate the migration and proliferation of ECs to sprout and form new vasculatures [3, 4, 6]. VEGF-A, which is secreted by tumor cells, plays a key role in this process [11].
The PI3 K/AKT signaling pathway plays an important role in regulating the vasculature and the process of angiogenesis. Several studies have demonstrated that the PI3K activation regulates the VEGF-A expression level in different types of cancer cells through HIF-1, ERK1/2, and NF-кB activation to induce tumor angiogenesis [16, 22, 23]. Various inhibitors that target the PI3K or mTOR signaling pathways have been developed to decrease VEGF-A secretion and angiogenesis [23].
The genetic alterations in the PI3K signaling molecules activate the PI3K signaling pathway involved in various kinds of human cancers, such as ovarian, breast, colon, and thyroid. These alterations include the amplification or mutation of PIK3CA, which encodes p110α, PTEN loss, AKT1 mutation, p85α mutation, p110β amplification, and so on [2, 21, 44, 48, 50].
In our previous study, the p55PIK promoted cell cycle progression and tumor development of colorectal cancer, leukemia, and thyroid cancer [19, 43]. Recent studies showed increased p55PIK mRNA expression and protein levels in majority of ovarian cancers [49]. We also found that p55PIK mRNA expression and protein levels increased in colorectal and gastric cancers (data not shown, Guihua Wang and Junbo Hu). In our present study, we found that p55PIK promoted tumor angiogenesis by enhancing the expression of VEGF-A.
The VEGF-A expression level is regulated by HIF-1α [15]. HIF-1α is a transcription factor involved in cellular adaptation to hypoxia. HIF-1α is inactive when oxygen is abundant but activated in hypoxic conditions [12]. Overexpression of HIF-1α was observed in many solid tumors [28]. In this study, we found that p55PIK may regulate the VEGF-A expression by up-regulating HIF-1α. Moreover, p55PIK was involved only in HIF-1α transcription but not in regulating HIF-1α stabilization (Fig. 4).
Previous studies showed that both PI3K/AKT and ERK could activate HIF-1α in tumor angiogenesis [10, 31]. However, the results of this study showed that the AKT and ERK phosphorylation showed no change by either up-regulating or down-regulating the p55PIK expression level. In addition, the inhibition of AKT phosphorylation did not affect the regulation of p55PIK on HIF-1α when p55PIK was overexpressed or knocked down in cells. These results showed that the regulation of HIF-1α by p55PIK was independent of the PI3K/AKT or ERK pathways.
Previous studies reported that NF-κB is a direct modulator of HIF-1α expression, which regulates HIF-1α transcription during inflammation [41]. Many studies suggested that activated p65 translocates into the nucleus to activate NF-κB. The phosphorylation of p65 (ser536) is necessary for this process. In this study, we found that p55PIK promoted the phosphorylation of p65 (ser536) through an AKT-independent manner. The regulation of HIF-1α expression by p55PIK may depend on the NF-κB pathway activity.
To further confirm whether p55PIK promoted the cancer cell-induced tumor angiogenesis, we used various CMs prepared from different p55PIK expression levels to detect its influence on EC growth, migration, and tube formation. The data showed that p55PIK could regulate the tumor cell-mediated EC proliferation, migration, and tube formation.
PI3K contains several isoforms of regulatory and catalytic subunits. Since the catalytic subunits of PI3K can bind to each regulatory subunit (p85, p55, and p50) with similar affinity, the signaling specificity may be mediated by the heterodimerization of the p110 catalytic subunits with different PI3K regulatory subunits [5]. Previous studies showed that PI3K regulatory subunits bind to p110 catalytic subunits and fix p110 in cell membranes. When growth factors stimulate cells, the catalytic subunits are released from regulatory subunits and activate downstream signaling [14]. Several studies showed that overexpression of p85 regulatory subunit inhibits PI3K cell proliferation [1]. However, the results showed that the p55PIK regulatory subunit had different functions from other regulatory subunits. p55PIK contains a unique N-terminal and mediates several PI3K-specific signaling [47]. p55PIK can bind to Rb by the N-terminal and positively regulate cell cycle progression and proliferation. The inhibition of the function of p55PIK can block the cell cycle progression and inhibit tumor growth. These results suggested that p55PIK mediates the PI3K-specific pathway signaling. Different from the classic PI3K/AKT pathway, p55PIK could promote NF-κB p65 phosphorylation and activate NF-κB signaling pathway. Given that the PI3K/AKT and NF-κB pathways contain several cross talks, AKT can also activate the NF-κB pathway [25]. However, we found that activation of the NF-κB signaling pathway by p55PIK did not depend on AKT. p55PIK mediated a specific PI3K downstream signaling in an NF-κB pathway-dependent manner but not AKT.
