Abstract
As one of biological particles, pollen grains could be suspended in air and cause health risks such as pollinosis and rhinitis. Air pollutants can be absorbed on the pollen grains which would increase allergenicity. However, few studies have been focused on characterization of polycyclic aromatic hydrocarbons (PAHs), one kind of toxic pollutants absorbed on pollen grains, and their relationships with the allergenic proteins. In this study, fresh Platanus pollen samples were collected in the central parking area and the outer ring area of Shanghai in the spring of 2023. Mass concentration of allergenic protein (Pla a3) released from the samples collected at the two sampling sites were investigated by using western blot assay and enzyme linked immunosorbent assay, while mass levels of PAHs absorbed on the surface of flower inflorescence and pollen grains were measured by using GC-MS. Our data showed that the mass level of Pla a3 released from the pollen grains collected in the parking area was significantly higher than that collected in the outer ring area. Mass level of PAHs on the flower inflorescence and pollen grains in the parking area was 2 times higher than that in the outer ring area. The molecular docking results indicated that four disulfide bonds (Cys4-Cys52, Cys14-Cys29, Cys30-Cys75, Cys50-Cys89) in the Pla a3 protein structure could interact with the PAHs by hydrogen bonds. This interaction could increase the structural stability of the protein and enhance the allergenicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Many researches have focused on the physicochemical characterization of ambient particles, including organic carbon (OC), elemental carbon (EC), heavy metals, polycyclic aromatic hydrocarbon (PAHs), etc. (Lu et al., 2019). Few studies have reported the biological component, which is one of the important compositions of particulate matters (PMs) (Misiukiewicz-Stepien & Paplinska-Goryca, 2021.). Biological components in PMs include bacteria, viruses, and pollen grains. Bacteria could be in the PM2.5 fraction, while viruses are generally included in PM1 (Huffman et al., 2010). In recent years, pollen pollution has attracted widespread attention due to the increase in the urban greening area and the occurrence of pollinosis (Plaza et al., 2016; Zhou et al., 2019). In the meantime, epidemiological investigation and experimental research showed that air pollutants had a significant effect on pollen allergy (Grewling et al., 2020; Grundström et al., 2017). Exposure to both pollen and air pollutants would have more major health effects than exposure to only pollen or air pollutants (Peden & Reed, 2010).
Previous studies have shown that pollen-induced respiratory allergies are caused by allergenic proteins (Zhou et al., 2021). Allergenic pollens include Ragweed, Birch, Platanus and Poaceae etc. To date, 987 different allergens have been officially described, of which 195 are registered as plant-derived airborne allergens (https://www.allergen.org). The pollen of Platanus is a major source allergen in Shanghai in spring. Our previous study showed that the amount of pollen detected in the atmosphere of Shanghai in the spring of 2009 reached 13.8% of the total annual pollen in Shanghai, which greatly increased the risk of pollen sensitization in the atmosphere during the flowering period of the Platanus (Feng et al., 2011). In particular, air pollutants, such as PMs, NOx, O3, etc., could alter the chemical composition of the pollen surface or alter the expression of pollen allergenic proteins, thus enhancing the allergenicity caused by pollen proteins (Zhou et al., 2021).
Sedghy et al. (2017) reported that Plannatus pollen allergenic proteins (Pla a3) in pollen grains collected from polluted areas were higher than those from unpolluted areas, exacerbating allergic disease infections. Vehicle emissions are one of the main sources of fine particulate matter, accounting for about 15–50% of the total aerosol mass in urban areas (Harrison & Pant, 2013). A large number of studies on traffic pollutants such as heavy metals (mercury, lead and cadmium), transition metals (nickel, chromium and manganese) and PAHs have been conducted from the perspective of environmental pollution (Ugale & Tidke, 2022), but few studies have been carried out on the relationship between these pollutants and pollen allergenicity. Due to the PAHs have clear carcinogenic effects and absorbed on pollen grains (Lambert et al., 2012), studies on relationships between PAHs and allergenic protein could provide usful information for assessement of allergenicty induced by the pollen protein. In this study, fresh pollen inflorescence was collected from Planatus tree planted in a city parking area and outer ring area, respectively. Plannatus pollen protein (Pla a3) and PAHs absorbed in pollen samples were investigated by immunochemical assay and gas chromatography-mass spectrometry respectively. In addition, the molecular docking technique was employed to investigate the binding ability between Pla a3 and PAHs. Our data could provide unique information for the assessment of potential allergenicity induced by Planatus pollen protein and PAHs.
