Abstract
Airborne pathogens pose a great threat to public health, and their appearance in bioaerosol also increases the contiguousness due to the long survival time and transmitting range. Real-time monitoring and rapid detection methods provide more effective prevention and control of airborne pathogens. The whole procedure could be divided into bioaerosol collection and detection processes. This review presents the basic principles and recent advances in commonly used methods for each of these two steps. We categorized four different kinds of collection methods based on their principles and discussed possible enrichment methods against a small amount of targets. Four different detection methods were compared regarding their ability to perform rapid testing. In the final section, we analyzed the latest trend in combining all these steps to set up a single device or platform for rapid, automated, and continuous on-site bioaerosol monitoring to overcome time and space constraints and increase the speed of the entire monitoring process. We conclude that an integrated all-in-one system using a microfluidic platform is the most promising solution for real-time monitoring of airborne pathogens, since they are capable of simplifying operational steps, efficient collection, and high-throughput detection, demonstrating the strong potential of field-deployable platforms.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Bioaerosols are collections of solid or liquid particles containing bacteria, viruses, pollens, and fungi suspended in a gas under various natural and anthropogenic activities (Fröhlich-Nowoisky et al., 2016; Robinson et al., 2023; Yu et al., 2021). Monitoring levels of airborne pathogens in bioaerosols is of increasing importance for the outbreak and prevention of airborne epidemics. After sampling bioaerosols from the environment or indoor spaces, the safety of bioaerosols is assessed through sample processing and detection of target harmful microorganisms.
1.1 Airborne pathogens and airborne hazards
Airborne pathogens include various infectious and dangerous microorganisms: bacteria, fungi, viruses, mycoplasmas, etc., with the most common contamination sources being soil, water, and human activity (Kalavakonda et al., 2021; Triadó-Margarit et al., 2022). For example, Mycobacterium tuberculosis (Mtb) and Streptococcus pneumoniae could cause respiratory disease and even invade other organs through airborne transmission (Freschi et al., 2021; Zhao et al., 2019). Aspergillus accounts for about 12% of airborne fungi, and its infection manifests as non-necrotizing granulomatous lesions in multiple organs, especially in lungs (Ijaz et al., 2016). Airborne viruses have caused several notable pandemics in human history, including the Spanish influenza pandemic in 1918–1919, the Severe Acute Respiratory Syndrome (SARS) in 2002, the influenza A virus in 2009, and the most recent COVID-19 pandemic. The US Centers for Disease Control and Prevention (CDC) estimated that influenza viruses have caused 35.6 to 92 million illnesses and 12,000 to 56,000 deaths annually since 2010 (Kim et al., 2020a). There is growing evidence of a significant correlation between cancer subjects and exposure to bioaerosols (Humbal et al., 2018; Wang et al., 2019a). Airborne pathogens pose a serious threat to human health since their transmission in bioaerosols increases both the time and travel distance of contagious period (Wang et al., 2022), thus significantly increasing the risk of airborne infectious diseases in public places (Ahmadipour et al., 2019; Bennett & Parks, 2006; Emerson et al., 2015; Gladding et al., 2020; Glushakova et al., 2022; Madhwal et al., 2020; Ye et al., 2021) and healthcare settings (Ge et al., 2020; Innes et al., 2021; Melzow et al., 2022; Oksanen et al., 2022; Putzer et al., 2022; Wong et al., 2021; Workman et al., 2020), while it has also been shown that dust can act as support material for pathogenic microorganisms in bioaerosols which leads to enhancement of pathogen viability(Noda et al., 2022). Pathogens that exhibit resistance against therapeutic drugs are frequently observed in recent studies (Howard & Khader, 2020), which makes prevention of transmission a more important subject in public health.
Humans, animals, plants, and the environment are interconnected. Pathogenic microorganisms in bioaerosols not only affect the economic income of farming (Meyer et al., 2017) and animal husbandry (Alonso et al., 2015; Cheng et al., 2020; Hou et al., 2020; Kaplan et al., 2020; Lu et al., 2020; Poonsuk et al., 2018; Prost et al., 2019; Reicks, 2019; Wei et al., 2023; Yu et al., 2016a), but at the same time, two-thirds of all new and emerging infectious diseases are now zoonotic (Kotwal & Yadav, 2021), with pathogens such as avian influenza viruses (Bertran et al., 2017; Li et al., 2018a), H3 swine influenza viruses (Kaplan et al., 2020), and Mycobacterium burnetii (Gregory et al., 2019; Winter et al., 2021). It is worrisome that findings in recent years have confirmed the hypothesis that bioaerosols are a potential pathway for the spread of bacterial diseases in plants (Berry et al., 2015; Chai et al., 2020), and infectious viruses pose a threat to human health through the food chain by contaminating edible produce (Haddow & Watt, 2020). These pathogens have pandemic potential, which not only directly affects overall food security and livestock safety, but also seriously threatens the safety of all human beings. Figure 1 provides a general illustration of bioaerosol sources and hazards.
1.2 Strategies for detection of airborne pathogens
In Fig. 2, we show a conventional strategy for the process of bioaerosol “collection-pretreatment-detection.” Bioaerosol collection techniques could be divided either by their collection principle or the medium on which bioaerosol is collected. The principles of collection in existing literature include filtration, centrifugation, impact, and electrostatic precipitation. After aerosol is collected, it is sprayed onto a solid or liquid medium. The advantage of a solid medium is that it could be directly used for further colonization, whereas a liquid medium is more suitable for direct quantification or enrichment to lower detection limits. The target components in bioaerosols diffuse in a larger volume of air, usually at very low levels, and the amount of sample may not reach the minimum level detectable by the detection equipment (Lee et al., 2023). Therefore, by adjusting the parameters of the sampler to obtain a sample solution with a high concentration ratio (Cho et al., 2019, 2020; Heo et al., 2021), some research groups have designed various enrichment channels and devices based on the principle of electrostatic adsorption and biomolecular recognition effects to adsorb and intercept targets for enrichment purposes (Kim et al., 2020b; Liu et al., 2019; Su et al., 2021).
A overview of the “collection-pretreatment-detection” strategies for bioaerosols analysis. Bioaerosol samples are collected onto solid- or liquid-state substrate for detection purpose, but several pretreatment procedures are necessary depending on the application. Some studies use enrichment methods to sort and concentrate the targets and achieve more precise detection. Lysis process is required if the detection is targeting proteins or nucleic acids inside the cells. Various detection methods are available for detection, and researchers should select optimal collection and pretreatment methods based on their application
Traditional culture-based detection methods rely on the continuous growth and multiplication of microorganisms in a suitable matrix to determine the concentration of a bioaerosol in terms of the number of colonies formed (Ngashangva et al., 2022). Immunological methods use the substance to be tested as an antigen or antibody, which can occur on paper strips, magnetic beads, and electrode surfaces, and determine the concentration of the substance to be tested based on the color, fluorescence, and electrical signals produced by the immune binding of the antigen and antibody (Kim et al., 2018; Lee et al., 2020; Li et al., 2018b; Xu et al., 2017; Yakoh et al., 2021). Nucleic acid detection methods are capable of distinguishing the specific nucleic acid sequence of the microorganism to be tested, with a lower limit of detection and higher specificity. With the pandemic of novel coronavirus pneumonia, rapid nucleic acid testing has attracted widespread attention (Wang et al., 2023). The common polymerase chain reaction (PCR), which involves denaturation, annealing, and extension processes to achieve amplification of the target nucleic acid fragments, requires high levels of equipment and technicians (Espí et al., 2021; Santarpia et al., 2020). Therefore, isothermal amplification methods have become preferable for point-of-care testing (POCT), including loop-mediated isothermal amplification (LAMP) (Wang et al., 2019b; Xiong et al., 2021) and recombinase polymerase amplification (RPA) (Nguyen et al., 2021; Sun et al., 2022), which have been used for the detection of a wide range of pathogens in bioaerosols (Lu et al., 2022; Wang et al., 2019b). Figure 3 provides a general illustration of common the isothermal nucleic acid amplification principles. However, it cannot be ignored that the selection of target sequences and the design of primers are difficult problems in the application of isothermal nucleic acid amplification method.

Reproduced with permission from Elsevier. C A method for detecting SARS-CoV-2 using LAMP and CRISPR/Cas12a technology that effectively avoids aerosol contamination from amplicon formation that may be caused by re-opening the lid after amplification (Chen et al., 2020). Reproduced with permission from Elsevier. D A microfluidic microarray platform based on loop-mediated isothermal amplification (LAMP) that enables simultaneous and immediate testing of Streptococcus pneumoniae and Mycoplasma pneumoniae (Wang et al., 2019b). Reproduced with permission from Elsevier. And E The RPA-Cas12a-based platform combined with digitized microfluidics (DMF) enables automated, rapid detection of influenza virus and SARS-CoV-2 (Sun et al., 2022). Reproduced with permission from Springer Nature
A Principles of several methods of nucleic acid amplification. B A dual PCR assay for the specific detection of the pathogenic bacterium Legionella pneumophila in outdoor air samples with low DNA concentrations to activate the appropriate public alerts to prevent disease outbreaks (Sánchez-Parra et al., 2019).
1.3 The significance of this article
To ensure that the threat of airborne pathogens is avoided or minimized, several collection and monitoring devices based on different principles or targeting different airborne pathogens have been developed in the last decades. Many review papers have been published on this topic. Existing articles focus on different specific aspects of the topic, concentrating on the two aspects of collection (Kesavan & Sagripanti, 2015; Mainelis, 2020) and detection (Lee et al., 2020; Ma et al., 2021) for bioaerosol monitoring, as well as on the detection of airborne microorganisms based on microfluidic platforms (Lee et al., 2023; Wang et al., 2021). However, these articles are limited to some of the steps in bioaerosol monitoring and do not address the need for continuous, real-time monitoring techniques for potentially airborne pathogens. In this direction, some reviews focused on these segments but limited their studies to selected airborne pathogens (Bhardwaj et al., 2021; Pan et al., 2019). Li’s team (2021a) reviewed recent advances in bioaerosol collection and detection methods, but lacked descriptions of aerosol sample enrichment, preprocessing, etc., as well as tremendous advances in the field of bioaerosols in recent years, which need to be updated.
The purpose of this paper is to provide a comprehensive and systematic review of the latest developments in this subject, particularly in terms of technology over the last five years, including the principles of bioaerosol sample collection, enrichment, and detection methods, with corresponding examples. We provide an overview of a wide range of sampling techniques and concentrate on the post-sampling sample enrichment and processing aspect, which has not been mentioned in any review in the last three years. In addition, we present bioaerosol detection systems that integrate a sampler with a detection device, although such examples are rare at the moment and discuss key issues in their combination and technical challenges in the development of miniaturized, integrated bioaerosol collection and detection integrated systems.
2 Bioaerosol collection and enrichment
The use of effective samplers to collect and quantify airborne pathogens and hazardous substances is an important part of bioaerosol monitoring. Koch first proposed the use of nutrient agar plates to naturally collect settling bioaerosols for incubation and enumeration of bacteria in 1881, but its utility was limited by its low collection efficiency, as well as its susceptibility to external environmental factors (Emerson et al., 2015; Glushakova et al., 2022; Mhuireach & Johnson, 2016). Bioaerosol sampling techniques have made tremendous progress over the past 20 years. Currently, the common collection methods for bioaerosols include filtration, centrifugation, impact, and electrostatic precipitation.
2.1 Collection
2.1.1 Filtration
Bioaerosols are deposited on the membrane of the filter by diffusion, interception, and impact. Filter sheets can be examined directly using a microscope or observed after incubation, or they can be eluted using a buffer (e.g., phosphate-buffered saline Taghvaee et al., 2019, purified water Santarpia et al., 2020) or chemically degraded (Viana et al., 2021) for subsequent technical analysis. Due to its simple procedure and easy manipulation, filtration is one of the most commonly used methods for collecting bioaerosols, including polytetrafluoroethylene (PTFE), polyvinyl chloride (PVC), and gelatin filters. Ahmadipour’s team (2019) used filters in cabs as mobile high-volume bioaerosol samplers to characterize the air pollution to which people are exposed within the city.
The cutoff size of the filter is an important factor limiting its use in biological environments. For example, the filters used within the air conditioning and ventilation systems (Al-abdalall et al., 2019) are very inefficient due to their large filter holes, with estimated filtration rates of 2% and 50% for bacteria and fungi, respectively, so filters with higher collection efficiency, wider cutoff sizes, reusable, and long-term collection have become a hot research topic. With high porosity and less exposure to gravitational bias from relative humidity and electrostatic charges, PTFE filters exhibit top-notch filtration performance even in low-temperature and high-humidity environments (Afshar-Mohajer et al., 2021; Shim et al., 2022), which opens up the possibility of routine monitoring in industrial, agricultural, or public health facilities. However, the collection of bioaerosol samples using PTFE requires a large initial equipment cost, while false-positive results indicate that the sensitivity and specificity of the sampler need to be further improved (Prost et al., 2019). Based on the fact that the Coulomb force can substantially increase the deposition of biological particulate matter when charged bioaerosol passes through a filter with a strong electric field (Sim et al., 2015), Choi’s team (2018) reported an electrically conductive polyester/aluminum (PET/Al) fiber filter with a high capture efficiency of ~ 99.99% for E. coli and Staphylococcus epidermidis. In addition, the thin oxide layer formed by this filter was able to improve its antimicrobial performance, and its capture and antimicrobial performance remained high upon repeated use.
2.1.2 Centrifugation
The centrifugal collection method involves injecting bioaerosols into a specially designed chamber, which generates a rotating airflow that causes inertial collisions of the bioaerosols through centrifugal force and surface tension, separating and collecting airborne particles according to their varying qualities into a liquid or wall while transporting them to an outlet. The addition of bacterial staining reagents to the sampling solution and the combination of microscopic images allow for the differentiation of dust and live/dead bacteria in the collected samples (Lee et al., 2021).
Most of the existing cyclone samplers have low sampling rates and small sampling flow rates, which require long sampling times. Lane’s team (2021) used a cyclone sampler with a sampling flow rate of 3.5 L/min for 6 h in the area of a patient diagnosed with COVID-19 or a symptomatic patient waiting for a result, and the 528 aerosol samples obtained were negative, especially in the emergency biosafety scenario, the conventional cyclone sampler has a significant disadvantage. Li’s team (2021b) designed and evaluated a high-capacity cyclone sampler, named Yao-CSpler, with an experimental cutoff size of 0.58 µm and a sampling flow rate of 400 L/min, which was able to achieve the collection of SARS-CoV-2 viruses in a clinical setting with a detection concentration of as low as 9–219 viruses/m3.
