Abstract
In this study, the effect of the thermophilic fungi of composts was analysed on the fungal composition of the air above. Air samples were collected with an Andersen air sampler at 1.5 m height in three large industrial composting facilities treating different waste types. Repetition was collected on three calm and rain-free days of three consecutive weeks in October 2011, in January, April and July 2012; five plates were exposed successively per sampling day. Compost samples were also collected (averaging 1 kg/compost piles). Air and compost samples were cultured at 50 °C. The thermophilic fungal composition of the air near the compost piles of different waste types differed significantly (p < 0.05) from that of the control site above a grassland ecosystem at each sampling time. Seasonal differences could be detected regarding the total number of thermophilic fungi in the air near the agricultural and horticultural compost types, but smaller differences were found near the municipal compost type. A total of 13 and 11 fungal species were detected in the compost and air samples where the dominant species were Thermomyces lanuginosus and Rasamsonia emersonii, respectively. The concentration of airborne thermophilic fungi was higher near the horticultural compost type and lower near the municipal compost. The results suggest that the differences between the incidences of some species in composts and associated aerosols refer to spore ontogeny and biological mechanisms of spore liberation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The majority of terrestrial fungi produce anemophilous spores during their life cycle (Gregory 1973; Lacey and West 2006). The airborne dispersal of fungi depends on some abiotic and biotic factors, such as climatic and vegetation effects (Ingold 1971; Jones and Harrison 2004).
The ecophysiological group of thermophilic fungi is defined on the basis of their temperature demand for growth. Their minimum growth temperature is about 20 °C, and the maximum, extraordinarily in eukaryotic world, is between 50 and 60 °C (Cooney and Emerson 1964; Crisan 1964). Thermophilic fungi comprise 55–60 species of the more than 100,000 fungal species (Mouchacca 2000). This group is taxonomically quite diverse; the majority belongs to the Sordariales, Eurotiales and Onygenales in the Ascomycota and some to the Mucorales in the Zygomycota (Morgenstern et al. 2012). In lack of a meiotic process, some of them are known only as anamorphic fungus. Recently, Thermoascus aurantiacus, Thermomyces lanuginosus and Rhizomucor pusillus besides other thermophilic species have attracted interest for their ability to secrete enzymes that deconstruct biomass at high temperatures for the biomass conversion to biofuels (Li et al. 2010; McClendon et al. 2012; Azad et al. 2013). On the other hand, some case studies reported mycoses caused by thermophilic fungi (e.g. Myceliophthora thermophila and R. pusillus, mostly in immunocompromised patients (Kimura et al. 2009; Hadaschik et al. 2012; Farina et al. 1998; Morio et al. 2011), but these fungi are rarely responsible for human infections.
Thermophilic fungi are less studied in aerobiological aspect than mesophilic ones on account of their minor importance in human hygiene and phytopatology (Breitenbach et al. 2002; Cox 1987; Pyrri and Kapsanaki-Gotsi 2007; Reiss et al. 2011). Thus, the majority of our knowledge on aeromycology is based on studies performed on mesophilic taxa (Grinn-Gofroń and Strzelczak 2013; Tilak and Pande 2005), and only a few studies reported the presence of thermophilic fungi in aerosol. Evans (1972) reported the appearance of 11 thermophilic fungi in the air 4 km from a coal spoil tip. The most common species were T. lanuginosus and R. pusillus. Lacey (1975) studied the airborne spores in pastures with the special interest of thermophilic fungi. He isolated six thermophilic fungal species and found T. lanuginosus to be the most frequent one. Thakur can be considered to be the first researcher who investigated the thermophilic fungal content of air in connection with a composting plant. He found nine thermophilic species in the air at Bombay, 6 km from a milk colony’s cattle farm which was well known for its waste compost system. The propagule of R. pusillus and Scytalidium thermophilum was detected in the greatest amount (Thakur 1977). Numerous studies deal with the bioaerosol of composting facilities (reviewed by Wéry 2014). Concerning the mould content of bioaerosols, investigations mainly focus on the thermotolerant Aspergillus fumigatus (Millner et al. 1980; Fischer et al. 1999; Ryckeboer et al. 2003; Taha et al. 2007), since this fungal species can cause allergenic symptoms or invasive infection and even characterised as opportunistic pathogen. However, recent studies using molecular methods proved that culture-based methods overestimate the proportion of Aspergillus species in the air of composting facilities (LeGoff et al. 2010; Langarica-Fuentes et al. 2014), and thermophilic microorganisms still occurred in large quantity in the mature compost (Bru-Adan et al. 2009; Langarica-Fuentes et al. 2014) where they can liberate into the atmosphere from. Furthermore, according to Wéry (2014), there is a major lack of knowledge concerning the dispersal of airborne microorganisms emitted by composting plants, and further studies are needed to explain the differences recorded between diversity in compost and that in the associated aerosols.