2 Materials and methods
2.1 Sampling collection
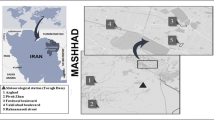
Sampling site A (121°42′15.55″, 31.28′93.09″N) was located in the urban area and sampling site B (121°37′53.13″, 31°32′25.99″) was located in outer ring area. The distance between the two sampling sites are about 20 km (Fig. 1). Branches with pollen grains were cut from the Platanus trees using 5 m long branch shears and the pollen inflorescence was collected in clean self-sealing polyethylene plastic bags. This sampling campaign was carried out in on the 5th of April, 2023. The pollen inflorescences were spread on a flat surface covered with clean tinfoil on the lab desk and dried naturally. After 2 days, pollen grains released from the inflorescences were collected. The pollen grains were separated by sieving using a 360-mesh sieve. The sieved pollen grains and pollen residue were kept in 15 ml eppendorf tubes, labeled and stored in the refrigerator at − 20 °C until use.
2.2 Immunofluorescence experiments
2.2.1 Measurement of total protein from pollen grains using bicinchoninic acid (BCA)
Pollen protein concentration was determined by BCA method by moving 1.5 ml of pollen sample into a 15 ml eppendorf tube, adding 7.5 ml of PBS, mixing for 10 and 30 min at 4 °C and 13,000 × g centrifuging for 15 min, and removing the supernatant. Total protein was quantified using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher, USA).
2.2.2 Measurement of pollen sub pollen particles (SPPs) release by flow cytometry
Pollen grain samples of 0.1 g were taken in a 2 ml centrifuge tube. The pollen grains were suspended with ddH2O, 1 m CaCl2 and 1 mM (NH4)2SO4, respectively, and incu-bated at 4 °C for 30 min. The release rate of SPPs was measured by Flow Cytometer (FCM) (MoFlo XDP, BECKMAN COULTER TER HK LTD). In the experiment, 10,000 particles from each sample were set to be measured.
2.2.3 ELISA assay for measuring Pla a3
The total protein extracted in the 2.2.1 section was diluted to 400 μg/mL, 100 μl of antigen was added to each well of a 96-well plate, and the concentration of Pla a3 protein and Tublin protein (one of the main components that make up the cytoskeleton, with relatively constant expression that does not change in response to external conditions, Hong et al., 2018) in the samples were measured separately. The internal reference antibody was used as a positive control (internal reference) at 4 °C in the experiment, and protected from light overnight; then, the plate was washed 3 times with PBS (5 min each time), followed by 5% skim milk (Each well was incubated with 100 μl of rabbit serum (diluted 1000 times)/Tublin’s antibody (diluted 1500 times) at 37 °C for 1 h. After 5 washes, 100 μl of peroxidase-labeled anti-rabbit IgG secondary antibody (diluted 10,000 times) was added and incubated for 1 h at 37 °C, with protection from light, followed and washed 7 times. 200 μl of TMB substrate color development solution was added to each well, incubated at 37 °C for 30 min, and the reaction was terminated by adding 50 μl of 2 M H2SO4. Finally, the absorbance of the reaction wells was read at 450 nm using a microplate marker (Bio-Tek Instruments, Winooski, VT, USA).