However, the stability and uniformity of the liquid film can significantly affect the extent of particle loss in a wet cyclone. In general, when the flow rate of the sampling air is maintained at a high level, an excessive flow rate of the liquid medium can lead to rapid evaporation and affect the stability of the liquid film. Conversely, a low liquid flow rate will cause the liquid to float or rupture in the cyclone. Since the formation of a stable and uniform film on the cyclone sampler wall is crucial for the collection of airborne particles, Heo’s team (2021) produced long-lasting super hydrophilicity and mechanical durability by coating silica nanoparticles on the inner surface of the wet sampler to optimize two-phase fluids (air/liquid), which promotes the formation of a thin and homogeneous liquid film inside. It was experimentally verified that the aerosol-to-liquid transfer rate (ALTR) increased to 99.99% after surface coating with silica nanoparticles. Therefore, the treatment with this coating not only reduces particle wall loss, but also does not affect the performance of the cyclone sampler. While the sampler demonstrated excellent collection performance for bacterial bioaerosols, further studies are needed to confirm sampling performance for viruses and fungi.
The cyclone air sampler can participate in the development and application of automated systems because of its portability and compatibility. By using a wireless control panel for remote operation, Cho’s team (2019) achieved control of air sampling, liquid supply, discharge flow rate, and sample transportation. Some research teams have also coupled the developed samplers with robots to realize unmanned air sampling based on predetermined routes (Li et al., 2021b).
2.1.3 Impact
Impact methods are commonly used to collect bioaerosols onto the sampling medium based on inertia. When the gas containing microbial particles passes through the narrow aperture in the sampler, the airflow is deflected with the channel, and the microbial particles with larger inertia will directly impact the sampling medium due to inertia, while the bioaerosols with smaller inertia will escape with the airflow. Solid impact samplers generally use agar medium as sampling plates (Farling et al., 2019; Knowlton & Boles, 2018), which can be directly biochemical culture to obtain colonies for research. The Andersen air sampler, the most widely used impact sampler, collects bioaerosols in a range of particle sizes that cover the deposition patterns in different areas of the human respiratory system (Madhwal et al., 2020). Therefore, it is commonly used to characterize the size distribution of bioaerosols at the research site as well as microbial diversity to gain a deeper understanding of the composition of microbial communities and species information in the environment (Gladding et al., 2020; Ye et al., 2021). To solve the problem of particles bouncing from impact, Xu's team (Xu et al., 2013) used mineral oil-coated agar plates to reduce the impact stresses and also reduced bioaerosol drying, which ultimately improved the biocollection efficiency of the Andersen air sampler. The virtual impactor uses a receiving nozzle instead of a receiving plate (Chen et al., 2019; Wu et al., 2020). After the bioaerosol is accelerated by the nozzle, particles smaller than the cutting particle size will be deflected by 90° with the main flow, while particles larger than the cutting particle size will not be deflected due to large inertia and will move directly forward into the receiving nozzle, concentrating into the small flow. When sampling bioaerosols using an impact sampler, different collection media may affect the rate of loss of bioaerosol particles. Horve's team (2021) investigated the ability of flocked swabs, cotton swabs, glass fiber filters, FTA cards, and AerosolSense Capture Media (ACM) for nucleic acid collection. Experimentally, ACM and bee lint swabs were determined to be the most efficient media, detecting SARS-CoV-2 RNA at concentrations of 0.32 gc/L and 3.2 gc/L, respectively, and were easily integrated into typical molecular workflows. Flocked swabs are preferred in clinical diagnostics during periods of extreme demand.
The principle of the liquid impact sampler is similar to that of the solid impact sampler, in that the air stream carrying microbial particles enters the sampler and rushes through a high-speed channel into the liquid collection medium, which can be used directly as an analytical sample without the need to extract microorganisms from the surface of the solid medium. All-Glass-Impinger (AGI) and SKC BioSampler (commercially available product at SKC, Inc.), for example, have been used for the detection of airborne viruses in hospitals (Kenarkoohi et al., 2020), bacteria and fungi in public places (Nguyen et al., 2022), and air quality evaluation (Campbell et al., 2022).
The bioaerosol impact distance inside the AGI-30 sampler is 30 mm and the localized impact velocity can reach up to 265 m/s. The higher impact velocity and longer sampling time can significantly increase the diversity of the samples, so the AGI-30 proved to have a higher performance in the collection of airborne E. coli compared to the Andersen air impactor (Nguyen et al., 2022), and AGI-30 also successfully captured viruses in the COVID-19 viral bioaerosol (Kenarkoohi et al., 2020). It should not be overlooked that the faster the impact speed during sampling, the greater the possibility of damage to the microorganisms in the bioaerosol, and the large number of bubbles caused by the impact will evaporate the sampling solution, which ultimately affects the sampling efficiency of the AGI. In contrast to AGI, which injects air through a single nozzle pointing toward the bottom of the flask, the BioSampler provides three tangential acoustic nozzles, which generate a rotational motion of the collection fluid to minimize re-atomization of microbial particles and gently collect bioaerosol particles. Not only is it highly efficient at collecting bacteria and fungi, but the SKC BioSampler sampler also preserves the integrity of viruses for the detection of respiratory viruses in clinical settings (Truyols et al., 2022). The AGI-30 and BioSampler samplers have similar size-resolved sampling efficiencies, but smaller particles are collected at very low efficiencies, with collection efficiencies below 30% (Guo et al., 2022; Wei et al., 2010). Yu’s team (2016b) found that filling the liquid impact sampler with glass beads improves its collection efficiencies for ultrafine particles and submicron viral in bioaerosols. Since it takes more time for the gas to pass through the thicker particle bed, there is a longer contact time for more particles to be collected by diffusion, settling, or impaction. However, it also increases the pressure drop across the sampler, placing a greater load on the sampling pump.
2.1.4 Electrostatic precipitation (ESP)
Bioaerosols are charged through a metal needle at the inlet of the electrostatic collector, creating a corona discharge that forces them to move in the direction of the electrode with the opposite charge (Tan et al., 2011). Corona discharge has been reported to cause bioaerosols to carry more charge (Li et al., 2021a), and bioaerosol particles with more charge are more susceptible to electrostatic deposition, which makes it easier to collect bioaerosols onto the electrodes, thus providing high collection efficiency. Noti’s team (2012) recovered submicron-sized infectious influenza viruses from cough bioaerosols generated by simulated patients using electrostatic deposition, demonstrating the broad applicability of ESP to effectively collect bioaerosol particles ranging from sub microns to tens of microns. In addition, electrostatic sampling also has the advantage of high sample output concentration. Filter samplers and impact samplers have large sample volumes, typically a few milliliters (Gladding et al., 2020; Li et al., 2018c; Prost et al., 2019; Ye et al., 2021). If there is only a small amount of target microorganisms in the air, the large sample volume can have a significant dilution effect, making these methods unsuitable for low LoD measurements. Instead, using an ESP sampler allows bioaerosol particles to be captured directly into an integrated liquid collector miniaturized to a volume of 150 μL (Ladhani et al., 2017).
As the voltage of the electrostatic sampler increases, bioaerosol particles can obtain more charge and higher electrostatic velocities in stronger electric fields. In particular, the ion yield and electromobility are higher at negative voltages (Niewulis et al., 2013). However, it should not be ignored that the high voltage of corona discharge is a potential risk that leads to a decrease in the activity of microorganisms to be tested in bioaerosols. Bhardwaj’s team (2020) used the hemagglutinin (HA) surface proteins and nucleoprotein (NP) of influenza A (H1N1) virus as targets for immune sensors for the rapid quantification of airborne H1N1 influenza viruses after EPC sampling and ultimately demonstrated that the outer shell proteins of the H1N1 viruses were damaged or degraded due to the combined effects of corona charging, non-pulsed electrostatic fields, and bioaerosol generation. It has also been suggested that electrostatic fields disrupt the lipid bilayer of Gram-negative bacteria thereby leading to inactivation of the bacteria (Yao et al., 2005). Pirhadi’s team (2020) increased the sampling flow rate of the electrostatic sampler, which resulted in a significant reduction in the ozone concentration produced by the ESP corona at higher flow rates, while Ma’s team (2016) used a carbon-fiber ionizer that could produce corona discharges at relatively low applied voltages, which resulted in negligible ozone production. In contrast, Kim’s team (2020a) used phosphate-buffered saline to cover the grounded electrode to achieve mitigation of the damage caused by the discharge electrode to viruses in the bioaerosol during sampling.
2.2 Bioaerosol enrichment
The probability that a target microorganism will be detected in bioaerosols is a function of the LoD of the sensor as well as the concentration of the collected sample added to the detector (Coudron et al., 2019). The gold standard for SARS-CoV-2 diagnosis is RT-PCR and qRT-PCR based on the detection of target nucleic acids (< 100 copies/mL) (Arnaout et al., 2020; Gupta et al., 2021). However, even during the COVID-19 pandemic, airborne virus concentrations in isolation facilities with confirmed patients were relatively low, with total SARS-CoV-2 concentrations ranging from 1.84 × 103 to 3.38 × 103 RNA copies/m3 of air in indoor environments (Chia et al., 2020), which complicates the monitoring of pathogenic microorganisms in bioaerosols. Therefore, collecting and concentrating pathogenic microorganisms dispersed in bioaerosols into a small volume of liquid above the limit of detection can reduce the time required to prepare detectable samples. Wet cyclones collect suspended bioaerosols into a liquid film on the inner wall of the cyclone, which increases their particle concentration due to the decrease in volume from air to liquid. Therefore, to obtain the highest possible particle concentration, the least amount of liquid sampling medium should be used and the ratio between air intake and liquid discharge should be increased. For example, the bioaerosol sampler developed by Cho’s team (2019) maintains a high particle enrichment rate based on a constant high air-to-liquid flow ratio (~ 1.4 × 105), using a continuous inlet airflow (16L/min) and outlet liquid flow (7 mL/h). Theoretically, this Automated and Real-time Bioaerosol Sampler based on Wet-cyclone (ARBSW) captured 11 times higher concentrations of microorganisms in 20 min than a biological sampler under the same environmental conditions. After further validation experiments, the sampler coefficients were set to a continuous inlet airflow (16L/min) and outlet liquid flow (~ 17 μL/min), which ultimately increased the concentration ratio of the collected bioaerosol samples to ~ 9.6 × 105 times (Cho et al., 2020). Electrostatic collectors can similarly obtain samples with high concentration ratios based on the principle of increasing the air flow rate and decreasing the liquid flow rate (Choi et al., 2017; Foat et al., 2016). A high flow rate electrostatic air sampler (HAFES) proposed by Kim’s team (2021) has a high recovery rate for viral bioaerosols, with enrichment values of 105 or more. When the device was used in a simulated viral epidemic, sampling for 20 min was able to produce detectable samples for real-time qRT-PCR. Table 1 shows some recent bioaerosol enrichments using cyclone and electrostatic samplers.
Versatile Aerosol Concentration System (VACES) is one of the most widely used bioaerosol concentrators. When used to collect air samples, coarse particles can penetrate directly into the water, while fine particles pass through a saturated condensation system, where droplets of 2–3 μm form on the surface of the particles due to the condensation of water vapor and are then passed through a virtual impactor to produce a highly concentrated liquid suspension. VACES is capable of effectively concentrating ambient particles of varying particle sizes by a factor of 20–40 (Ntziachristos et al., 2007), while the collected samples retain the physical and chemical characteristics of the ambient bioaerosols for use in in vitro studies (Liu et al., 2019). The study by Viana’s team (2021) confirmed the representativeness of the VACES system for bioaerosol samples collected in an occupational industrial environment and its validity in terms of mass concentration in high-exposure scenarios. In their study, the enrichment coefficient of VACES was 31. The applicability of this instrument in practical industrial scenarios is debatable due to its large size which interferes with the factory production process and limits the collection of large numbers of samples. In recent years, researchers have been optimizing the VACES device from various perspectives. The VACES/aerosol-into-liquid-collector not only captures water-soluble particles, but also water-insoluble particles directly to the bottom of the collector in a concentrated slurry, effectively recovering all components of environmental particles (2019). An improved VACES integrated into an optical instrument improves particle concentration and amplifies the signal-to-noise ratio during measurements, and this new technology reduces the detection limit by an order of magnitude at high temporal resolution (2 h) and a small sampling flow rate (6 L/min) (Kang et al., 2022). Wubulihairen’s team (2015) introduced a vapor generator, which is small and portable compared to VACES for different field sampling applications.
Other than microbial components, a large number of dust, droplets, salt, and other particles exist in air samples. In addition to adjusting the performance and parameters of the sampler, effective collection, capture, and separation of the target particles from the background environment can be equally useful for enrichment purposes. Su’s team (2021) designed a biochip based on polydopamine-co-chitosan (PDA-co-CS) composite gel-modified microelectrodes. During the enrichment process, the enrichment efficiency of microorganisms was 99.9% under the synergy of the adsorption capacity of mussel protein-like catechol functional groups contained in PDA, the molecular interaction between chitosan and microbial cells, electrostatic adsorption, and other synergistic effects. Kim’s team (2020b) enrichment channel-integrated handheld system, Hydrosol-to-hydrosol (HTH), was able to adsorb bacteria entering the channel onto pre-immobilized Concanavalin A-coated magnetic particles (CMPs), and the enrichment channel was surrounded by magnet blocks so that CMPs were immobilized on the inner wall of the channel by magnetic force. Preparation of real-time qPCR detectable bacterial samples in an on-site environment requires an enrichment capacity of at least 106 levels, and this device has a total enrichment capacity of 1.192 × 106. The system can be used not only for bacterial enrichment, but also for more than tenfold enrichment of nebulized test viruses, including Human Coronavirus 229E (HCoV-229E), Influenza A Virus Subtype H1N1 (A/H1N1), and Influenza A Virus Subtype H3N2 (A/H3N2) (Kim et al., 2020a). Most impressively, the device was able to turn undetectable virus samples collected during a viral epidemic scenario into detectable samples within 10 min.
2.3 Summary
Air disperses a wide range of biological, chemical, and physical components, of which airborne pathogens are only a portion, leading to a complex composition in collected sample. In order to facilitate bridging downstream assays for efficient pathogen analysis, post-collection bioaerosol pretreatment is necessary. Examples include filtration of impurities, concentration of the sample solution, and elution of target analytes. The collection and enrichment processes are collectively referred to as pretreatments, with lysis sometimes required in studies regarding nucleic acids. When detecting airborne pathogens in the field, consideration should be given to the consistency of collection and detection to ensure the viability and structural integrity of target analyte. The collection techniques of settling, filtering, and impinging on solid media described previously have the advantages of simple operation and inexpensive cost, but they require extraction of target analytes from solid surfaces or filters, which may result in damage or loss of airborne pathogens, thus affecting the accuracy of downstream detection and false-negative results. Meanwhile, the method of direct incubation followed by microscopic detection takes long time and has low efficiency, and the elution process of the filter is not easy to be integrated inside the automated equipment, so the filtration method is preferred as a strategy for air purification. The strategy of using liquid matrices to collect bioaerosols without the need for elution and extraction processes can significantly improve the stability and viability of airborne pathogens and reduce the loss of target analytes. Moreover, liquid samples facilitate the integration of downstream assay steps and reduce sample preparation time. Pre-incorporation of magnetic beads or nanoparticles into the liquid collection matrix achieves purification and enrichment of target pathogens through intermolecular specific recognition interactions and accomplishes rapid integrated detections (Han et al., 2023; He et al., 2017; Shafagati et al., 2015). Furthermore, for nucleic acid detection, the liquid collection matrix itself could be lysis solution, which would benefit from the integration of lysis process with the pretreatment step. There are also recent reviews about pretreatment process, readers could find more details if interested (Wang et al., 2023).