Thus, the goal of our study was to approach the species composition and diversity of thermophilic fungal propagula near the composts of different origin and to reveal the aerosol-forming nature of the propagula of different species with the comparison of their incidence in the composts and in the air in the surrounding.
2 Materials and methods
2.1 Air sampling
The abundance of thermophilic fungi was examined in the air of three composting plants, each treating wastes of different origin (municipal, agricultural and horticultural) in Hungary. The agricultural and horticultural composts comprised of plant residues where the latter contained potting soil and green wastes as well. Domestic waste (paper, food residuals, plastics, etc.) was the raw material for municipal compost. The background concentration was measured in a natural (pasture) environment. Air samples were collected with an Andersen air sampler (Andersen 1958) at 1.5 m above the ground level; the distance from the compost pile was 1 m. The sampler was loaded with 90-mm Petri dishes containing Martin’s medium (Martin 1950) potato dextrose agar (PDA) supplemented with streptomycin of 100 mg/l. Results were expressed as colony-forming units per cubic metre of air (CFU/m3) calculated using the positive-hole correction method (Feller 1950). Repetition was collected on three calm and rain-free days of three consecutive weeks in October 2011, in January, April and July 2012; five plates were exposed successively per sampling day. The operating time of the sampler was based on calibrating test measurements at a flow rate of 28.3 l/min performed before each sampling. The exposed Petri dishes were incubated for 7 days at 50 °C. The plates were investigated daily to detect fungal appearance. To study colony morphology and microscopic characteristics, all isolates were subcultured on PDA.
2.2 Compost sampling
In order to quantify the amount of thermophilic fungi in the examined composts, samples averaging 1 kg were taken from the compost piles after the air samplings in October. The samples were stored at 4 °C until further analysis. The quantity of thermophilic fungi in the examined composts was determined using the decimal serial dilution method. Ten grams of the compost samples was dissolved in 90 ml sterile distilled water and subjected to ultrasonic treatment for 5 min in order to break up the aggregates. Aliquots of 100 μl of the dilutions were spread on PDA supplemented with streptomycin of 100 mg/l. The plates were incubated for 7 days at 50 °C and fungal isolates were treated as described above. The results were expressed as colony-forming units per gram of compost (CFU/g).
2.3 Identification and data analysis
Fungal isolates were morphologically identified according to the criteria of Cooney and Emerson (1964) and Mouchacca (1997), and the results were confirmed with the analysis of the internal transcribed spacer (ITS) region sequence (Bellemain et al. 2010). DNA was extracted from the mycelia using the Masterpure™ yeast DNA purification kit (Epicentre Biotechnologies) according to the instructions of the manufacturer. The ITS region was amplified by PCR using the primer pairs ITS1 and ITS4 according to White et al. (1990). The purified PCR products were used in sequencing reactions using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA). Sequencing was performed on an ABI 310 Genetic Analyzer (Applied Biosystems, USA). Sequences were compared with those of all known fungal species available from the NCBI GenBank Sequence Database (http://www.ncbi.nlm.nih.gov). We used the current name of the fungi based on the online CABI databases, Index Fungorum (http://www.indexfungorum.org/names/names.asp).
For statistical analyses, nonparametric methods were used because the data did not appear to be normally distributed. Kruskal–Wallis ANOVA was applied to determine the differences in group characteristics regarding compost types. Spearman’s rank order correlation was used to show the relationship between occurrence of the thermophilic fungal species in the compost and in the air. The general fungal diversity, including richness and evenness, was expressed by the Shannon diversity index (Shannon and Weaver 1963) and Simpson’s reciprocal index (Simpson 1949).
3 Results
3.1 Diversity of thermophilic fungi in the air of composting facilities
A total of 2638 thermophilic fungal isolates belonging to 11 species were obtained from the air samples. The compost samples contained a total of 13 thermophilic fungal species (495 isolates). The most fungal species were found in the air of municipal composting facility, being the only site where Malbranchea cinnamomea was found in the air. The air of horticultural composting site contained the fewest thermophilic fungi, which is not surprising since the least species could be detected in this compost. Rasamsonia thermophila and Paecilomyces variotii proved to be the most common species in the air samples of all the three sampling sites. It is interesting to note that the cumulative incidence of these two species exceeded 50 % in every sites. T. lanuginosus (the most common species in all the three compost types) was found to be the third in the order of the frequency in the air of both agricultural and horticultural composting facilities, while the frequency of Acremonium alabamense as well as R. pusillus exceeded T. lanuginosus in the air surrounding the municipal compost. Chaetomium thermophilum, R. pusillus and T. aurantiacus were also isolated in significant amount from the air of the composting sites. The air samples taken from the agricultural and the horticultural composting sites also seemed to be similar concerning the two rarest species (M. thermophila and Penicillium dupontii). In the air of municipal composting site, P. dupontii and M. cinnamomea occurred in the lowest incidence (Table 1). Melanocarpus albomyces and S. thermophilum were not present in the air samples.