2.2.4 Western blot
The detailed protocol of the assay can be found in our previous report (Zhou et al., 2021). Briefly, Pla a3 protein lysates were separated on 15% SDS-PAGE electrophoresis and blotted onto the NC membrane by the method of electrical transfer. The membrane was then sealed by adding 1% BSA for 1 h. After that, the blocked membranes were incubated with sensitized rat IgG antiserum (1:2000 dilution) at 37 °C for 1 h. After washing the membrane three times with PBST buffer (pH 7.4, 0.05% Tween), the binding of the primary antibody was detected with mouse HRP-conjugated anti-rat IgG poly-clonal antibody (1:10,000 dilution, YESEN Biotechnology Co. Ltd), and the membrane was incubated at 37 °C for 1 h. Subsequently, the membranes were washed with PBST buffer (pH 7.4) and incubated with substrate TMB at 37 °C for 30 min for color develop-ment.
2.3 Determination of polycyclic aromatic hydrocarbons (PAHs)
The analytical procedure for PAHs has been described previously (Liu et al., 2017; Wang et al., 2012) and modified for the pollen inflorescences experiment. Briefly, 2 g of pollen sample and inflorescence residue were moved to separate test tubes, For the recovery, a mixture of compounds containing deu-terated naphthalene (Nap-d8, m/z136), deuterated acenaphthene (Ace-d10, m/z 164), deuterated phenanthrene (Phe-d10, m/z 188), deuterated cheyenne (Chr-d12, m/z 240) and deuterated perylene (Per-d12, m/z 264) were added to all samples prior to extraction. Recoveries of the target compounds were over 70%.
Extraction was carried out using dichloromethane (DCM) as the extracting solvent for 48 h. The total extract was concentrated to about 5 ml using a rotary evaporator. Then, the solvent was exchanged to hexane (HEX) and the samples were further concentrated to about 2 ml with a stream of highly purified N2. The concentrated extracts were se-quentially purified in the chromatography column filled with 3 cm of deactivated alumina (Al2O3), 3 cm of silica gel (SiO2) and 1 cm of anhydrous sodium sulfate (Na2SO4) and eluted with 20 ml of DCM/HEX (1:1, v:v). A sample vial (2 ml) was prepared for the final concentrated extract, and hexamethylbenzene (HMB) was used as an internal standard to quantify the PAHs. The analytical conditions of the analytical instrument are important parameters for the qualitative analysis of PAHs using GC-MS (GC/MS—QP2010Plus, Shimadzu, Japan), and in this experiment, reference was made to the qualitative analytical parameters available in our laboratory.
2.3.1 Chromatographic conditions and mass spectrometry condtion
Chromatographic column: DB-5MS capillary column (30 m × 0.25 mm i.d × 0.25 μm film thickness); carrier gas: 99.999% helium; flow rate: 1 mL/min; inlet temperature: 270 °C; injection: non-split injection, injection volume of 2 μL; ramp-up procedure: the initial column temperature of 90 °C (held for 1 min), ramping up to 180 °C at a rate of 8 °C/min, and finally ramping up to 280 °C at a rate of 15 °C/min (held for 15 min). The column was ramped up to 180 °C at a rate of 8 °C/min, and finally to 280 °C at a rate of 15 °C/min (held for 15 min).
Ion source: EI (electron bombardment source); ion source temperature: 260 °C; in-terface temperature: 200 °C; ionization voltage: 70 eV; qualitative analysis: full scan mode (SCAN), 40–350 amu; quantitative analysis: selected ion detection (SIM).
2.3.2 Quality control
Procedural blanks, standard-spiked blanks and matrices were analyzed. The tar-geted 16 PAH compounds were not detected in the procedural blank; the PAH recov-eries of the standard-spiked matrix ranged from 85 to 95%; and the paired duplicate samples agreed within 15% of the measured values (n = 10). Sample results are shown as a blank correction by subtracting an average blank from each sample. During the injec-tion process, a quality control standard (QC) was performed every 4 samples to check instrument stability, correct the retention time, and validate the response.