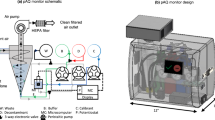
The collection of bioaerosols is a prerequisite for the monitoring of airborne pathogenic microorganisms, and the use of bioaerosol samplers has been shown to reduce the intensity of work and destructiveness to organisms involved in sample collection (Prost et al., 2019). Accurate quantification and identification of pathogens present in bioaerosols is highly dependent on the sampling equipment used. Improperly selected air samplers may fail to collect human respirable chemical hazardous substances, toxins, pathogenic microorganisms, etc., from bioaerosols (Kesavan & Sagripanti, 2015), thus underestimating the risk of accidental or intentional contamination of public environments by infectious or toxic bioaerosols, and even leading to unnecessary loss of life. Most of the commonly used bioaerosol samplers are based on one or more collection mechanisms of gravity deposition, interception, impact, and electrostatic attraction. Figure 4 provides a general description of collectors commonly used for bioaerosols. Although there is a big difference in appearance design and size, they all share some basic structures, including: an air inlet, transfer pipeline, and sample collection area. The air inlet can be designed as a pump or an air separator or an unpowered device, and the design of the transfer pipeline with a concentrating device can increase the concentration of microorganisms in the bioaerosol. Most of the collection plates for bioaerosols are designed as liquids to increase the recovery rate from the collection matrix (Lee et al., 2020).
Schematic of several of the most common bioaerosol collectors. Pictures include: A Filter sampler. Bioaerosols are deposited on the filter membrane by diffusion, interception, and impaction. B Solid impact sampler. Inertia-based collection of bioaerosols into solid media. C Electrostatic sampler. Bioaerosols are collected using the electrostatic adsorption effect. D All Glass Impactor (AGI). Bioaerosols impinge and are deposited in the liquid collection medium. E The SKC BioSampler. Impact sampling equipment using liquids to capture bioaerosols designed by SKC Ltd. and F Cyclone air sampler. Separation of particles in bioaerosols by centrifugal force
Table 2 shows an assessment of the sampling efficiency of bioaerosol samplers in recent years. Wang’s team (2019a) compared the efficiencies of filter and cyclone samplers for collecting bacterial and fungal samples, where the cyclone sampler performed better in collecting bacteria and both samplers showed almost the same collection efficiencies and community structure of sampled bacteria for fungal collection. The filter sampler is not dependent on liquids and can operate at low temperatures, but requires additional treatment of the initial sample, which has a significant impact on fragile microbial activity and can greatly affect subsequent studies. Cyclone samplers can extract low concentrations of airborne microorganisms into a running buffer that can be conveniently analyzed without additional treatment of the filter sample, with limitations on environmental temperature. Impactors are an effective way to collect large particle samples (Farling et al., 2019; Gladding et al., 2020; Knowlton et al., 2018; Madhwal et al., 2020; Ye et al., 2021); however, the rebound and re-suspension of larger, heavier particles due to inertial impacts can introduce large errors into the collection results of target particles with relatively small particle sizes. Not to be overlooked, the mechanical stresses generated during sampling may damage the structure and even the DNA of target bacteria, viruses, etc. Previous studies have reported that the recovery rate of influenza viruses is generally lower than that of MS2 phage when the inertial impact sampling method is used (Kim et al., 2021). Electrostatic samplers have the lowest sampling velocity and therefore low damage to bacteria, fungi, and viruses, and their collection efficiency is high over a wide range of particle sizes (Mbareche et al., 2018), providing better coverage of the microbial diversity in the air sampler under test. Due to the gentle and abundant sampling, the concentration of infectious T3 phages captured in electrostatic particle concentrators is thousands of times higher than that collected by impact samplers (Jang et al., 2022), and their collection efficiency is comparable to that of the advanced impact bioaerosol samplers, with the most notable advantage of providing samples with at least 10 times higher concentration (Pardon et al., 2015).
Compared to traditional samplers, research teams have been designing and manufacturing portable, automated samplers (Afshar-Mohajer et al., 2021; Foat et al., 2016; Hong et al., 2016; Ladhani et al., 2017), and although the scale of the research is limited at this time, the trend toward their clinical application as a noninvasive sampling device cannot be ignored (Ladhani et al., 2020). Integrated microfluidic electrostatic samplers capture bioaerosol particles into liquid samples, with an effective collection efficiency of about 20–40% for airborne particles (Ma et al., 2016). The microfluidic-based sampling system “MicroSampler” is driven by the difference in particle inertia through the stable formation of a stratified flow of two-phase fluids, air and liquid, in a curved microfluidic channel. The MicroSampler achieves particle collection efficiencies of 80–98% at microfluidic airflows of 0.2–0.6 L/min, and exhibits recovery rates of > 90% when tested with bacterial bioaerosols (Choi et al., 2017). A multichannel microfluidic chip for the detection of Mycobacterium tuberculosis is currently being developed that can obtain a 175 μL sample in only 2 min, allowing initial screening of TB patients without the need for specialized personnel (Ma et al., 2022). Based on various technical and application challenges, bioaerosol sampling techniques have made significant progress in a relatively short period, and prioritizing the capture and preservation of certain bioaerosol particles may be a direction for future exploration.
3 Bioaerosol detection
Traditional detection through microbial culture requires a long time of cultivation, which makes the result not time effective. This is extremely limiting in matters such as public health events or environment monitoring, despite its advantage in easy manipulation and low cost. Moreover, it is not possible to detect viruses or VBNC (viable but non-culture) bacteria through this method. Here, we discussed four popular novel detection methods for bioaerosol.
3.1 Lateral flow assay
The lateral flow test strip is the most commercially viable and proven option. Lateral flow strips typically consist of a sample pad, a splice pad that has been coated with a labeled antibody, two “lines” (nitrocellulose membranes that have been coated with antigen), and an absorbent pad. The sample is first added to the sample pad, and the sample binds to the labeled (usually gold nanoparticles) antibody in the paper splice pad (Lu et al., 2023). Through capillary action, it then flows into the test strip channel, which is pre-coated with gold-labeled secondary antibodies, forming a sandwich structure. Finally, this composite structure is captured by the recognition probe, deposited and colored at the detection line, and the redundant label is captured at the control line. The lateral flow test achieves visualization of the assay and can often be used for qualitative testing. Figure 5 provides a general description of the test strip assay.

Reproduced with permission from American Chemical Society. C Yu et al., 2020. Reproduced with permission from Elsevier. D Li et al., 2022. Reproduced with permission from Frontiers. E Xiao et al., 2021. Reproduced with permission from Springer Nature. and F Seok et al., 2021. Reproduced with permission from MDPI
A Schematic diagram of the basic structure of a lateral flow test strip. B-F Illustration of several recently developed devices based on the lateral flow test strip detection principle. Images are adapted from: B Lee et al., 2020.
Analytical devices based on lateral flow test strips have been widely used in recent years in studies for the detection of microorganisms in the atmosphere or breath due to their simplicity, portability, biocompatibility, and the ability to provide rapid detection of small sample volumes without the need for a pump, making them ideal for use in restricted environments. A paper-based kit designed by Seok’s team (2021) was tested and analyzed for Staphylococcus aureus within three days of air from different seasons, and the target bacteria were successfully detected within 15 min. Currently used lateral flow assays (LFA) have lower detection limits than commercial enzyme-linked immunosorbent assays (ELISA). To reduce the non-specific binding of analytes, which produces a high background signal, Mohd Amiruddin’s team (2020) used 5% (w/v) BSA as a blocking agent to enable lateral flow test strips to detect Mtb 16 kDa antigen (Ag16) down to 125 ng, which is lower than the presence of soluble forms of Ag16 in the cerebrospinal fluid of patients with tuberculous meningitis, at a concentration of ~ 15–20 µg/mL (Haldar et al., 2012), with sensitivity and specificity comparable to PCR. Lee's team (Lee et al., 2020) used a highly specific near-infrared signaling nanoprobe to improve the sensitivity of LFA while pre-treating the sample pad to enhance the solubility of the transfer solution, ultimately achieving a paper test strip that was more effective in detecting airborne target pathogens than ELISA in 20 min.
Paper-based materials are easily combustible and can be safely disposed of after use without any risk of contamination (Suntornsuk et al., 2020), becoming one of the detection methods for medical wearable technology. Nguyen’s team (2021) designed a wearable, autonomously functioning mask sensor for the detection of SARS-CoV-2 in exhaled bioaerosols. The wearer only needs to press a button to activate a reservoir containing nuclease-free water, and the flow action of the wicking material moves the collected breath sample to the downstream reaction zone where the virus lysis to release the SARS-CoV-2 vRNA, S gene for RT-RPA amplification. The final results are visualized by lateral flow analysis strips. The entire process takes only about 1.5 h and has a detection limit of 500 copies (17 aM) of SARS-CoV-2 in vitro transcribed (IVT) RNA, which is compatible with the standard laboratory-based RT-PCR assay recognized by the World Health Organization. However, the detection platform needs to face challenges such as biological contamination in sweat, signal loss, disposable nature, and inability to work in specific environmental conditions (e.g., high humidity or underwater).
Most notably, lateral flow analysis makes it difficult to obtain accurate quantitative results, and most endpoint detection methods are only qualitative. Due to the lack of quantitative sensing devices, these methods more frequently confirm results with the naked eye.
3.2 Electrochemical detection
Electrochemical detection techniques can convert chemical and biological signals into electrical signals, including impedance, current, and potential, with the strength of the signal correlating with the amount of the object being detected. The main advantages of electrochemical detection methods are low power, high resolution, good selectivity and repeatability, ppm-level detection accuracy (Khan et al., 2019), and fast response. Because the electrochemical analytical platform is immune to environmental contaminants such as proteins and endotoxins, non-target microbial particles, as well as sample turbidity or fluorescent compounds contamination interference, it is widely used for the detection of various gaseous molecules and biomolecules (Chen et al., 2018; Giordano et al., 2021). It has been realized to detect a variety of airborne pathogens and biomolecules in the air, including: Influenza A (H1N1) virus (Kim et al., 2018), Escherichia coli (E. coli) (Lee et al., 2021), Mycobacterium Tuberculosis (Ma et al., 2022), oxidative stress biomarkers (Ito et al., 2019), etc. Yakoh’s team (2021) designed a folded novel coronavirus immunoglobulin (represented by IgG and IgM) detection tool for electrochemical detection in the presence of a redox indicator ([Fe(CN)6]3−/4−). The device consists of three paper-folded layers, each with a hydrophilic center confined by a wax barrier, with three electrodes screen printed on the back of the device and the folded layers held tightly in place by double-sided tape during use. The paper platform is capable of rapid (30 min) detection of SARS-CoV-2 antibodies with a detection limit of 1 ng/mL and has 100% sensitivity and 90% specificity for clinical use, which is 3 orders of magnitude more sensitive than colorimetric LFA, but has not yet reached the level of detection of real nasal swab specimens (down to pg/mL).
In research, properly designed probes can improve the sensitivity of the system and reduce the limit of detection. Antibodies and enzymes are commonly used probes, while nucleic acid chains are also widely used as recognition elements for electrochemical biosensors because of their simple fabrication and convenient storage. Wang's team (Wang et al., 2022) used ATP as a biomarker for the detection of total bioaerosols based on the fact that the specific binding of ATP to its aptamer forms a g-quadruplex structure, which prevents the electron transfer from the surface of the electrodes and produces a strong electrochemical impedance change. The corresponding electrochemical behavior was characterized by the EIS technique with a detection limit of 0.11 nM over a wide linear range from 0.1 to 100 nM.
With the increasing demand for rapid on-site detection methods, electrochemical detection platforms have tended to be miniaturized and convenient (Lee et al., 2023), while the emergence of nano- and micromechanical devices has provided new strategies for building highly integrated electrochemical detection devices capable of carrying out high-throughput tests. Duarte’s team (2021) designed a microfluidic device based on nanoelectrode-activated microwell arrays for the capture and quantification of airborne fungal pathogen Sclerotinia sclerotiorum. Each column in the device array can be measured individually, which allows for the detection of individual particles in a single microwell, showing great potential for application to rapid detection in the field. The content of target substances in bioaerosols will change over time, providing sensitive and accurate assessment evidence for population health management. The ability to monitor and analyze airborne biological contaminants and the risk of respiratory disease in real time or online using electrochemical detection platforms enables early and rapid screening and reporting in clinical applications (Chen et al., 2018; Nigatu et al., 2015), enabling relatively simple, highly selective, and interactive health care that may revolutionize the study of contaminant health effects as well as diagnosis and monitoring of respiratory-transmitted diseases at the bedside. Figure 6 gives an example of some recent electrochemical detection platforms.

Reproduced with permission from MDPI. B An electrochemical sensor was constructed utilizing the fact that the presence of the SARS-COV-2 antibody would interrupt the redox conversion of the redox indicator, resulting in a reduced current response (Yakoh et al., 2021). Reproduced with permission from Elsevier. And C A device comprising a nanothick aluminum electrode structures integrated with picoliter trap arrays for dielectrophoresis-driven spore capture and on-chip quantitative detection using impedimetric sensing (Duarte et al., 2021). Reproduced with permission from American Chemical Society
A An electrochemical aptasensor for the detection of adenosine triphosphate was constructed using nanohybrids containing zeolite imidazolate frameworks and covalent organic frameworks (Wang et al., 2022).
3.3 Fluorescent and colorimetric detection
The principle of optical detection methods is to convert the signal of the target to be detected into optical signals such as fluorescence and color and identify the presence of certain substances by using optical instruments or color changes visible to the naked eye. Among them, fluorescence and colorimetric methods are currently the most commonly used optical detection methods. The unique photophysical properties of fluorescent dyes, which produce visible light directly and enhance the light signal when excited by UV light, make it possible to use fluorescent dyes to label bioaerosols with substances to be detected with direct visual confirmation of the results, thus enabling real-time detection. It has been used commercially in many fully bioaerosol monitors. Lu’s team (2022) prepared a real-time fluorescent nucleic acid detection platform using 3D printing technology, which takes only 90 min from sample input to readout. The platform contains three LAMP reaction chambers that can work independently, and its detection limit is 4 × 104 spores/sample, which can meet the requirements for rapid early warning detection of airborne fungi. Li’s team (2018b) established an immunofluorescence intensity—Aspergillus niger spore number semi-quantitative standard curve to calculate the number of target spores based on the intensity of the fluorescence signal. The detection limit is ~ 20 spores, which is equivalent to ~ 300 spores/m3 of the relevant target in air. Meanwhile, optical detection methods have been widely used for high-throughput testing, capable of meeting test throughputs of thousands of samples/day or more (Patterson et al., 2020; Sun et al., 2022; Xiong et al., 2021).