Taxonomic diversity of the airborne fungi at the composting facilities was expressed with Shannon–Weaver’s simple and Simpson’s reciprocal diversity indices. The values are shown in Table 2.
3.2 Seasonal variation of thermophilic fungal levels in the air of composting facilities
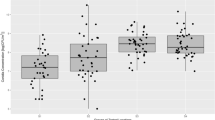
The total number of thermophilic fungi in the air samples taken in composting facilities was significantly higher (p < 0.05) than those in the air samples taken at the control site (grassland) in every season and showed obvious seasonal changes (Fig. 1).
At the municipal waste-composting facility, the total concentration of thermophilic fungi in the air was 320, 510, 870 and 980 CFU/m3 in January, April, July and October, respectively.
In the air samples collected in the surroundings of composted agricultural waste, the levels of thermophilic fungi were 292 CFU/m3 in January, 966 CFU/m3 in April, 3150 CFU/m3 in July and 2700 CFU/m3 in October. Their concentrations at this place in April showed a significant increase, and the summer maxima slightly exceeded even the values of October.
In the air near the horticultural compost, the concentration of thermophilic fungi was 285, 830, 3750 and 2950 CFU/m3 in January, April, July and October, respectively. In the surrounding of the composts of both agricultural and horticultural origin, the quantity of the airborne thermophilic fungi was markedly higher in April than in January, and after reaching particularly high peak values in July, there were some decreases in October at both places. In the control environment, thermophilic fungi could be found only scarcely in the air in every season.
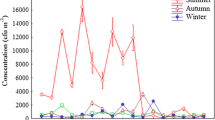
The seasonal changes of the concentration of five species (dominant species and fungi representing Ascomycota and Zygomycota) in the air near the municipal compost are shown in Fig. 2. Their airborne concentrations appeared to be low in winter (in January). Moreover, the quantity of R. pusillus, Peacilomyces variotii and R. emersonii was experienced below 20 CFU/m3 at that time. The airborne level of the latter three species started to rise in spring (in April) (22, 53 and 69 CFU/m3, respectively), became even higher in summer (in July) (53, 139 and 227 CFU/m3, respectively) and reached the maximum value in autumn (in October) (71, 177 and 346 CFU/m3, respectively), and thereafter their concentration decreased notably in winter. The representatives of another group of the thermophilic fungi, C. thermophilum and T. lanuginosus could be found in the air in the surrounding of the compost piles even in winter in the quantity of 69 and 91 CFU/m3. On the other hand their level remained low in every season.
4 Discussion
The airborne concentration of thermophilic fungi in the surroundings of mature compost piles at different composting facilities shows marked seasonal variations. In January, a relatively low level of thermophilic fungi was found in all three sites. The air samples were taken in dry and calm weather, below 5 °C, so it is supposed that self-heating compost piles are important sources of the thermophilic fungal aerosol because fungal spores can be aerosolised by convection. We suppose that no other air current than upright flow by convection is available for the spores to escape from the piles. Shedding of spores in convection currents is a known passive liberation mechanism of fungal spores. Convection is generated by self-heating and the insolation onto the dark pile surface. Having a moderate force, convective air currents are able to detach and lift up spores adhered loosely to their own hyphae or any other surfaces (Lacey 1979). In general, it could carry away small-sized, light, one-celled spores with a hydrophobic surface (Gregory 1973; Magyar 2007; Vestlund et al. 2014).
Composting process is independent from the effect of climatic factors. For this reason, high concentrations of air propagula could be found even in winter. Seasonal variation of the airborne levels of different thermophilic fungal species is considered to depend, first of all, on the quantity of viable propagula in reservoir compost. R. emersonii, P. variotii and R. pusillus that are known as outstanding decomposers, can prolong a moderate growth even in mature compost, and their propagula are present in great number in the composts. Contrariwise, propagula of C. thermophilum and T. lanuginosus are produced in smaller number.