2.3.3 Data processing
The mass concentration of each PAH was calculated from the peak of the spectrum and divided by the mass of the corresponding flower inflorescence/pollen grains (μg/g).
2.4 Statistical analysis
Data were obtained from at least three independent experiments and expressed as mean ± standard error (SE). Statistical analyzes were performed using SPSS version 11.5 for paired Student’s t-test and ANOVA. Differences were considered statistically sig-nificant when p < 0.05.
2.5 Molecular docking
Molecular docking is extensively applied in computer-aided drug design to dock small molecules with macromolecules in order to find leading molecules with desired bio-performance. Blind molecular docking of PAHs into Pla a3 protein was achieved in silico using PyRx software version 0.8 (San Diego, CA, USA) to assess the docking pat-tern (docking manner and sites), binding affinity (BA) score, and inhibition constant (Ki) between allergens and PAHs.
2.5.1 Establishment of Pla a3 Protein structure
Based on the amino acid sequence of the Pla a3 protein (Fig. S1), a similar protein structure to the Pla a3 protein can be found in the SWISS-MODEL database (https://swissmodel.expasy.org).
The template for the non-specific lipid transfer protein Pun g 1.0101 in pomegran-ate was selected as the predicted structural model for the Pla a3 protein in Fig. S2 (in supplementary material).
2.5.2 Determination of binding sites and major affinities of PAHs to the Pla a3 protein
Three-dimensional structure data files (SDFs) of PAHs were obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov). The PAHs were docked using AutoDock Vina in PyRx software version 0.8. The blind docking method was selected to contact the PAHs (binding free energy of each PAHs) with the ligand of Plaa3 at the lowest energy. The best conformation for the receptor-ligand interaction was selected, and the type of protein–ligand interaction was displayed using Biovia Discovery Studio 4.5 software.
3 Results
3.1 BCA measurement of total protein
As shown in Fig. 2A, after 30 min of soaking in ddH2O, the total protein con-centrations of pollen samples from the inner-city parking lot and the outer ring area were 31.03 mg/ml and 23.67 mg/ml, respectively. Our previous data showed that sub pollen particles (SPPs) could be released from pollen grains after soaking in PBS (Zhou et al., 2019). It was obvious that visual differences in the yellow color intensity could be found between the different samples, the solution color of the samples from site A was darker than the samples from site B (Fig. 2B). In this study, the results of flow cytometry showed that the proportion of SPPs released after 30 min of soaking in Platanus pollen in the urban parking area was 3.58%, and that in the outer ring area was 1.26%. Apparently, more SPPS and total proteins were released from pollen grains collected from the urban park area (Fig. 2C).
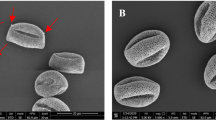
Subpollen particles released from Plannatus pollen grains. A: Total protein concentration from the samples after immersing in water in 30 min. (**p < 0.01) B: Images of SPPs extracts (left-samples from park area, right- samples from outer ring area), C: percentage of SPPs released from the pollen grains by using of Flow Cytometer
3.2 Mass level of Pla a3 measured by ELISA assay
The ELISA results showed that the mass level of Pla a3 protein in the pollen grain collected from the urban park area (5.13 ± 0.23 μg/ml) was significantly higher than that in the pollen collected from the outer ring area (4.26 ± 0.08 μg/ml) (**p < 0.01) (Fig. 3) when the same adjustment was made for the mass level of Tublin protein in both sam-ples.
3.3 Western blot assay
The results of Western blot assay showed that the sample collected from the parking area and the outer ring area induced gray value ratios of 0.88 ± 0.23 and 0.34 ± 0.09, respectively (Fig. 4). The gray value ratio of Pla a3 protein to Tublin protein in the sample collected from the park area was higher than that of the outer ring area.