PI3Ks have been considered as promising targets for the treatment of cancer and cardiovascular, inflammatory, and autoimmune diseases [8]. A number of PI3K/AKT pathway inhibitors have been developed. These inhibitors showed higher efficacy against tumor angiogenesis and growth. However, since PI3K/AKT pathways were involved in many normal cellular processes, new drugs need to be developed to minimize the adverse effects associated with other PI3K/AKT-dependent cellular processes [27]. In this study, we showed that the PI3K regulatory subunit p55PIK regulates tumor angiogenesis in an AKT-independent and NF-κB-dependent manner. This result may provide a novel insight that the regulation of tumor angiogenesis by PI3K and inhibition of p55PIK signaling may have advantages over the present PI3K/AKT pathway inhibitors, which may give a new molecular target for tumor therapy in p55PIK-overexpressed tumors.
References
Anderson DH (2010) p85 Plays a critical role in controlling flux through the PI3K/PTEN signaling axis through dual regulation of both p110 (PI3K) and PTEN. Cell Cycle 9:2055–2056
Berenjeno IM, Vanhaesebroeck B (2009) PI3K regulatory subunits lose control in cancer. Cancer Cell 16:449–450
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3:401–410
Bikfalvi A (2006) Angiogenesis: health and disease. Ann Oncol 17(Suppl 10):x65–x70
Bunney TD, Katan M (2010) Phosphoinositide signalling in cancer: beyond PI3K and PTEN. Nat Rev Cancer 10:342–352
Carmeliet P (2003) Angiogenesis in health and disease. Nat Med 9:653–660
Cook KM, Figg WD (2010) Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 60:222–243
Courtney KD, Corcoran RB, Engelman JA (2010) The PI3K pathway as drug target in human cancer. J Clin Oncol 28:1075–1083
Dayan F, Bilton RL, Laferriere J et al (2009) Activation of HIF-1alpha in exponentially growing cells via hypoxic stimulation is independent of the Akt/mTOR pathway. J Cell Physiol 218:167–174
Du J, Xu R, Hu Z et al (2011) PI3K and ERK-induced Rac1 activation mediates hypoxia-induced HIF-1alpha expression in MCF-7 breast cancer cells. PLoS One 6:e25213
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364:656–665
Fang J, Ding M, Yang L et al (2007) PI3K/PTEN/AKT signaling regulates prostate tumor angiogenesis. Cell Signal 19:2487–2497
Foukas LC, Berenjeno IM, Gray A et al (2010) Activity of any class IA PI3K isoform can sustain cell proliferation and survival. Proc Natl Acad Sci U S A 107:11381–11386
Grothey A, Galanis E (2009) Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nat Rev Clin Oncol 6:507–518
Hamada K, Sasaki T, Koni PA et al (2005) The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 19:2054–2065
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23:1011–1027
Hu J, Liu S, Wang J et al (2005) Overexpression of the N-terminal end of the p55gamma regulatory subunit of phosphatidylinositol 3-kinase blocks cell cycle progression in gastric carcinoma cells. Int J Oncol 26:1321–1327
Hu J, Xia X, Cheng A et al (2008) A peptide inhibitor derived from p55PIK phosphatidylinositol 3-kinase regulatory subunit: a novel cancer therapy. Mol Cancer Ther 7:3719–3728
Hubbard J, Grothey A (2010) Antiangiogenesis agents in colorectal cancer. Curr Opin Oncol 22:374–380
Jia S, Liu Z, Zhang S et al (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454:776–779
Jiang BH, Liu LZ (2009) PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res 102:19–65
Karar J, Maity A (2011) PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci 4:51
Kerbel RS (2008) Tumor angiogenesis. N Engl J Med 358:2039–2049
Kloo B, Nagel D, Pfeifer M et al (2011) Critical role of PI3K signaling for NF-kappaB-dependent survival in a subset of activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 108:272–277
Lawrence T, Bebien M, Liu GY et al (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434:1138–1143