There have also been some research teams in recent years that have chosen to use smartphones to quantify detection results. Kim’s team (2022) produced a smartphone-based fluorescence microscope with LEDs as the light source for the excitation of fluorescent particles, capable of being used to detect COVID-19. The obtained fluorescence microscope image was divided into three color channels (red, green, and blue), and the degree of fluorescent particle aggregation was quantified by optimizing the intensity threshold, of the fluorescence signal and removing background noise. The total cost of parts and consumables for the entire fluorescence microscope was only $46.60. Chen’s team (2020) used a smartphone to observe the fluorescence produced by the RT-LAMP reaction, and the entire amplification detection process could be completed in 40 min with a sensitivity of up to 20 copies of SARS-CoV-2 RNA. Fluorescent dyes can detect not only amplicons directly, but also by-products produced by DNA amplification, such as pyrophosphate ions. Under UV light (365 nm), the complex formed by the binding of calcein to manganese ions (Mn2+) remains quenched. During LAMP amplification, pyrophosphate ions deprive calcein-bound Mn2+ and produce fluorescence. At the same time, free calcein readily binds to magnesium ions (Mg2+) in the reaction mixture, resulting in a more pronounced fluorescence indicating the presence of target genes. Wang’s team (2019b) recorded and transmitted this fluorescent information via the device's Bluetooth Wi-Fi camera to an application on a smartphone that specializes in receiving and analyzing it, ultimately enabling instant identification of Mycobacterium pneumoniae and Mycoplasma pneumoniae.
Ultraviolet laser-induced fluorescence (UV-LIF) is based on the display of intrinsic fluorescence or autofluorescence by specific organic molecules of living organisms (e.g., proteins, coenzymes, pigments) and was initially used in military research for the rapid detection of biological warfare agents (Kwaśny et al., 2023). Currently, they show great potential in the detection and characterization of environmental bioaerosols (Tian et al., 2020), potentially harmful viruses in the environment (Owoicho et al., 2021), etc. However, the integration of portable detection platforms is a challenge that limits its development. Figure 7 illustrates some recent optical signal-based detection platforms.

Reproduced with permission from Elsevier. B A smartphone-based fluorescence microscope isolated and counted the immunoagglutinated particles on a paper chip for direct airborne detection of SARS-CoV-2 (Kim et al., 2022). Reproduced with permission from Elsevier. And C Semiquantitative detection of Aspergillus niger spores based on immunofluorescence analysis (Li et al., 2018b). Reproduced with permission from American Chemical Society
A A visual detection method for SARS-CoV-2 using a smartphone and a portable 3D-printed instrument to see the fluorescence produced with the naked eye without any specialized instrumentation (Chen et al., 2020).
3.4 Non-fluorescent optical detection
Raman spectroscopy is an optical bioimaging method based on the principle of inelastic scattering of light. Compared with fluorescence spectroscopy, Raman spectroscopy can represent hundreds of different vibrational frequencies, therefore, detection devices based on Raman spectroscopy have been used for viruses and various biomarkers (Desai et al., 2020). However, surface-enhanced Raman spectroscopy (SERS) techniques capable of an order-of-magnitude signal enhancement of analytes are evolving due to the weak conventional Raman scattering intensity of atmospheric bioaerosol particles (Ben-Jaber et al., 2016). Choi’s team (2020) used the plasmon resonance effects of silver nanoparticles (AgNPs) and SERS to amplify the Raman signal up to 1011 times. SERS combined with machine learning algorithms of support vector machine (SVM) can distinguish between samples carrying influenza A virus, influenza B virus (Tabarov et al., 2022), and those not carrying the virus, with an accuracy of 93% for virus detection.
The phenomenon of incident light excitation of free electron resonance on the surface of metallic materials is known as surface plasmon resonance (SPR), and SPR biosensors are often used as initial diagnostic tools due to their short detection time and label-free nature. In recent years, noble metal nanoparticles with strong localized surface plasmon resonance (LSPR) in the visible region have shown special chemical and physical properties, such as gold or silver nanoparticles (Guo et al., 2015; Yin & Tong, 2021). The LSPR is highly sensitive to changes in refractive index, and in addition, metal nanoparticles neither flash nor bleach, which is favorable for long-term applications, and is considered to be an ideal candidate for a new generation of virus detection methods (Lathika et al., 2021; Qiu et al., 2020; Takemura, 2021; Yang & Murray, 2022). Wang’s team (2018) premixed ESAT-6 with antibodies and added them to gold nanoparticles (GNPs) for salt-induced aggregation with NaCl. In the presence of divalent ions, the space between the GNPs was filled, and the solution changed from red to blue with good visual detection sensitivity, which could reach a detection limit of 1.25 pM. The human eye is insensitive to changes in optical density of the same color, and to improve the sensitivity of visual detection, Xu’s team (2017) developed a colorimetric assay for H5N1 viruses showing multiple color changes. The reduced Ag was deposited on the surface of gold nanobipyramids (Au NBPs) to change the refractive index, and the color of the solution changed from brownish yellow to green, dark blue, and dark red with the increase of the virus concentration, and the vivid color changes could easily achieve the semi-quantitative detection by the naked eye with the limit of detection (LOD) of 1 pg/mL. In Fig. 8, we exemplify the most recent and relevant recent reports.

Reproduced with permission from Springer Nature. B A biosensor combining the plasma photothermal effect and localized surface plasmon resonance sensing transduction (Qiu et al., 2020). Reproduced with permission from American Chemical Society. C An optofluidic surface-enhanced Raman spectroscopy platform for real-time detection of airborne microorganisms (Choi et al., 2020). Reproduced with permission from Elsevier. D A highly sensitive human angiotensin-converting enzyme 2 protein (ACE2) functionalized silver nanotriangle (AgNT) array localized surface plasmon resonance (LSPR) sensor for rapid detection of coronaviruses (Yang et al., 2022). Reproduced with permission from Elsevier
A Photo-induced-enhanced Raman spectroscopy, in which the combination of plasmonic nanoparticles and photoactivated substrates produces a large signal enhancement for small molecules (Ben-Jaber et al., 2016).
3.5 Summary
Conventional detection methods, such as colony culture and microscopic observation, are not capable of rapid and accurate bioaerosol detection in the field, although the results are reliable. Table 3 shows a variety of detection programs or equipment in recent years. LFA requires no expensive instruments for visual detection and is suitable for on-site detection of the presence of pathogens, as it offers lower production costs, ease of operation, real-time readout of results, and significant advantages in the normalization of detection platforms. Optical detection methods have a high degree of accuracy for the detection of airborne pathogens and are capable of quantitative analysis. However, external interference from the background environment during on-site detection needs to be considered to effectively analyze the target pathogens. Electrochemical detection techniques with high sensitivity are proficiently used for the detection of airborne pathogens. The electrical signals generated by the interaction between the electrodes and the target can be measured by various strategies, but the design of the relevant devices needs to take into full consideration the conversion effect of the signals when applied in the field.
With the continuous progress of science and technology, multidisciplinary collaboration has provided various bioaerosol detection solutions covering molecular biology, smart materials, communication technology, nanotechnology, and microscale technology, including microfluidics (Duarte et al., 2021; Ma et al., 2022; Sun et al., 2022; Wang et al., 2019b; Xiong et al., 2021), microelectromechanical systems (MEMS) (Chellasivalingam et al., 2020; Chen et al., 2019; Gong et al., 2023), 3D printing (Chen et al., 2020; Lu et al., 2022), and other technologies, which are continuously optimized in terms of detection efficiency, economic cost, performance indicators, practicality, convenience, and other aspects. Nanomaterials are capable of amplifying electrical, optical, and visual signals to further improve the platform's performance in detecting trace amounts of infectious airborne microorganisms. For example, carbon nanomaterials, including graphene and carbon nanotubes, are also emerging as promising candidates for rapid diagnostic techniques due to their good mechanical properties, scalability, unique optoelectronic properties, and size comparable to biomolecules such as DNA, proteins, or viruses (Bardhan et al., 2021; Lu et al., 2024). The high selectivity generated by protein-based receptors (e.g., antibodies) when combined with highly sensitive carbon nanotubes can be used for real-time detection of airborne Bacillus cereus (Yoo et al., 2017), allergenic fungi associated with various allergenic and pulmonary diseases (Kim et al., 2016). In a recent report, Pinals’s team (2021) constructed a single-walled carbon nanotube (SWCNT) sensor based on non-covalent functionalization of ACE2, and the SARS-CoV-2 spiking protein induced a twofold fluorescence enhancement within 90 min of exposure. Preloading or embedding a mixture of primers and enzymes on the detection unit can facilitate on-site operation and system transportation without affecting detection efficiency (Liu et al., 2018a, 2018b).
4 Integrated sampling and detection systems and future perspectives
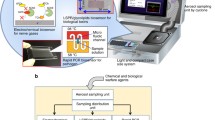
With the rapid development of sample collection and detection methods, the idea of an integrated device for both steps has emerged. The most important benefit is to avoid contamination during transfer process, including contamination to the sample that could affect test results and those potential harm to operating personnel. It also provides an automatic way of monitoring bioaerosols in real time. More importantly, an all-in-one system could minimize influence on bioactivity of collected bioaerosols, which is vital to accurate detection. However, these integrated platforms are flawed in battery run time, sample flow rate or level of integration, etc. Figure 9 presents a direct view to recent examples of integrated platforms.

Reproduced with permission from American Chemical Society. B Knowlton et al., 2018. Reproduced with permission from Springer Nature. C Chen et al., 2018. Reproduced with permission from American Chemical Society. D Seok et al., 2021. Reproduced with permission from MDPI. And E Kim et al., 2016. Reproduced with permission from American Chemical Society
Graphical illustrations of several recently developed integrated devices. Images are adapted from: A Cho et al., 2020.
Microfluidics technology requires only nanoliter or even picoliter volumes of sample to detect in a micrometer-scale structure, significantly increasing the specific surface area of the reaction chamber, thus facilitating the heat transfer effect of the biochemical reaction and enabling rapid reactions. This assay reduces the time, sample size, and reagent volume of traditional methods, allows for automated sample manipulation on a chip, and also requires less personnel handling skills, making it ideal for quick and easy monitoring of bioaerosols in the field. Laboratory-on-a-chip based on the microfluidic principle has been used individually for bioaerosol collection and detection techniques. Commercial samplers tend to be large and require external pumps becoming a challenge for simultaneous sampling and detection using microfluidic chips. Research on microfluidic platforms integrating capture, enrichment, and detection technologies has recently been reported (Duarte et al., 2021; Lee et al., 2020; Liu et al., 2018b; Ryu et al., 2020; Sun et al., 2022). A high degree of integration minimizes system complexity and enables real-time and continuous detection of bioaerosols, enabling rapid response to unexpected risks. They enriched bioaerosols in a small volume of liquid by designing a capture structure or reducing the sampling volume of the sampler, etc., which was integrated with a downstream detection platform on a microfluidic chip, ultimately realizing in situ sampling of bioaerosol particles. The capture efficiencies and detection limits of the individual chips met expectations, making them a promising candidate for the immediate detection of trace pathogens in environmental samples (POCT). Figure 10 illustrates the idea of microfluidic chips for integrated monitoring of bioaerosols.
Microfluidic technology is becoming more and more mature, and as a miniaturized and rapid experimental platform, it is widely used in molecular diagnosis, biochemical analysis and other fields. However, the design and fabrication of microfluidic platforms have a high threshold, requiring repeated testing and modification of their structures and functions by specialized personnel, and high manufacturing costs, which hinder the promotion of microfluidic technology. Machine learning (ML) is a promising approach to identify patterns and predict future behaviors using trainable statistical models to automate the design-test-optimization process of microfluidic platforms, reduce the knowledge and experience gaps of non-professionals in the research and development process, and facilitate the commercialization and diffusion of microfluidic platforms (McIntyre et al., 2022; Riordon et al., 2019). Typically, machine learning requires large-scale raw data input and computation to achieve accurate automated operations, an inefficient and time-consuming approach, and a team of researchers is currently using design of experiments and numerical methods with machine learning algorithms to accelerate experimental optimization of platforms without the need for extensive experimentation (Ahmadi et al., 2022). Furthermore, mapping design and operational parameters to ML models enables redesigning the functionality of microfluidic platforms, while integration with computer-aided design (CAD) frameworks enables automating the design of microfluidic platforms (Tsur et al., 2020; Yiannacou et al., 2022).
Artificial intelligence (AI) is an emerging technology with extremely powerful computational and analytical capabilities, and the integration of AI and microfluidic technology is a hot spot in current research. The large amount of data generated after high-throughput testing of the microfluidic platform can promote the deep learning of AI, while the powerful processing capability of AI can quickly complete the analysis of the test results of the microfluidic platform, and the fusion and synergy between the two technologies can enable real-time risk assessment and evidence-based public health interventions for pathogenic microorganisms in bioaerosols(Fang et al., 2023; Liu et al., 2021, 2023). Integrating AI and microfluidics facilitates significantly improved analysis of massive datasets obtained from high-throughput and multiplexed microfluidic platforms (Zare Harofte et al., 2022), while deploying high-density detection devices can output real-time maps of airborne pathogen distribution and predict transmission pathways, translating bioaerosol monitoring results into potential individual infection risk probability, providing support for public health interventions and decision-making.
Although research on integrated bioaerosol monitoring platforms has made some progress, there is still some room for improvement. Firstly, most devices validate their monitoring performance in simulated environments of single or complex bioaerosols, and there is a lack of precision and reliability testing of the devices using field samples. Among them, enzymes, fluorescent probes, and biochemical reagents are easily affected by environmental factors or operational steps and lose their activity. Strategies of immobilization or pre-embedding can be adopted to reduce liquid transport and thus improve stability. Secondly, current detection methods lack a step to determine dead microorganisms in collected samples, which can lead to an overestimation of the amount of pathogenic microorganisms in bioaerosols. Bacterial stains (LIVE/DEAD®BacLight™), DNA dyes (propidium monomethyl azide (PMA) and ethidium monomethyl azide (EMA)), etc., can be used to determine microbial activity, but reasonable concentrations for use need to be considered to avoid toxicity. Thirdly, a wide range of biological and chemical reactions in a very small volume of reaction systems characterize microfluidic chips, and their potential for high-throughput, multiplexed detection should be fully demonstrated in future design and fabrication to better meet the needs of practical applications.
In summary, we reviewed the recent advances in collection, enrichment, and detection of bioaerosol samples and compared their pros and cons. The future trend in on-site monitoring of disease-causing pathogens in bioaerosols involve the use of miniaturized and integrated multiplexed detection platforms to realize real-time detection. In recent years, numerous achievements have been made in the field of bioaerosol collection and detection in conjunction with emerging science and technology. The rapid growth in microfluidic chips and artificial intelligence provided more versatile solutions. The future challenge lies in how to cost effectively minimize the influence of interfering components in collected sample and how to establish reliable validation standard to test these systems. The first challenge is mostly related to development of microfabrication and the design of microfluidic devices. The high price is due to the constant changing chip design and low requirement in amount of chips. Once the design is well tested, there are many ways to reduce the cost of microfluidic chips, such as injection molding. The second challenge is due to the complex composition of real field samples. There are microorganisms, organic molecules, inorganic compounds, and particles in collected real sample that might affect the activity of recognition sites and cause error in detection process, but the standard sample used during research and development do not mimic such effect. As the result, the systems based on standard sample may not perform well when dealing with real samples. This poses high requirement to enrichment procedure, especially in selectivity and recover rate of targets. Another aspect is the varying working environment, including temperature, light, radiation, humidity, contaminates that may cause unfavorable condition in working media (such as pH change), etc. A portable on-site monitoring device should be tested under different situations so that the results are not influenced with unconventional working environment. Last but not least, researchers should combine both direct and indirect validation to ensure reliable results. Direct validation could be achieved by building a secure chamber and release real sample containing pathogens, and the chamber could be fully sterilized after validation of monitoring effect. The indirect validation is based on non-pathogenic surrogate or mock target, which are similar to pathogens but are not as dangerous as real pathogens.