3.4 Mass concentration of polycyclic aromatic hydrocarbon in the pollen inflorescences and in pollen grains
Mass concentrations of PAHs in the pollen samples from the parking area were higher than in the samples from the outer ring area (Table 1). The total concentration of PAHs on pollen grains collected in the parking area (102.74 ± 7.49 ng/g) was about 5 times higher than in the outer ring area (19.11 ± 0.63 ng/g). Six kinds of PAHs were de-tected in pollen grains collected from an urban parking area, namely naphthalene (17.25 ± 2.89 ng/g), acenaphthene (11.19 ± 1.8 ng/g), fluorine (14.38 ± 2.1 ng/g), phenanthrene (31.39 ± 3.45 ng/g), fluoranthene (12.25 ± 0.82 ng/g), and pyrene (16.28 ± 0.92 ng/g); while four PAHs were present in pollen grains collected from the outer area, namely, naphthalene (4.28 ± 0.07 ng/g), acenaphthene (1.1 ± 0.56 ng/g), phenanthrene (4.13 ± 0.62 ng/g), and pyrene (9.59 ± 0.12 ng/g). The average concentration of PAHs in pollen inflorescence in the park area was 12.17 ± 1.25 ng/g, which was higher than that in pollen grains (3.99 ± 0.15 ng/g), while in the outer ring area, the average concentration of PAHs in the pollen inflorescence and grains was 4.78 ± 0.16 ng/g and 5.28 ± 0.39 ng/g, respectively. This phenomenon may suggest the possible entry of PAHs into the pollen inflorescence and their deposition in pollen grains.
3.5 Molecular docking
Molecular docking technique, i.e., the docking of small molecules to macromolec-ular compounds with specific biological functions, is widely used in computer-aided drug design (Dallakyan & Olson, 2015). This has been applied to studies of the allergen Der f 2 using Todomatsu essential oil (Lin et al., 2022). Therefore, we worked to find the binding site of Pla a3 protein structure with PAHs to provide useful information for Pla a3 allergen-induced allergenicity.
In the optimal template of the Pla a3 protein (Figs. S1 and S2), four disulfide bonds, Cys4-Cys52, Cys14-Cys29, Cys30-Cys75, and Cys50-Cys89, can be found (Fig. S3), which play a role in protein stability. Our docking data demonstrated that the four disulfide bonds could bind to PAHs via hydrogen bonds, van der Waals forces, hydrophobic interactions, π-S interactions, and π-σ interactions. Pyrene, fluorene, flu-oranthene, and benzo(b)fluoranthene interact with Cys49 in a sulfur π-bond, whereas anthracene has an alkyl-hydrophobic interaction with Cys14, and indeno(1,2,3-cd) py-rene has an alkyl-hydrophobic interaction with Cys49 (Table 2). The interaction between the Plaa3 protein and the PAHs could cause changes in the di-tertiary structure of the protein and affect the allergenicity of the protein.
4 Discussion
In this study, fresh Planatus pollen inflorescences in the urban park area (point A) and outer ring area (point B) were collected. BCA results showed that pollen samples collected at sampling site A released more total protein than the samples collected at sampling site B. More SPPs were released from pollen grains at the sampling site A, implying that air pollutants, such as NOx and PM, play an important role in SPPs release. We previously reported that pollen grain wall could be broken by NO2 and O3, leading to an increased release of Pla a3 allergen into the atmosphere in the form of SPPs (Zhou et al., 2021). Therefore, traffic pollution affects the ability of pollen to release SPPs.
ELISA assay (Fig. 3) and Western blot assay (Fig. 4) revealed that the expres-sion of Pla a3 protein from the pollen grain collected at the parking area was higher than that of the outer ring area (Fig. 3) and the pollen protein had a stronger IgG-binding ability (Fig. 4). Our results are in agreement with those of Ghiani et al. (2012), who reported that Artemisa pollen proteins collected from high traffic areas had a higher IgE binding signal.