Marone R, Cmiljanovic V, Giese B et al (2008) Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 1784:159–185
Maxwell PH, Dachs GU, Gleadle JM et al (1997) Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A 94:8104–8109
Menakuru SR, Brown NJ, Staton CA et al (2008) Angiogenesis in pre-malignant conditions. Br J Cancer 99:1961–1966
Mitchell DC, Bryan BA (2010) Anti-angiogenic therapy: adapting strategies to overcome resistant tumors. J Cell Biochem 111:543–553
Mylonis I, Chachami G, Paraskeva E et al (2008) Atypical CRM1-dependent nuclear export signal mediates regulation of hypoxia-inducible factor-1alpha by MAPK. J Biol Chem 283:27620–27627
Okumura N, Yoshida H, Kitagishi Y et al (2012) PI3K/AKT/PTEN signaling as a molecular target in leukemia angiogenesis. Adv Hematol 2012:843085
Pufe T, Lemke A, Kurz B et al (2004) Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol 164:185–192
Rhodes DR, Yu J, Shanker K et al (2004) Large-scale meta-analysis of cancer microarray data identifies common transcriptional profiles of neoplastic transformation and progression. Proc Natl Acad Sci U S A 101:9309–9314
Rhodes DR, Yu J, Shanker K et al (2004) ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia 6:1–6
Sakurai H, Chiba H, Miyoshi H et al (1999) IkappaB kinases phosphorylate NF-kappaB p65 subunit on serine 536 in the transactivation domain. J Biol Chem 274:30353–30356
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C (T) method. Nat Protoc 3:1101–1108
Skrzypczak M, Goryca K, Rubel T et al (2010) Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. Plos One 5:e13091
Tian T, Nan KJ, Wang SH et al (2010) PTEN regulates angiogenesis and VEGF expression through phosphatase-dependent and -independent mechanisms in HepG2 cells. Carcinogenesis 31:1211–1219
Tsuzuki Y, Fukumura D, Oosthuyse B et al (2000) Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha–> hypoxia response element–> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res 60:6248–6252
Van Uden P, Kenneth NS, Rocha S (2008) Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem J 412:477–484
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M et al (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Bio 11:329–341
Wang G, Deng Y, Cao X et al (2012) Blocking p55PIK Signaling inhibits proliferation and induces differentiation of leukemia cells. Cell Death Differ 19:1870–1879
Wee S, Wiederschain D, Maira SM et al (2008) PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci U S A 105:13057–13062
Weidner N, Semple JP, Welch WR et al (1991) Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 324:1–8
Winder T, Lenz HJ (2010) Vascular endothelial growth factor and epidermal growth factor signaling pathways as therapeutic targets for colorectal cancer. Gastroenterology 138:2163–2176
Xia X, Cheng A, Akinmade D et al (2003) The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol Cell Biol 23:1717–1725
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27:5497–5510
Zhang L, Huang J, Yang N et al (2007) Integrative genomic analysis of phosphatidylinositol 3′-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin Cancer Res 13:5314–5321
Zhao L, Vogt PK (2008) Class I PI3K in oncogenic cellular transformation. Oncogene 27:5486–5496
Acknowledgments
This study was supported by grants of foundation of “973” Program (No. 2009CB521802), National Natural Science foundation (No. 30872472, No.30973496, No.81172512, No.81272278, and No.31000612). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Guihua Wang and Cheng Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, G., Chen, C., Yang, R. et al. p55PIK-PI3K stimulates angiogenesis in colorectal cancer cell by activating NF-κB pathway. Angiogenesis 16, 561–573 (2013). https://doi.org/10.1007/s10456-013-9336-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-013-9336-y