Data availability and materials
Data availability is not applicable to this article as no new data were created or analyzed in this study.
References
Afshar-Mohajer, N., Foos, R., Ramachandran, G., & Volckens, J. (2021). Field evaluation of the ultrasonic personal aerosol sampler (UPAS) for respirable dust exposure in a taconite mine. Annals of Work Exposures and Health, 65(1), 127–135. https://doi.org/10.1093/annweh/wxaa094
Ahmadi, F., Simchi, M., Perry, J. M., Frenette, S., Benali, H., Soucy, J. P., Massarweh, G., & Shih, S. C. C. (2022). Integrating machine learning and digital microfluidics for screening experimental conditions. Lab on a Chip, 23(1), 81–91. https://doi.org/10.1039/d2lc00764a
Ahmadipour, F., Esmaeili Sari, A., & Bahramifar, N. (2019). Characterization, concentration and risk assessment of airborne particles using car engine air filter (case study: Tehran metropolis). Environmental Geochemistry and Health, 41(6), 2649–2663. https://doi.org/10.1007/s10653-019-00319-1
Al-abdalall, A. H., Al-dakheel, S. A., & Al-Abkari, H. A. (2019). Impact of air-conditioning filters on microbial growth and indoor air pollution. In T. Morosuk & M. Sultan (Eds.), Low-temperature technologies (pp. 179–206). InTech. https://doi.org/10.5772/intechopen.88548
Alonso, C., Raynor, P. C., Davies, P. R., & Torremorell, M. (2015). Concentration, size distribution, and infectivity of airborne particles carrying swine viruses. PLoS ONE, 10(8), e0135675. https://doi.org/10.1371/journal.pone.0135675
Arnaout, R., Lee, R. A., Lee, G. R., Callahan, C., Yen, C. F., Smith, K. P., Arora, R., & Kirby, J. E. (2020). SARS-CoV2 testing: The limit of detection matters. bioRxiv, 167, 131144. https://doi.org/10.1101/2020.06.02.131144
Bardhan, N. M., Jansen, P., & Belcher, A. M. (2021). Graphene, carbon nanotube and plasmonic nanosensors for detection of viral pathogens: Opportunities for rapid testing in pandemics like COVID-19. Frontiers in Nanotechnology, 3, 733126. https://doi.org/10.3389/fnano.2021.733126
Ben-Jaber, S., Peveler, W. J., Quesada-Cabrera, R., Cortés, E., Sotelo-Vazquez, C., Abdul-Karim, N., Maier, S. A., & Parkin, I. P. (2016). Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules. Nature Communications, 7, 12189. https://doi.org/10.1038/ncomms12189
Bennett, A., & Parks, S. (2006). Microbial aerosol generation during laboratory accidents and subsequent risk assessment. Journal of Applied Microbiology, 100(4), 658–663. https://doi.org/10.1111/j.1365-2672.2005.02798.x
Berry, E. D., Wells, J. E., Bono, J. L., Woodbury, B. L., Kalchayanand, N., Norman, K. N., Suslow, T. V., López-Velasco, G., & Millner, P. D. (2015). Effect of proximity to a cattle feedlot on Escherichia coli O157:H7 contamination of leafy greens and evaluation of the potential for airborne transmission. Applied and Environmental Microbiology, 81(3), 1101–1110. https://doi.org/10.1128/AEM.02998-14
Bertran, K., Balzli, C., Kwon, Y. K., Tumpey, T. M., Clark, A., & Swayne, D. E. (2017). Airborne transmission of highly pathogenic influenza virus during processing of infected poultry. Emerging Infectious Diseases, 23(11), 1806–1814. https://doi.org/10.3201/eid2311.170672
Bhardwaj, J., Kim, M. W., & Jang, J. (2020). Rapid airborne influenza virus quantification using an antibody-based electrochemical paper sensor and electrostatic particle concentrator. Environmental Science and Technology, 54(17), 10700–10712. https://doi.org/10.1021/acs.est.0c00441
Bhardwaj, S. K., Bhardwaj, N., Kumar, V., Bhatt, D., Azzouz, A., Bhaumik, J., Kim, K. H., & Deep, A. (2021). Recent progress in nanomaterial-based sensing of airborne viral and bacterial pathogens. Environment International, 146, 106183. https://doi.org/10.1016/j.envint.2020.106183
Campbell, J. R., Battaglia, M., Jr., Dingilian, K., Cesler-Maloney, M., St Clair, J. M., Hanisco, T. F., Robinson, E., DeCarlo, P., Simpson, W., Nenes, A., Weber, R. J., & Mao, J. (2022). Source and chemistry of hydroxymethanesulfonate (HMS) in Fairbanks, Alaska. Environmental Science and Technology, 56(12), 7657–7667. https://doi.org/10.1021/acs.est.2c00410
Chai, A., Yuan, L., Li, L., Shi, Y., Xie, X., Wang, Q., & Li, B. (2020). Aerosol transmission of Pseudomonas amygdali pv. lachrymans in greenhouses. The Science of the Total Environment, 748, 141433. https://doi.org/10.1016/j.scitotenv.2020.141433
Chellasivalingam, M., Imran, H., Pandit, M., Boies, A. M., & Seshia, A. A. (2020). Weakly coupled piezoelectric MEMS resonators for aerosol sensing. Sensors, 20(11), 3162. https://doi.org/10.3390/s20113162
Chen, H., Li, J., Zhang, X., Li, X., Yao, M., & Zheng, G. (2018). Automated in vivo nanosensing of breath-borne protein biomarkers. Nano Letters, 18(8), 4716–4726. https://doi.org/10.1021/acs.nanolett.8b01070
Chen, H., & Yao, M. (2018). A high-flow portable biological aerosol trap (HighBioTrap) for rapid microbial detection. Journal of Aerosol Science, 117, 212–223. https://doi.org/10.1016/j.jaerosci.2017.11.012
Chen, T., Sun, J., Ma, T., Li, T., Liu, C., Zhu, X., & Xue, N. (2019). Design and analysis of particulate matter air-microfluidic grading chip based on MEMS. Micromachines, 10(8), 497. https://doi.org/10.3390/mi10080497
Chen, Y., Shi, Y., Chen, Y., Yang, Z., Wu, H., Zhou, Z., Li, J., Ping, J., He, L., Shen, H., Chen, Z., Wu, J., Yu, Y., Zhang, Y., & Chen, H. (2020). Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection. Biosensors and Bioelectronics, 169, 112642. https://doi.org/10.1016/j.bios.2020.112642
Cheng, Y., Sihua, Z., Lu, Q., Zhang, W., Wen, G., Luo, Q., Shao, H., & Zhang, T. (2020). Evaluation of young chickens challenged with aerosolized Salmonella pullorum. Avian Pathology, 49(5), 507–514. https://doi.org/10.1080/03079457.2020.1783433
Chia, P. Y., Coleman, K. K., Tan, Y. K., Ong, S. W. X., Gum, M., Lau, S. K., Lim, X. F., Lim, A. S., Sutjipto, S., Lee, P. H., Son, T. T., Young, B. E., Milton, D. K., Gray, G. C., Schuster, S., Barkham, T., De, P. P., Vasoo, S., Chan, M., et al. (2020). Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nature Communications, 11(1), 2800. https://doi.org/10.1038/s41467-020-16670-2
Cho, Y. S., Hong, S. C., Choi, J., & Jung, J. H. (2019). Development of an automated wet-cyclone system for rapid, continuous and enriched bioaerosol sampling and its application to real-time detection. Sensors and Actuators b, Chemical, 284, 525–533. https://doi.org/10.1016/j.snb.2018.12.155
Cho, Y. S., Kim, H. R., Ko, H. S., Jeong, S. B., Chan Kim, B., & Jung, J. H. (2020). Continuous surveillance of bioaerosols on-site using an automated bioaerosol-monitoring system. ACS Sensors, 5(2), 395–403. https://doi.org/10.1021/acssensors.9b02001
Choi, D. Y., Heo, K. J., Kang, J., An, E. J., Jung, S. H., Lee, B. U., Lee, H. M., & Jung, J. H. (2018). Washable antimicrobial polyester/aluminum air filter with a high capture efficiency and low pressure drop. Journal of Hazardous Materials, 351, 29–37. https://doi.org/10.1016/j.jhazmat.2018.02.043
Choi, J., Hong, S. C., Kim, W., & Jung, J. H. (2017). Highly enriched, controllable, continuous aerosol sampling using inertial microfluidics and its application to real-time detection of airborne bacteria. ACS Sensors, 2(4), 513–521. https://doi.org/10.1021/acssensors.6b00753
Choi, J., Lee, J., & Jung, J. H. (2020). Fully integrated optofluidic SERS platform for real-time and continuous characterization of airborne microorganisms. Biosensors and Bioelectronics, 169, 112611. https://doi.org/10.1016/j.bios.2020.112611
Coudron, L., McDonnell, M. B., Munro, I., McCluskey, D. K., Johnston, I. D., Tan, C. K. L., & Tracey, M. C. (2019). Fully integrated digital microfluidics platform for automated immunoassay; A versatile tool for rapid, specific detection of a wide range of pathogens. Biosensors and Bioelectronics, 128, 52–60. https://doi.org/10.1016/j.bios.2018.12.014
Desai, S., Mishra, S. V., Joshi, A., Sarkar, D., Hole, A., Mishra, R., Dutt, S., Chilakapati, M. K., Gupta, S., & Dutt, A. (2020). Raman spectroscopy-based detection of RNA viruses in saliva: A preliminary report. Journal of Biophotonics, 13(10), e202000189. https://doi.org/10.1002/jbio.202000189
Duarte, P. A., Menze, L., Shoute, L., Zeng, J., Savchenko, O., Lyu, J., & Chen, J. (2021). Highly efficient capture and quantification of the airborne fungal pathogen Sclerotinia sclerotiorum employing a nanoelectrode-activated microwell array. ACS Omega, 7(1), 459–468. https://doi.org/10.1021/acsomega.1c04878
Emerson, J. B., Keady, P. B., Brewer, T. E., Clements, N., Morgan, E. E., Awerbuch, J., Miller, S. L., & Fierer, N. (2015). Impacts of flood damage on airborne bacteria and fungi in homes after the 2013 Colorado Front Range flood. Environmental Science and Technology, 49(5), 2675–2684. https://doi.org/10.1021/es503845j
Espí, A., Del Cerro, A., Oleaga, Á., Rodríguez-Pérez, M., López, C. M., Hurtado, A., Rodríguez-Martínez, L. D., Barandika, J. F., & García-Pérez, A. L. (2021). One Health Approach: An Overview of Q Fever in Livestock, Wildlife and Humans in Asturias (Northwestern Spain). Animals, 11(5), 1395. https://doi.org/10.3390/ani11051395
Fang, W., Wu, J., Cheng, M., Zhu, X., Du, M., Chen, C., Liao, W., Zhi, K., & Pan, W. (2023). Diagnosis of invasive fungal infections: Challenges and recent developments. Journal of Biomedical Science, 30(1), 42. https://doi.org/10.1186/s12929-023-00926-2
Farling, S., Rogers, T., Knee, J. S., Tilley, E. A., Brown, J., & Deshusses, M. A. (2019). Bioaerosol emissions associated with pit latrine emptying operations. The Science of the Total Environment, 648, 1082–1086. https://doi.org/10.1016/j.scitotenv.2018.08.147
Foat, T. G., Sellors, W. J., Walker, M. D., Rachwal, P. A., Jones, J. W., Despeyroux, D. D., Coudron, L., Munro, I., McCluskey, D. K., Tan, C. K. L., & Tracey, M. C. (2016). A prototype personal aerosol sampler based on electrostatic precipitation and electrowetting-on-dielectric actuation of droplets. Journal of Aerosol Science, 95, 43–53. https://doi.org/10.1016/j.jaerosci.2016.01.007
Freschi, L., Vargas, R., Jr., Husain, A., Kamal, S. M. M., Skrahina, A., Tahseen, S., Ismail, N., Barbova, A., Niemann, S., Cirillo, D. M., Dean, A. S., Zignol, M., & Farhat, M. R. (2021). Population structure, biogeography and transmissibility of Mycobacterium tuberculosis. Nature Communications, 12(1), 6099. https://doi.org/10.1038/s41467-021-26248-1
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., Lang-Yona, N., Burrows, S. M., Gunthe, S. S., Elbert, W., Su, H., Hoor, P., Thines, E., Hoffmann, T., Després, V. R., & Pöschl, U. (2016). Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmospheric Research, 182, 346–376. https://doi.org/10.1016/j.atmosres.2016.07.018
Ge, Z. Y., Yang, L. M., Xia, J. J., Fu, X. H., & Zhang, Y. Z. (2020). Possible aerosol transmission of COVID-19 and special precautions in dentistry. Journal of Zhejiang University. Science. B, 21(5), 361–368. https://doi.org/10.1631/jzus.B2010010
Giordano, G. F., Freitas, V. M. S., Schleder, G. R., Santhiago, M., Gobbi, A. L., & Lima, R. S. (2021). Bifunctional metal meshes acting as a semipermeable membrane and electrode for sensitive electrochemical determination of volatile compounds. ACS Applied Materials and Interfaces, 13(30), 35914–35923. https://doi.org/10.1021/acsami.1c07874
Gladding, T. L., Rolph, C. A., Gwyther, C. L., Kinnersley, R., Walsh, K., & Tyrrel, S. (2020). Concentration and composition of bioaerosol emissions from intensive farms: Pig and poultry livestock. Journal of Environmental Management, 272, 111052. https://doi.org/10.1016/j.jenvman.2020.111052
Glushakova, A. M., Kachalkin, A. V., Prokof’eva, T. V., & Lysak, L. V. (2022). Enterobacteriaceae in soils and atmospheric dust aerosol accumulations of Moscow city. Current Research in Microbial Sciences, 3, 100124. https://doi.org/10.1016/j.crmicr.2022.100124
Gong, X., Kuo, Y. C., Zhou, G., Wu, W. J., & Liao, W. H. (2023). An aerosol deposition based MEMS piezoelectric accelerometer for low noise measurement. Microsystems and Nanoengineering, 9, 23. https://doi.org/10.1038/s41378-023-00484-5
Gregory, A. E., van Schaik, E. J., Russell-Lodrigue, K. E., Fratzke, A. P., & Samuel, J. E. (2019). Coxiella burnetii intratracheal aerosol infection model in mice, guinea pigs, and nonhuman primates. Infection and Immunity, 87(12), e00178-e219. https://doi.org/10.1128/IAI.00178-19
Guo, J., Zheng, X., Qin, T., Lv, M., Zhang, W., Song, X., Qiu, H., Hu, L., Zhang, L., Zhou, D., Sun, Y., & Yang, W. (2022). An experimental method for efficiently evaluating the size-resolved sampling efficiency of liquid-absorption aerosol samplers. Scientific Reports, 12(1), 4745. https://doi.org/10.1038/s41598-022-08718-8
Guo, L., Jackman, J. A., Yang, H. H., Chen, P., Cho, N. J., & Kim, D. H. (2015). Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today, 10(2), 213–239. https://doi.org/10.1016/j.nantod.2015.02.007