Total concentration of PAHs was nearly 5 times higher in samples collected in the urban area than in the outer ring area. Six PAHs were detected in pollen samples at the parking area sampling site and four PAHs were detected in pollen samples at the outer ring sampling site. Lambert et al. (2012) reported that mass level of four PAHs (PAH4: sum of benzo[a]pyrene(BaP), benzo[a]anthracene (BaA), benzo[b]fluoranthene (BbF) and chrysene (CHR) varied from 0.330 to 129.410 µg/ kg in pollen in several apiaries located in west France. Smiljanic et al. (2019) reported that two PAHs were detected on pollen collected in unpolluted areas away from urban areas: naphthalene (104.8 ± 14.9 ng/g) and phenanthrene (319.3 ± 39.9 ng/g); while four PAHs were detected on pollen collected near a chemical plant (both chemically polluted and traffic-polluted): naph-thalene (20.4 ± 2.9 ng/g), acenaphthylene (20.9 ± 3.0 ng/g), phenanthrene (171.3 ± 21.4 ng/g), and fluoranthene (14.2 ± 1.8 ng/g). Yue et al. (2016) tested the mass levels of PAHs in bee pollen samples collected from different areas of China, including Shanxi, Shandong and Beijing places (Table 1): Lingshi County in Shanxi Province, Mengyin County in Shandong Province, and Miyun Mountain District in Beijing. The environmental quality of the three sampling sites varied (more coal washeries, coking plants, magne-sium plants and aluminum plants) in Lingshi County (Shanxi) and emitting a large number of toxic and harmful gases caused serious pollution to the natural environment. The sampling of Melissa yard in Mengyin County (Shandong) is close to a small marble factory or beside the highway, and the environmental pollution is moderate. Miyun Mountain District is far away from large industrial factories and has a large green cov-erage, thus differing in the mass level of the polycyclic aromatic hydrocarbons in the pollen samples. It was observed that fluorene, phenanthrene and naphthalene were abundant in the samples collected in Lingshi county. Our data also showed that mass levels of phenanthrene, pyrene and naphthalene were high in pollen grains. Two kinds of PAHs with more than three benzene rings were found in the pollen grain collected from the parking area, fluoranthene (12.25 ± 0.82 ng/g) and pyrene (16.28 ± 0.92 ng/g); however, only one PAH, pyrene (9.59 ± 0.12 ng/g) was detected in the pollen grain samples collected in the outer ring area, and our data may provide useful information that air quality around the inner-city parking area can enhance allergenic effects induced by the pollen protein.
To investigate the interrelationships between PAHs and Pla a3 proteins, the mo-lecular docking technique was employed. Our data show that six PAHs can interact with Cys14 and Cys49 amino acid residues, Cys14-Cys28 and Cys49-Cys88, respectively, which affects two pairs of disulfide bonds of Cys14-Cys28 and Cys49-Cys88, and results in changes in protein structure and thus changes in protein sensitization. As shown in Table 2, the main covalent binding forces of PAHs to amino acid residues are hydrogen bonding, van der Waals forces, hydrophobic interactions, π-S interactions, and π-σ in-teractions, and the Gibbs free energy (ΔG) of PAHs interbinding with multiple amino acid residues is < 0, which indicates that the interbinding of the two is a spontaneous entropy-driven exothermic reaction and an ordered entropy-reducing process.
Hydrophobic interactions are the main driving force for protein folding and are essential for maintaining protein stability and biological activity. PAHs are strongly hydrophobic and can enhance amino acid hydrophobicity when bound to amino acid residues, and hydrophobic interactions also promote the formation of the protein’s hydrophobic nucleus and the most overall force throughout the folding, which is the force that maintains the structure of biological systems. Considering that PAHs have a wide range of covalent bonding interactions with amino acid residues, such as hydrogen bonds, van der Waals forces, and hydrophobic interactions, PAHs could modify protein molecules, leading to changes in the protein macromolecular structure, and thus alter-ing protein sensitization, requiring further research to be studied in the near future.
5 Conclusions
Fresh Planatus pollen grains in Shanghai different areas were collected. Plannatus allergenic pollen protein (Pla a3) and PAHs absorbed in pollen samples were investi-gated. Relationships between the Pla a3 and the PAHs were analysized. The conclusions were following as.
-
(1)
Immunochemical analysis of Pla a3 protein expression by Western blot assay and Elisa method showed that the expression of Pla a3 protein in the pollen of Platanus in the park area was significantly higher than that in the outer ring area.
-
(2)
The mass concentration of PAHs in Planatus inflorescence and pollen grains collected in the turban area was higher than that in the outer ring area.
-
(3)
The molecular docking results showed that PAHs could interact with Pla a3 protein, increasing its structural stability and may enhance the sensitization of the protein.
References
Dallakyan, S., & Olson, A. J. (2015). Small-molecule library screening by docking with PyRx. Methods in Molecular Biology, 1263, 243–250.
Feng, M., Lu, S., Zhang, R., Shen, C., Huang, J., Wang, Y., Wu, L., Feng, J., & Wu, M. (2011). Preliminary studies on complex pollution caused by ambient allergenic pollens and urban particulate matters in Shanghai. China Environmental Science, 31(7), 1095–1101.
Ghiani, A., Aina, R., Asero, R., Bellotto, E., & Citterio, S. (2012). Ragweed pollen collected along high-traffic roads shows a higher aller-genicity than pollen sampled in vegetated areas. Allergy, 67, 887–894.
Grewling, Ł, Bogawski, P., Kostecki, Ł, Nowak, M., Szymańska, A., & Frątczak, A. (2020). Atmospheric exposure to the major Ar-temisia pollen allergen (Art v 1): Seasonality, impact of weather, and clinical implications. Science of the Total Enviroment. https://doi.org/10.1016/j.scitotenv.2020.136611
Grundström, M., Dahl, Å., Ou, T., Chen, D., & Pleijel, H. (2017). The relationship between birch pollen, air pollution and weather types and their effect on antihistamine purchase in two Swedish cities. Aerobiologia (Bologna), 33, 457–471.
Harrison, R., & Pant, P. (2013). Estimation of the contribution of road traffic emissions to particulate matter concentrations from field measurements: A review. Atmospheric Environment, 77, 78–97.
Hong, Q., Zhou, S., Zhao, H., Peng, J., Li, Y., Shang, Y., Wu, M., Zhang, W., Lu, S., Li, S., Yu, S., Wang, W., & Wang, Q. (2018). Allergenicity of recombinant Humulus japonicus pollen allergen 1 after combined exposure to ozone and nitrogen dioxide. Environmental Pollution, 234, 707–715.
Huffman, J. A., Treutlein, B., & P¨oschl, U. (2010). Fluorescent biological aerosol particle concentrations and size distributions measured with an ultraviolet aerodynamic particle sizer (UV-APS) in central Europe. Atmospheric Chemistry and Physics, 10, 3215–3233. https://doi.org/10.5194/acp-10-3215-2010
Lambert, O., Veyrand, B., Durand, S., Marchand, P., Bizec, B., Piroux, M., Puyo, S., Thorin, C., Delbac, F., & Pouliquen, H. (2012). Polycyclic aromatic hydrocarbons: Bees, honey and pollen as sentinels for environmental chemical contaminant. Chemosphere, 86, 98–104.
Lin, Y., Enyoh, C. E., Wang, Q., Lu, S., Zhang, W., Xiao, K., Zhou, S., Kaneko, T., Seguchi, A., Wang, W., & Guo, Y. (2022). Novel approaches for inhibiting the indoor allergen Der f 2 excreted from house dust mites by todomatsu oil produced from woodland residues. International Journal of Environmental Research and Public Health, 19(17), 10881. https://doi.org/10.3390/ijerph191710881
Liu, Y., Yan, C., Ding, X., Wang, X., Fu, Q., Zhao, Q., Zhang, Y., Duan, Y., Qiu, X., & Zheng, M. (2017). Sources and spatial distribution of particulate polycyclic aromatic hydrocarbons in Shanghai, China. Science of the Total Environment, 584–585, 307–317.