Gupta, N., Augustine, S., Narayan, T., O’Riordan, A., Das, A., Kumar, D., Luong, J. H. T., & Malhotra, B. D. (2021). Point-of-care PCR assays for COVID-19 detection. Biosensors, 11(5), 141. https://doi.org/10.3390/bios11050141
Haddow, A. D., Watt, T. R., Bloomfield, H. A., Shamblin, J. D., Dyer, D. N., & Harbourt, D. E. (2020). Stability of SARS-CoV-2 on produce following a low-dose aerosol exposure. The American Journal of Tropical Medicine and Hygiene, 103(5), 2024–2025. https://doi.org/10.4269/ajtmh.20-1033
Haldar, S., Sankhyan, N., Sharma, N., Bansal, A., Jain, V., Gupta, V. K., Juneja, M., Mishra, D., Kapil, A., Singh, U. B., Gulati, S., Kalra, V., & Tyagi, J. S. (2012). Detection of Mycobacterium tuberculosis GlcB or HspX antigens or devR DNA impacts the rapid diagnosis of tuberculous meningitis in children. PLoS ONE, 7(9), e44630. https://doi.org/10.1371/journal.pone.0044630
Han, Y., Li, F., Yang, L., Guo, X., Dong, X., Niu, M., Jiang, Y., Li, L., Li, H., & Sun, Y. (2023). Imunocapture magnetic beads enhanced and ultrasensitive CRISPR-Cas13a-assisted electrochemical biosensor for rapid detection of SARS-CoV-2. Biosensors, 13(6), 597. https://doi.org/10.3390/bios13060597
He, H., Li, R., Chen, Y., Pan, P., Tong, W., Dong, X., Chen, Y., & Yu, D. (2017). Integrated DNA and RNA extraction using magnetic beads from viral pathogens causing acute respiratory infections. Scientific Reports, 7, 45199. https://doi.org/10.1038/srep45199
Heo, K. J., Ko, H. S., Jeong, S. B., Kim, S. B., & Jung, J. H. (2021). Enriched aerosol-to-hydrosol transfer for rapid and continuous monitoring of bioaerosols. Nano Letters, 21(2), 1017–1024. https://doi.org/10.1021/acs.nanolett.0c04096
Hong, S., Bhardwaj, J., Han, C. H., & Jang, J. (2016). Gentle sampling of submicrometer airborne virus particles using a personal electrostatic particle concentrator. Environmental Science and Technology, 50(22), 12365–12372. https://doi.org/10.1021/acs.est.6b03464
Hong, S., Kim, M. W., & Jang, J. (2021). Physical collection and viability of airborne bacteria collected under electrostatic field with different sampling media and protocols towards rapid detection. Scientific Reports, 11(1), 14598. https://doi.org/10.1038/s41598-021-94033-7
Horve, P. F., Dietz, L., Northcutt, D., Stenson, J., & Van Den Wymelenberg, K. (2021). Evaluation of a bioaerosol sampler for indoor environmental surveillance of Severe Acute Respiratory Syndrome Coronavirus 2. PLoS ONE, 16(11), e0257689. https://doi.org/10.1371/journal.pone.0257689
Hou, P., Xu, Y., Wang, H., & He, H. (2020). Detection of bovine viral diarrhea virus genotype 1 in aerosol by a real time RT-PCR assay. BMC Veterinary Research, 16(1), 114. https://doi.org/10.1186/s12917-020-02330-6
Howard, N. C., & Khader, S. A. (2020). Immunometabolism during Mycobacterium tuberculosis infection. Trends in Microbiology, 28(10), 832–850. https://doi.org/10.1016/j.tim.2020.04.010
Humbal, C., Gautam, S., & Trivedi, U. (2018). A review on recent progress in observations, and health effects of bioaerosols. Environment International, 118, 189–193. https://doi.org/10.1016/j.envint.2018.05.053
Ijaz, M. K., Zargar, B., Wright, K. E., Rubino, J. R., & Sattar, S. A. (2016). Generic aspects of the airborne spread of human pathogens indoors and emerging air decontamination technologies. American Journal of Infection Control, 44(9 Suppl), S109–S120. https://doi.org/10.1016/j.ajic.2016.06.008
Innes, N., Johnson, I. G., Al-Yaseen, W., Harris, R., Jones, R., Kc, S., McGregor, S., Robertson, M., Wade, W. G., & Gallagher, J. E. (2021). A systematic review of droplet and aerosol generation in dentistry. Journal of Dentistry, 105, 103556. https://doi.org/10.1016/j.jdent.2020.103556
Ito, T., Ogino, K., Nagaoka, K., Takemoto, K., Nishiyama, R., & Shimizu, Y. (2019). Detection of 3-nitrotyrosine in atmospheric environments via a high-performance liquid chromatography-electrochemical detector system. Journal of Visualized Experiments. https://doi.org/10.3791/58371.10.3791/58371
Jang, J., Bhardwaj, J., & Jang, J. (2022). Efficient measurement of airborne viable viruses using the growth-based virus aerosol concentrator with high flow velocities. Journal of Hazardous Materials, 434, 128873. https://doi.org/10.1016/j.jhazmat.2022.128873
Jiang, X., Jing, W., Sun, X., Liu, Q., Yang, C., Liu, S., Qin, K., & Sui, G. (2016). High-throughput microfluidic device for LAMP analysis of airborne bacteria. ACS Sensors, 1(7), 958–962. https://doi.org/10.1021/acssensors.6b00282
Kalavakonda, R. R., Masna, N. V. R., Mandal, S., & Bhunia, S. (2021). A smart mask for active defense against airborne pathogens. Scientific Reports, 11(1), 19910. https://doi.org/10.1038/s41598-021-99150-x
Kang, H., Shang, X., Abdumutallip, M., Chen, Y., Li, L., Wang, X., Li, C., Ouyang, H., Tang, X., Wang, L., Rudich, Y., & Chen, J. (2022). Accurate observation of black and brown carbon in atmospheric fine particles via a versatile aerosol concentration enrichment system (VACES). The Science of the Total Environment, 837, 155817. https://doi.org/10.1016/j.scitotenv.2022.155817
Kaplan, B. S., Kimble, J. B., Chang, J., Anderson, T. K., Gauger, P. C., Janas-Martindale, A., Killian, M. L., Bowman, A. S., & Vincent, A. L. (2020). Aerosol transmission from infected swine to ferrets of an H3N2 Virus collected from an agricultural fair and associated with human variant infections. Journal of Virology, 94(16), e01009-e1020. https://doi.org/10.1128/JVI.01009-20
Kenarkoohi, A., Noorimotlagh, Z., Falahi, S., Amarloei, A., Mirzaee, S. A., Pakzad, I., & Bastani, E. (2020). Hospital indoor air quality monitoring for the detection of SARS-CoV-2 (COVID-19) virus. The Science of the Total Environment, 748, 141324. https://doi.org/10.1016/j.scitotenv.2020.141324
Kesavan, J., & Sagripanti, J. L. (2015). Evaluation criteria for bioaerosol samplers. Environmental Science. Processes and Impacts, 17(3), 638–645. https://doi.org/10.1039/c4em00510d
Khan, M. A. H., Rao, M. V., & Li, Q. (2019). Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors, 19(4), 905. https://doi.org/10.3390/s19040905
Kim, H. R., An, S., & Hwang, J. (2020a). An integrated system of air sampling and simultaneous enrichment for rapid biosensing of airborne coronavirus and influenza virus. Biosensors and Bioelectronics, 170, 112656. https://doi.org/10.1016/j.bios.2020.112656
Kim, H. R., An, S., & Hwang, J. (2020b). Aerosol-to-hydrosol sampling and simultaneous enrichment of airborne bacteria for rapid biosensing. ACS Sensors, 5(9), 2763–2771. https://doi.org/10.1021/acssensors.0c00555
Kim, H. R., An, S., & Hwang, J. (2021). High air flow-rate electrostatic sampler for the rapid monitoring of airborne coronavirus and influenza viruses. Journal of Hazardous Materials, 412, 125219. https://doi.org/10.1016/j.jhazmat.2021.125219
Kim, H. U., Min, J., Park, G., Shin, D., Sung, G., Kim, T., & Lee, M. H. (2018). Electrochemical detection of airborne influenza virus using air sampling system. Aerosol Air Qual, 18, 2721–2727. https://doi.org/10.4209/aaqr.2018.06.0221
Kim, J., Jin, J. H., Kim, H. S., Song, W., Shin, S. K., Yi, H., Jang, D. H., Shin, S., & Lee, B. Y. (2016). Fully automated field-deployable bioaerosol monitoring system using carbon nanotube-based biosensors. Environmental Science and Technology, 50(10), 5163–5171. https://doi.org/10.1021/acs.est.5b06361
Kim, S., Akarapipad, P., Nguyen, B. T., Breshears, L. E., Sosnowski, K., Baker, J., Uhrlaub, J. L., Nikolich-Žugich, J., & Yoon, J. Y. (2022). Direct capture and smartphone quantification of airborne SARS-CoV-2 on a paper microfluidic chip. Biosensors and Bioelectronics, 200, 113912. https://doi.org/10.1016/j.bios.2021.113912
Knowlton, S. D., Boles, C. L., Perencevich, E. N., Diekema, D. J., Nonnenmann, M. W., & Epicenters Program, C. D. C. (2018). Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrobial Resistance and Infection Control, 7, 16. https://doi.org/10.1186/s13756-018-0301-9
Kotwal, A., & Yadav, A. (2021). Biothreat & One Health: Current scenario & way forward. The Indian Journal of Medical Research, 153(3), 257–263. https://doi.org/10.4103/ijmr.IJMR_583_21
Kwaśny, M., Bombalska, A., Kaliszewski, M., Włodarski, M., & Kopczyński, K. (2023). Fluorescence methods for the detection of bioaerosols in their civil and military applications. Sensors, 23(6), 3339. https://doi.org/10.3390/s23063339
Ladhani, L., Pardon, G., Meeuws, H., van Wesenbeeck, L., Schmidt, K., Stuyver, L., & van der Wijngaart, W. (2017). Sampling and detection of airborne influenza virus towards point-of-care applications. PLoS ONE, 12(3), e0174314. https://doi.org/10.1371/journal.pone.0174314
Ladhani, L., Pardon, G., Moons, P., Goossens, H., & Van der Wijngaart, W. (2020). Electrostatic sampling of patient breath for pathogen detection: A pilot study. Frontiers in Mechanical Engineering, 6, 40. https://doi.org/10.3389/fmech.2020.00040
Lane, M. A., Brownsword, E. A., Babiker, A., Ingersoll, J. M., Waggoner, J., Ayers, M., Klopman, M., Uyeki, T. M., Lindsley, W. G., & Kraft, C. S. (2021). Bioaerosol sampling for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a Referral Center with Critically Ill Coronavirus Disease 2019 (COVID-19) patients March-May 2020. Clinical Infectious Diseases, 73(7), e1790–e1794. https://doi.org/10.1093/cid/ciaa1880
Lathika, S., Raj, A., & Sen, A. K. (2021). LSPR based on-chip detection of dengue NS1 antigen in whole blood. RSC Advances, 11(53), 33770–33780. https://doi.org/10.1039/d1ra05009e
Lee, C. H., Seok, H., Jang, W., Kim, J. T., Park, G., Kim, H. U., Rho, J., Kim, T., & Chung, T. D. (2021). Bioaerosol monitoring by integrating DC impedance microfluidic cytometer with wet-cyclone air sampler. Biosensors & Bioelectronics, 192, 113499. https://doi.org/10.1016/j.bios.2021.113499
Lee, I., Jeon, E., & Lee, J. (2023). On-site bioaerosol sampling and detection in microfluidic platforms. Trends in Analytical Chemistry: TRAC, 158, 116880. https://doi.org/10.1016/j.trac.2022.116880
Lee, I., Seok, Y., Jung, H., Yang, B., Lee, J., Kim, J., Pyo, H., Song, C. S., Choi, W., Kim, M. G., & Lee, J. (2020). Integrated bioaerosol sampling/monitoring platform: Field-deployable and rapid detection of airborne viruses. ACS Sensors, 5(12), 3915–3922. https://doi.org/10.1021/acssensors.0c01531
Li, J., Leavey, A., Wang, Y., O’Neil, C., Wallace, M. A., Burnham, C. D., Boon, A. C., Babcock, H., & Biswas, P. (2018c). Comparing the performance of 3 bioaerosol samplers for influenza virus. Journal of Aerosol Science, 115, 133–145. https://doi.org/10.1016/j.jaerosci.2017.08.007
Li, M., Wang, L., Qi, W., Liu, Y., & Lin, J. (2021a). Challenges and perspectives for biosensing of bioaerosol containing pathogenic microorganisms. Micromachines, 12(7), 798. https://doi.org/10.3390/mi12070798
Li, S., Zhou, Y., Gao, S., Pang, Q., & Miao, Z. (2018a). A small-scale study on airborne transmission of H9N2 avian influenza virus under field conditions. Journal of Infection in Developing Countries, 11(12), 962–966. https://doi.org/10.3855/jidc.9013
Li, W., Li, M., Zhang, H., Li, C., Xu, H., Gong, B., Fu, J., Guo, Z., Peng, J., Zhou, G., Tian, Z., & Wang, Q. (2022). A Novel immunochromatographic strip based on latex microspheres for the rapid detection of North American-type porcine reproductive and respiratory syndrome virus. Frontiers in Microbiology, 13, 882112. https://doi.org/10.3389/fmicb.2022.882112
Li, X., Chen, H., Qi, X., Peng, Y., Zhou, L., Ma, J., & Yao, M. (2021b). A robot assisted high-flow portable cyclone sampler for bacterial and SARS-CoV-2 aerosols. Aerosol and Air Quality Research, 21(12), 210130. https://doi.org/10.4209/aaqr.210130
Li, X., Zhang, X., Liu, Q., Zhao, W., Liu, S., & Sui, G. (2018b). Microfluidic system for rapid detection of airborne pathogenic fungal spores. ACS Sensors, 3(10), 2095–2103. https://doi.org/10.1021/acssensors.8b00615
Liu, D., Mariman, R., Gerlofs-Nijland, M. E., Boere, J. F., Folkerts, G., Cassee, F. R., & Pinelli, E. (2019). Microbiome composition of airborne particulate matter from livestock farms and their effect on innate immune receptors and cells. The Science of the Total Environment, 688, 1298–1307. https://doi.org/10.1016/j.scitotenv.2019.06.217
Liu, H., Nan, L., Chen, F., Zhao, Y., & Zhao, Y. (2023). Functions and applications of artificial intelligence in droplet microfluidics. Lab on a Chip, 23(11), 2497–2513. https://doi.org/10.1039/d3lc00224a
Liu, L., Bi, M., Wang, Y., Liu, J., Jiang, X., Xu, Z., & Zhang, X. (2021). Artificial intelligence-powered microfluidics for nanomedicine and materials synthesis. Nanoscale, 13(46), 19352–19366. https://doi.org/10.1039/d1nr06195j
Liu, Q., Zhang, X., Li, X., Liu, S., & Sui, G. (2018a). A semi-quantitative method for point-of-care assessments of specific pathogenic bioaerosols using a portable microfluidics-based device. Journal of Aerosol Science, 115, 173–180. https://doi.org/10.1016/j.jaerosci.2017.10.010