Lu, S., Win, M., Zeng, J., Yao, C., Zhao, M., Xiu, G., Lin, Y., Dai, Y., Rao, L., Zhang, L., Yonemochi, S., & Wang, Q. (2019). A characterization of HULIS-C and the oxidative potential of HULIS and HULIS-Fe(II) mixture in PM2.5 during hazy and non-hazy days in Shanghai. Atmospheric Environment, 219, 117058.
Misiukiewicz-Stepien, P., & Paplinska-Goryca, M. (2021). Biological effect of PM10 on airway epithelium-focus on obstructive lung diseases. Clinical Immunology, 227, 108754.
Peden, D., & Reed, C. E. (2010). Environmental and occupational allergies. The Journal of Allergy and Clinical Immunology, 125, 150–160.
Plaza, M., Alcazar, P., Hernandez-Ceballos, M., & Galan, C. (2016). Mismatch in aeroallergens and airborne grass pollen concentrations. Atmospheric Environment, 144, 361–369.
Sedghy, F., Sankian, M., Moghadam, M., Ghasemi, Z., Mahmoudi, M., & Varasteh, A. R. (2017). Impact of traffic-related air pollution on the expression of Platanus orientalis pollen allergens. International Journal of Biometeorology, 61, 1–9.
Smiljanic, K., Prodic, I., Apostolovic, D., Cvetkovic, A., Veljovic, D., Mutic, J., Hage, M., Burazer, L., & Velickovic, T. (2019). In-depth quantitative profiling of post-translational modifications of Timothy grass pollen allergome in relation to environmental oxidative stress. Environment International, 126, 644–658.
Ugale, C., & Tidke, J. A. (2022). Air pollution effects on Datura innoxia mill. pollen structure, protein and germination. Aerobiologia, 38(3), 379–390.
Wang, X., Miao, Y., Zhang, Y., Li, Y., Wu, M., & Yu, G. (2012). Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: Occurrence, source apportionment and potential human health risk. Science of the Total Environment, 447, 80–89.
Yue, W., Chen, F., Xue, X., Cao, W., & Wu, L. (2016). Rapid determination of polycyclic aromatic hydrocarbons in bee pollen by GC-MS coupled with modified Qu ECh ERS. Journal of Chinese Institute of Food Science and Technology, 8, 2011–2017.
Zhou, S., Wang, X., Lu, S., Yao, C., Zhang, L., Rao, L., Liu, X., Zhang, W., Li, S., Wang, W., & Wang, Q. (2021). Characterization of allergenicity of Platanus pollen allergen a 3 (Pla a3) after ex-posure to NO2 and O3. Environmental Pollution, 278, 116913.
Zhou, S., Zhao, H., Peng, J., Hong, Q., Xiao, K., Shang, Y., Lu, S., Zhang, W., Wu, M., Li, S., Yu, S., & Wang, Q. (2019). Size distribution of Platanus acerifolia allergen 3 (Pla a3) in Shanghai ambient size-resolved particles and its allergenic effects. Atmospheric Environment, 198(1), 324–334.
Acknowledgements
This research was funded by the Science and Technology Committee of Shanghai (21DZ1202300)
Funding
This study was supported by the Science and Technology Committee of Shanghai (21DZ1202300).
Author information
Authors and Affiliations
Contributions
LS designed the study, instructed all experiments. ZL drafted the manuscript. LJ and ZS were responsible for the experiments. LX analyzed the data of PAHs. ZW and SY provided instruction on chemical analysis. LY, ECE checked grammars and revised the manuscript. WW, WQ revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. On behalf of the all authors, Senlin Lu.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Senlin, L., Lu, Z., Jin, L. et al. Expression of allergenic protein and polycyclic aromatic hydrocarbons in the Platanus pollen grains collected from different area of Shanghai. Aerobiologia (2024). https://doi.org/10.1007/s10453-023-09805-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10453-023-09805-7