Liu, Q., Zhang, X., Li, X., Liu, S., & Sui, G. (2018b). A novel microfluidic module for rapid detection of airborne and waterborne pathogens. Sensors and Actuators b: Chemical, 258, 1138–1145. https://doi.org/10.1016/j.snb.2017.11.113
Liu, Q., Zhang, Y., Jing, W., Liu, S., Zhang, D., & Sui, G. (2016). First airborne pathogen direct analysis system. The Analyst, 141(5), 1637–1640. https://doi.org/10.1039/c5an02367j
Lu, C., Fang, Z., Yang, S., Ning, K., Xu, M., Buhot, A., Hou, Y., Hu, P., & Xu, P. (2024). Innovations in measuring and mitigating phytohemagglutinins, a key food safety concern in beans. Food Quality and Safety. https://doi.org/10.1093/fqsafe/fyae003
Lu, C., Wang, J., Pan, L., Gu, X., Lu, W., Chen, D., Zhang, C., Ye, Q., Xiao, C., Liu, P., Tang, Y., Tang, B., Huang, G., Fang, J., & Jiang, H. (2023). Rapid detection of multiple resistance genes to last-resort antibiotics in Enterobacteriaceae pathogens by recombinase polymerase amplification combined with lateral flow dipstick. Frontiers in Microbiology, 13, 1062577. https://doi.org/10.3389/fmicb.2022.1062577
Lu, H., Zhu, J., Zhang, T., Zhang, X., Chen, X., Zhao, W., Yao, Y., Zhao, W., & Sui, G. (2022). A rapid multiplex nucleic acid detection system of airborne fungi by an integrated DNA release device and microfluidic chip. Talanta, 246, 123467. https://doi.org/10.1016/j.talanta.2022.123467
Lu, Y., Lu, Q., Cheng, Y., Wen, G., Luo, Q., Shao, H., & Zhang, T. (2020). High concentration of coagulase-negative staphylococci carriage among bioaerosols of henhouses in Central China. BMC Microbiology, 20(1), 21. https://doi.org/10.1186/s12866-020-1709-y
Ma, J., Du, M., Wang, C., Xie, X., Wang, H., & Zhang, Q. (2021). Advances in airborne microorganisms detection using biosensors: A critical review. Frontiers of Environmental Science and Engineering, 15(3), 47. https://doi.org/10.1007/s11783-021-1420-8
Ma, J., Jiang, G., Ma, Q., Wang, H., Du, M., Wang, C., Xie, X., Li, T., & Chen, S. (2022). Rapid detection of airborne protein from Mycobacterium tuberculosis using a biosensor detection system. The Analyst, 147(4), 614–624. https://doi.org/10.1039/d1an02104d
Ma, Z., Zheng, Y., Cheng, Y., Xie, S., Ye, X., & Yao, M. (2016). Development of an integrated microfluidic electrostatic sampler for bioaerosol. Journal of Aerosol Science, 95, 84–94. https://doi.org/10.1016/j.jaerosci.2016.01.003
Madhwal, S., Prabhu, V., Sundriyal, S., & Shridhar, V. (2020). Ambient bioaerosol distribution and associated health risks at a high traffic density junction at Dehradun city, India. Environmental Monitoring and Assessment, 192(3), 196. https://doi.org/10.1007/s10661-020-8158-9
Mainelis, G. (2020). Bioaerosol sampling: Classical approaches, advances, and perspectives. Aerosol Science and Technology : THe Journal of the American Association for Aerosol Research, 54(5), 496–519. https://doi.org/10.1080/02786826.2019.1671950
Mbareche, H., Veillette, M., Bilodeau, G. J., & Duchaine, C. (2018). Bioaerosol sampler choice should consider efficiency and ability of samplers to cover microbial diversity. Applied and Environmental Microbiology, 84(23), e01589-e1618. https://doi.org/10.1128/AEM.01589-18
McIntyre, D., Lashkaripour, A., Fordyce, P., & Densmore, D. (2022). Machine learning for microfluidic design and control. Lab on a Chip, 22(16), 2925–2937. https://doi.org/10.1039/d2lc00254j
Melzow, F., Mertens, S., Todorov, H., Groneberg, D. A., Paris, S., & Gerber, A. (2022). Aerosol exposure of staff during dental treatments: A model study. BMC Oral Health, 22(1), 128. https://doi.org/10.1186/s12903-022-02155-9
Meyer, M., Cox, J. A., Hitchings, M. D. T., Burgin, L., Hort, M. C., Hodson, D. P., & Gilligan, C. A. (2017). Quantifying airborne dispersal routes of pathogens over continents to safeguard global wheat supply. Nature Plants, 3(10), 780–786. https://doi.org/10.1038/s41477-017-0017-5
Mhuireach, G., Johnson, B. R., Altrichter, A. E., Ladau, J., Meadow, J. F., Pollard, K. S., & Green, J. L. (2016). Urban greenness influences airborne bacterial community composition. The Science of the Total Environment, 571, 680–687. https://doi.org/10.1016/j.scitotenv.2016.07.037
Mohd Amiruddin, M. N., Ang, G. Y., Yu, C. Y., Falero-Diaz, G., Otero, O., Reyes, F., Camacho, F., Chin, K. L., Sarmiento, M. E., Norazmi, M. N., Acosta, A., & Yean Yean, C. (2020). Development of an immunochromatographic lateral flow dipstick for the detection of Mycobacterium tuberculosis 16 kDa antigen (Mtb-strip). Journal of Microbiological Methods, 176, 106003. https://doi.org/10.1016/j.mimet.2020.106003
Ngashangva, L., Hemdan, B. A., El-Liethy, M. A., Bachu, V., Minteer, S. D., & Goswami, P. (2022). Emerging bioanalytical devices and platforms for rapid detection of pathogens in environmental samples. Micromachines, 13(7), 1083. https://doi.org/10.3390/mi13071083
Nguyen, P. Q., Soenksen, L. R., Donghia, N. M., Angenent-Mari, N. M., de Puig, H., Huang, A., Lee, R., Slomovic, S., Galbersanini, T., Lansberry, G., Sallum, H. M., Zhao, E. M., Niemi, J. B., & Collins, J. J. (2021). Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nature Biotechnology, 39(11), 1366–1374. https://doi.org/10.1038/s41587-021-00950-3
Nguyen, X. D., Zhao, Y., Evans, J. D., Lin, J., & Purswell, J. L. (2022). Survival of Escherichia coli in airborne and settled poultry litter particles. Animals : An Open Access Journal from MDPI, 12(3), 284. https://doi.org/10.3390/ani12030284
Niewulis, A., Berendt, A., Podliński, J., & Mizeraczyk, J. (2013). Electrohydrodynamic flow patterns and collection efficiency in narrow wire-cylinder type electrostatic precipitator. Journal of Electrostatics, 71(4), 808–814. https://doi.org/10.1016/j.elstat.2013.02.002
Nigatu, A. W., Bråtveit, M., Deressa, W., & Moen, B. E. (2015). Respiratory symptoms, fractional exhaled nitric oxide & endotoxin exposure among female flower farm workers in Ethiopia. Journal of Occupational Medicine and Toxicology, 10, 8. https://doi.org/10.1186/s12995-015-0053-x
Noda, J., Tomizawa, S., Takahashi, K., Morimoto, K., & Mitarai, S. (2022). Air pollution and airborne infection with mycobacterial bioaerosols: A potential attribution of soot. International Journal of Environmental Science and Technology : IJEST, 19(2), 717–726. https://doi.org/10.1007/s13762-021-03203-7
Noti, J. D., Lindsley, W. G., Blachere, F. M., Cao, G., Kashon, M. L., Thewlis, R. E., McMillen, C. M., King, W. P., Szalajda, J. V., & Beezhold, D. H. (2012). Detection of infectious influenza virus in cough aerosols generated in a simulated patient examination room. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America, 54(11), 1569–1577. https://doi.org/10.1093/cid/cis237
Ntziachristos, L., Ning, Z., Geller, M. D., Sheesley, R. J., Schauer, J. J., & Sioutas, C. (2007). Fine, ultrafine and nanoparticle trace element compositions near a major freeway with a high heavy-duty diesel fraction. Atmospheric Environment, 41(27), 5684–5696. https://doi.org/10.1016/j.atmosenv.2007.02.043
Oksanen, L. M., Sanmark, E., Sofieva, S., Rantanen, N., Lahelma, M., Anttila, V. J., Lehtonen, L., Atanasova, N., Pesonen, E., Geneid, A., & Hyvärinen, A. P. (2022). Aerosol generation during general anesthesia is comparable to coughing: An observational clinical study. Acta Anaesthesiologica Scandinavica, 66(4), 463–472. https://doi.org/10.1111/aas.14022
Owoicho, O., Olwal, C. O., & Quaye, O. (2021). Potential of laser-induced fluorescence-light detection and ranging for future stand-off virus surveillance. Microbial Biotechnology, 14(1), 126–135. https://doi.org/10.1111/1751-7915.13698
Pan, M., Eiguren-Fernandez, A., Hsieh, H., Afshar-Mohajer, N., Hering, S. V., Lednicky, J., Hugh Fan, Z., & Wu, C. Y. (2016). Efficient collection of viable virus aerosol through laminar-flow, water-based condensational particle growth. Journal of Applied Microbiology, 120(3), 805–815. https://doi.org/10.1111/jam.13051
Pan, M., Lednicky, J. A., & Wu, C. Y. (2019). Collection, particle sizing and detection of airborne viruses. Journal of Applied Microbiology, 127(6), 1596–1611. https://doi.org/10.1111/jam.14278
Pardon, G., Ladhani, L., Sandström, N., Ettori, M., Lobov, G., & van der Wijngaart, W. (2015). Aerosol sampling using an electrostatic precipitator integrated with a microfluidic interface. Sensors and Actuators b: Chemical, 212, 344–352. https://doi.org/10.1016/j.snb.2015.02.008
Patterson, B., Dinkele, R., Gessner, S., Morrow, C., Kamariza, M., Bertozzi, C. R., Kamholz, A., Bryden, W., Call, C., Warner, D. F., & Wood, R. (2020). Sensitivity optimisation of tuberculosis bioaerosol sampling. PLoS ONE, 15(9), e0238193. https://doi.org/10.1371/journal.pone.0238193
Pinals, R. L., Ledesma, F., Yang, D., Navarro, N., Jeong, S., Pak, J. E., Kuo, L., Chuang, Y. C., Cheng, Y. W., Sun, H. Y., & Landry, M. P. (2021). Rapid SARS-CoV-2 spike protein detection by carbon nanotube-based near-infrared nanosensors. Nano Letters, 21(5), 2272–2280. https://doi.org/10.1021/acs.nanolett.1c00118
Pirhadi, M., Mousavi, A., & Sioutas, C. (2020). Evaluation of a high flow rate electrostatic precipitator (ESP) as a particulate matter (PM) collector for toxicity studies. The Science of the Total Environment, 739, 140060. https://doi.org/10.1016/j.scitotenv.2020.140060
Poonsuk, K., Giménez-Lirola, L., & Zimmerman, J. J. (2018). A review of foot-and-mouth disease virus (FMDV) testing in livestock with an emphasis on the use of alternative diagnostic specimens. Animal Health Research Reviews, 19(2), 100–112. https://doi.org/10.1017/S1466252318000063
Prost, K., Kloeze, H., Mukhi, S., Bozek, K., Poljak, Z., & Mubareka, S. (2019). Bioaerosol and surface sampling for the surveillance of influenza A virus in swine. Transboundary and Emerging Diseases, 66(3), 1210–1217. https://doi.org/10.1111/tbed.13139
Putzer, D., Dammerer, D., Huber, C., Boschert, H., Thaler, M., & Nogler, M. (2022). Aerosol morphology and particle size distribution in orthopaedic bone machining: a laboratory worst-case contamination simulation. Is high-speed bone machining potentially harmful by pollution and quality schemes and what measures could be taken for prevention? International Orthopaedics, 46(7), 1647–1655. https://doi.org/10.1007/s00264-022-05398-x
Qiu, G., Gai, Z., Tao, Y., Schmitt, J., Kullak-Ublick, G. A., & Wang, J. (2020). Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano, 14(5), 5268–5277. https://doi.org/10.1021/acsnano.0c02439
Reicks, D. L. (2019). Effective biosecurity to protect North American studs and clients from emerging infectious disease. Theriogenology, 137, 82–87. https://doi.org/10.1016/j.theriogenology.2019.05.041
Riordon, J., Sovilj, D., Sanner, S., Sinton, D., & Young, E. W. K. (2019). Deep learning with microfluidics for biotechnology. Trends in Biotechnology, 37(3), 310–324. https://doi.org/10.1016/j.tibtech.2018.08.005
Robinson, J. M., & Breed, M. F. (2023). The aerobiome-health axis: A paradigm shift in bioaerosol thinking. Trends in Microbiology, 31(7), 661–664. https://doi.org/10.1016/j.tim.2023.04.007
Ryu, B., Chen, J., Kurabayashi, K., Liang, X., & Park, Y. (2020). Integrated on-site collection and detection of airborne microparticles for smartphone-based micro-climate quality control. The Analyst, 145(19), 6283–6290. https://doi.org/10.1039/d0an01147a
Sánchez-Parra, B., Núñez, A., & Moreno, D. A. (2019). Preventing legionellosis outbreaks by a quick detection of airborne Legionella pneumophila. Environmental Research, 171, 546–549. https://doi.org/10.1016/j.envres.2019.01.032
Santarpia, J. L., Rivera, D. N., Herrera, V. L., Morwitzer, M. J., Creager, H. M., Santarpia, G. W., Crown, K. K., Brett-Major, D. M., Schnaubelt, E. R., Broadhurst, M. J., Lawler, J. V., Reid, S. P., & Lowe, J. J. (2020). Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Scientific Reports, 10(1), 12732. https://doi.org/10.1038/s41598-020-69286-3
Seok, Y., Lee, J., & Kim, M. G. (2021). Paper-Based airborne bacteria collection and DNA extraction kit. Biosensors, 11(10), 375. https://doi.org/10.3390/bios11100375
Shafagati, N., Lundberg, L., Baer, A., Patanarut, A., Fite, K., Lepene, B., & Kehn-Hall, K. (2015). The use of Nanotrap particles in the enhanced detection of Rift Valley fever virus nucleoprotein. PLoS ONE, 10(5), e0128215. https://doi.org/10.1371/journal.pone.0128215
Shim, E., Noh, J., & Kim, Y. (2022). Development and performance evaluation of polytetrafluoroethylene-membrane-based automotive cabin air filter. ACS Omega, 7(48), 43738–43746. https://doi.org/10.1021/acsomega.2c04758
Sim, K. M., Park, H. S., Bae, G. N., & Jung, J. H. (2015). Antimicrobial nanoparticle-coated electrostatic air filter with high filtration efficiency and low pressure drop. The Science of the Total Environment, 533, 266–274. https://doi.org/10.1016/j.scitotenv.2015.07.003
Su, X., Ren, R., Wu, Y., Li, S., Ge, C., Liu, L., & Xu, Y. (2021). Study of biochip integrated with microelectrodes modified by poly-dopamine-co-chitosan composite gel for separation, enrichment and detection of microbes in the aerosol. Biosensors and Bioelectronics, 176, 112931. https://doi.org/10.1016/j.bios.2020.112931
Sun, Z., Lin, K. F., Zhao, Z. H., Wang, Y., Hong, X. X., Guo, J. G., Ruan, Q. Y., Lu, L. Y., Li, X., Zhang, R., Yang, C. Y., & Li, B. A. (2022). An automated nucleic acid detection platform using digital microfluidics with an optimized Cas12a system. Science China Chemistry, 65(3), 630–640. https://doi.org/10.1007/s11426-021-1169-1
Suntornsuk, W., & Suntornsuk, L. (2020). Recent applications of paper-based point-of-care devices for biomarker detection. Electrophoresis, 41(5–6), 287–305. https://doi.org/10.1002/elps.201900258
Tabarov, A., Vitkin, V., Andreeva, O., Shemanaeva, A., Popov, E., Dobroslavin, A., Kurikova, V., Kuznetsova, O., Grigorenko, K., Tzibizov, I., Kovalev, A., Savchenko, V., Zheltuhina, A., Gorshkov, A., & Danilenko, D. (2022). Detection of A and B influenza viruses by surface-enhanced raman scattering spectroscopy and machine learning. Biosensors, 12(12), 1065. https://doi.org/10.3390/bios12121065
Taghvaee, S., Mousavi, A., Sowlat, M. H., & Sioutas, C. (2019). Development of a novel aerosol generation system for conducting inhalation exposures to ambient particulate matter (PM). The Science of the Total Environment, 665, 1035–1045. https://doi.org/10.1016/j.scitotenv.2019.02.214
Takemura, K. (2021). Surface plasmon resonance (SPR)- and localized SPR (LSPR)-based virus sensing systems: Optical vibration of nano- and micro-metallic materials for the development of next-generation virus detection technology. Biosensors, 11(8), 250. https://doi.org/10.3390/bios11080250
Tan, M., Shen, F., Yao, M., & Zhu, T. (2011). Development of an automated electrostatic sampler (AES) for bioaerosol detection. Aerosol Science and Technology, 45(9), 1154–1160. https://doi.org/10.1080/02786826.2011.582193
Tian, J. H., Yan, C., Nasir, Z. A., Alcega, S. G., Tyrrel, S., & Coulon, F. (2020). Real time detection and characterisation of bioaerosol emissions from wastewater treatment plants. The Science of the Total Environment, 721, 137629. https://doi.org/10.1016/j.scitotenv.2020.137629
Triadó-Margarit, X., Cáliz, J., & Casamayor, E. O. (2022). A long-term atmospheric baseline for intercontinental exchange of airborne pathogens. Environment International, 158, 106916. https://doi.org/10.1016/j.envint.2021.106916
Truyols Vives, J., Muncunill, J., Toledo Pons, N., Baldoví, H. G., Sala Llinàs, E., & Mercader Barceló, J. (2022). SARS-CoV-2 detection in bioaerosols using a liquid impinger collector and ddPCR. Indoor Air, 32(2), e13002. https://doi.org/10.1111/ina.13002
Tsur, E. E. (2020). Computer-aided design of microfluidic circuits. Annual Review of Biomedical Engineering, 22, 285–307. https://doi.org/10.1146/annurev-bioeng-082219-033358
Viana, M., Salmatonidis, A., Bezantakos, S., Ribalta, C., Moreno, N., Córdoba, P., Cassee, F. R., Boere, J., Fraga, S., Teixeira, J. P., Bessa, M. J., & Monfort, E. (2021). Characterizing the chemical profile of incidental ultrafine particles for toxicity assessment using an aerosol concentrator. Annals of Work Exposures and Health, 65(8), 966–978. https://doi.org/10.1093/annweh/wxab011
Wang, F. A., Lakshmipriya, T., & Gopinath, S. C. B. (2018). Red spectral shift in sensitive colorimetric detection of tuberculosis by ESAT-6 antigen-antibody complex: A new strategy with gold nanoparticle. Nanoscale Research Letters, 13(1), 331. https://doi.org/10.1186/s11671-018-2753-5
Wang, H., Ma, Z., Qin, J., Shen, Z., Liu, Q., Chen, X., Wang, H., An, Z., Liu, W., & Li, M. (2019b). A versatile loop-mediated isothermal amplification microchip platform for Streptococcus pneumoniae and Mycoplasma pneumoniae testing at the point of care. Biosensors and Bioelectronics, 126, 373–380. https://doi.org/10.1016/j.bios.2018.11.011
Wang, J., Jiang, H., Pan, L., Gu, X., Xiao, C., Liu, P., Tang, Y., Fang, J., Li, X., & Lu, C. (2023). Rapid on-site nucleic acid testing: On-chip sample preparation, amplification, and detection, and their integration into all-in-one systems. Frontiers in Bioengineering and Biotechnology, 11, 1020430. https://doi.org/10.3389/fbioe.2023.1020430
Wang, L., Qi, W., Liu, Y., Essien, D., Zhang, Q., & Lin, J. (2021). Recent advances on bioaerosol collection and detection in microfluidic chips. Analytical Chemistry, 93(26), 9013–9022. https://doi.org/10.1021/acs.analchem.1c00908
Wang, P., Zhang, R., Wu, Y., Chang, Y., & Liu, M. (2022). An Electrochemical aptasensor integrating zeolitic imidazolate framework for highly selective detection of bioaerosols. Biosensors, 12(9), 725. https://doi.org/10.3390/bios12090725
Wang, Z., Li, J., Qian, L., Liu, L., Qian, J., Lu, B., & Guo, Z. (2019a). Composition and distribution analysis of bioaerosols under different environmental conditions. Journal of Visualized Experiments. https://doi.org/10.3791/58795.10.3791/58795
Wei, X., Aggrawal, A., Bond, R. F., & Atwill, E. R. (2023). Low to zero concentrations of airborne bacterial pathogens and indicator E. coli in proximity to beef cattle feedlots in Imperial Valley, California. Microorganisms, 11(2), 411. https://doi.org/10.3390/microorganisms11020411
Wei, Z., Rosario, R. C., & Montoya, L. D. (2010). Collection efficiency of a midget impinger for nanoparticles in the range of 3–100 nm. Atmospheric Environment, 44(6), 872–876. https://doi.org/10.1016/j.atmosenv.2009.11.037
Winter, F., Schoneberg, C., Wolf, A., Bauer, B. U., Prüfer, T. L., Fischer, S. F., Gerdes, U., Runge, M., Ganter, M., & Campe, A. (2021). Concept of an active surveillance system for Q fever in german small ruminants-conflicts between best practices and feasibility. Frontiers in Veterinary Science, 8, 623786. https://doi.org/10.3389/fvets.2021.623786
Wong, R., Bannerjee, P., & Kumaran, N. (2021). Aerosol generating procedures in intraocular surgery. Eye, 35(5), 1504–1505. https://doi.org/10.1038/s41433-020-0997-7
Workman, A. D., Jafari, A., Welling, D. B., Varvares, M. A., Gray, S. T., Holbrook, E. H., Scangas, G. A., Xiao, R., Carter, B. S., Curry, W. T., & Bleier, B. S. (2020). Airborne aerosol generation during endonasal procedures in the era of COVID-19: Risks and recommendations. Otolaryngology: Head and Neck Surgery, 163(3), 465–470. https://doi.org/10.1177/0194599820931805
Wu, B., Bai, X., Liu, W., Zhu, C., Hao, Y., Lin, S., Liu, S., Luo, L., Liu, X., Zhao, S., Hao, J., & Tian, H. (2020). Variation characteristics of final size-segregated PM emissions from ultralow emission coal-fired power plants in China. Environmental Pollution, 259, 113886. https://doi.org/10.1016/j.envpol.2019.113886
Wubulihairen, M., Jiang, Y., & Ning, Z. (2015). Prototype development and laboratory evaluation of an aerosol to hydrosol sampler. Aerosol and Air Quality Research, 15(3), 776–785. https://doi.org/10.4209/aaqr.2014.08.0175
Xiao, Y., Yang, F., Liu, F., Yao, H., Wu, N., & Wu, H. (2021). Antigen-capture ELISA and immunochromatographic test strip to detect the H9N2 subtype avian influenza virus rapidly based on monoclonal antibodies. Virology Journal, 18(1), 198. https://doi.org/10.1186/s12985-021-01671-4
Xiong, H., Ye, X., Li, Y., Qi, J., Fang, X., & Kong, J. (2021). Efficient microfluidic-based air sampling/monitoring platform for detection of aerosol SARS-CoV-2 on-site. Analytical Chemistry, 93(9), 4270–4276. https://doi.org/10.1021/acs.analchem.0c05154
Xu, S., Ouyang, W., Xie, P., Lin, Y., Qiu, B., Lin, Z., Chen, G., & Guo, L. (2017). Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Analytical Chemistry, 89(3), 1617–1623. https://doi.org/10.1021/acs.analchem.6b03711
Xu, Z., Wei, K., Wu, Y., Shen, F., Chen, Q., Li, M., & Yao, M. (2013). Enhancing bioaerosol sampling by Andersen impactors using mineral-oil-spread agar plate. PLoS ONE, 8(2), e56896. https://doi.org/10.1371/journal.pone.0056896
Yakoh, A., Pimpitak, U., Rengpipat, S., Hirankarn, N., Chailapakul, O., & Chaiyo, S. (2021). Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosensors and Bioelectronics, 176, 112912. https://doi.org/10.1016/j.bios.2020.112912
Yang, Y., Murray, J., Haverstick, J., Tripp, R. A., & Zhao, Y. (2022). Silver nanotriangle array based LSPR sensor for rapid coronavirus detection. Sensors and Actuators b, Chemical, 359, 131604. https://doi.org/10.1016/j.snb.2022.131604
Yao, M., Mainelis, G., & An, H. R. (2005). Inactivation of microorganisms using electrostatic fields. Environmental Science and Technology, 39(9), 3338–3344. https://doi.org/10.1021/es048808x
Ye, J., Qian, H., Zhang, J., Sun, F., Zhuge, Y., & Zheng, X. (2021). Combining culturing and 16S rDNA sequencing to reveal seasonal and room variations of household airborne bacteria and correlative environmental factors in Nanjing, southeast China. Indoor Air, 31(4), 1095–1108. https://doi.org/10.1111/ina.12807
Yiannacou, K., Sharma, V., & Sariola, V. (2022). Programmable droplet microfluidics based on machine learning and acoustic manipulation. Langmuir: the ACS Journal of Surfaces and Colloids, 38(38), 11557–11564. https://doi.org/10.1021/acs.langmuir.2c01061
Yin, S., & Tong, C. (2021). Europium (III)-Modified silver nanoparticles as ratiometric colorimetric and fluorescent dual-mode probes for selective detection of dipicolinic acid in bacterial spores and lake waters. ACS Applied Nano Materials, 4(5), 5469–5477. https://doi.org/10.1021/acsanm.1c00838
Yoo, M. S., Shin, M., Kim, Y., Jang, M., Choi, Y. E., Park, S. J., Choi, J., Lee, J., & Park, C. (2017). Development of electrochemical biosensor for detection of pathogenic microorganism in Asian dust events. Chemosphere, 175, 269–274. https://doi.org/10.1016/j.chemosphere.2017.02.060
Yu, G. L., Wei, L. M., Liu, Y. Y., Liu, J. Y., Wang, Y., Gao, J., Chai, T. J., & Cai, Y. M. (2016a). Influence of indoor microbial aerosol on the welfare of meat ducks. British Poultry Science, 57(1), 12–22. https://doi.org/10.1080/00071668.2015.1122739
Yu, J., Lin, Y., Cao, Y., Li, X., Liao, D., Ye, Y., Pan, M., Ye, J., Wei, Y., Xiao, L., Tang, J., Kang, R., Xie, J., & Zhou, L. (2020). Development and application of a colloidal gold test strip for the rapid detection of the infectious laryngotracheitis virus. Poultry Science, 99(5), 2407–2415. https://doi.org/10.1016/j.psj.2019.11.066
Yu, K. P., Chen, Y. P., Gong, J. Y., Chen, Y. C., & Cheng, C. C. (2016b). Improving the collection efficiency of the liquid impinger for ultrafine particles and viral aerosols by applying granular bed filtration. Journal of Aerosol Science, 101, 133–143. https://doi.org/10.1016/j.jaerosci.2016.08.002
Yu, Y., Liang, Z., Liao, W., Ye, Z., Li, G., & An, T. (2021). Contributions of meat waste decomposition to the abundance and diversity of pathogens and antibiotic-resistance genes in the atmosphere. The Science of the Total Environment, 784, 147128. https://doi.org/10.1016/j.scitotenv.2021.147128
Zare Harofte, S., Soltani, M., Siavashy, S., & Raahemifar, K. (2022). Recent advances of utilizing artificial intelligence in lab on a chip for diagnosis and treatment. Small (weinheim an Der Bergstrasse, Germany), 18(42), e2203169. https://doi.org/10.1002/smll.202203169
Zhao, W., Pan, F., Wang, B., Wang, C., Sun, Y., Zhang, T., Shi, Y., & Zhang, H. (2019). Epidemiology characteristics of Streptococcus pneumoniae from children with pneumonia in shanghai: A retrospective study. Frontiers in Cellular and Infection Microbiology, 9, 258. https://doi.org/10.3389/fcimb.2019.00258
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2600503) and the 26th Student Research Program of China Jiliang University (Nos. 2023X26095).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. CL organized the structure of the paper and was in charge of modification of the writing. XF was in charge of collecting related papers and writing the initial draft. The other authors contributed in providing insight opinion in bioaerosol collection and detection industry and provided some related papers. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
There is no conflict of interest to declare.
Ethical approval
This was a review study and did not require ethical approval.
Consent to participate
The study did not involve animal experiments and human subjects.
Consent to publish
The authors confirm that all participants gave informed consent for the publication of the article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Feng, X., Hu, P., Jin, T. et al. On-site monitoring of airborne pathogens: recent advances in bioaerosol collection and rapid detection. Aerobiologia (2024). https://doi.org/10.1007/s10453-024-09824-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10453-024-09824-y