All of the thermophilic fungal species we isolated from the air have also been found in the air by other researchers (Evans 1972; Lacey 1975; Thakur 1977; Sandhu and Singh 1985; LeGoff et al. 2010). Differences between the incidences of some species in aerosols and associated composts may depend on the different spore ontogeny and biological mechanisms of spore liberation. Spores of these fungi can escape from the thallus through different liberation mechanisms. Some of them discharge spores actively, but species with phialoconodia, arthroconidia or aleurioconidia have passive spore liberation mechanisms. R. pusillus had higher incidence in the air than in composts. As the single representative of the division Zygomycota, this fungus plays an important role as an r-strategist pioneer species in the thermophilous phase of composting. The sporangiophores of this fungus are tall, and after the rupture of the wall of the sporangium, the aerial dispersal of the sporangiophores becomes possible. Concerning the incidence of ascosporous species, certain differences were observed. In spite of their high level in composts, C. thermophilum (having dark ascospores in perithecial ascomata), as well as T. aurantiacus and Thermoascus crustaceus (having hyaline ascospores in cleistotecial ascomata), had relatively low concentration in the air. This discrepancy can be explained with their ascomata production. Ascospores ooze out from the ascomata into compost, but not directly into the air, having no active ascospore discharge mechanism. They do not have the ability to produce any asexual propagula that could be emitted directly into the air. The rare occurrence of M. albomyces and its lack in air can be traced back to its heterothallic sexual cycle and lack of any small-sized, easily detachable mitotic propagula. The difference between the occurrence of the closely related P. dupontii and R. emersonii in the air versus their similar incidence in composts can also be explained by their different propagule formation. The colonies of P. dupontii mostly consist of ascomata, where the ascospores are present in smaller numbers. Phialoconidia are also formed in a limited number by this species. On the other hand, R. emersonii produce phialoconidia abundantly. The concentrations of A. alabamense and P. variotii are higher in air than in compost, possibly due to their phialoconidial proliferation. Their conidia are produced by a permanent division of a meristematic cell (phialide) and the products, and the conidia are pushed out permanently (Cole and Samson 1979). M. cinnamomea and S. thermophilum reported to be common in composts (Cooney and Emerson 1964) were also found in all of the studied composts, but were not present in the air samples. The lack of their occurrence in the air could be explained with their conidium-ontogenic process. These two species, in contrast to the other isolated fungi, produce arthroconidia in limited number by fission of certain parts of the vegetative hyphae. Moreover, such conidia are detached from each other and get into the aerosol with difficulty. Further, two cosmopolitan species, M. thermophila and T. lanuginosus, were also found in all the three composts with high incidences due to their biodegrading activity on broad spectra of substrates. M. thermophila was the only species in which significant correlation was found between the relative incidence in compost and that in the air samples (Table 3). However, their concentration in the air may depend on various factors influencing the spore emission. Both species propagate with aleurioconidia originating from the terminal cells of hyphae, but with some differences. T. lanuginosus has rough-walled aleurioconidia, arising directly from the vegetative hyphae, and were therefore produced in limited number. The smooth-walled aleurioconidia of M. thermophila, on the other hand, form on characteristic, branching aerial hyphae in higher quantity.
A number of researches (Millner et al. 1980; Fischer et al. 1999; Hryhorczuk et al. 2001; Kampfer et al. 2002; Ryckeboer et al. 2003) report on the outstanding incidence of A. fumigatus in surroundings of compost piles as well. It is noteworthy to mention that the incidence of this thermotolerant species exceeded that of all thermophilic species in our experiments conducted on airborne fungi.
Although many studies focused on the thermophilic fungal content of composts (Anastasi et al. 2005; Salar and Aneja 2007; Langarica-Fuentes et al. 2014), the air of composting facilities was rarely investigated for these fungi. LeGoff et al. (2010) used culture-independent method to analyse bioaerosols of five composting plants. He experienced that T. lanuginosus and Rasamsonia byssochlamydoides were the most common thermophilic fungi. Contrary to this observation, R. emersonii and P. variotii were found to be the most common species in our air samples. The difference in the dominant species may be explained by the different substrates. In this study, 12, 13 and 10 thermophilic fungal species were found in the compost of municipal, agricultural and horticultural origins, respectively. The species compositions of the thermophilic fungi of the composts and those in the associated aerosol showed a high similarity: 91.6, 76.9 and 80.0 % of the species were present in the air samples collected in the surrounding of municipal, agricultural and horticultural composts, respectively. The total concentration of airborne thermophilic fungi in the composting facilities proved to be higher than at the control site, even in winter (Fig. 1). It is supposed that the compost piles are permanent reservoirs and sources of the airborne thermophilic fungi, since all of the species occurred in air samples could be detected in the related compost piles, too, but they are absent in natural ecosystems. Little differences between the Shannon–Weaver’s indices were found, possibly because the different waste types become similar a end product (i.e. mature compost) during the course of composting process. A similar conclusion can be drawn from the Simpson indices expressing the average rarity of the species.
5 Conclusions
Our results suggest that the differences between the incidences of some species in composts and associated aerosols refer to spore ontogeny and biological mechanisms of spore liberation. The difference between the occurrences of thermophilic fungal species in the air samples and in the compost reservoirs can be summarised as follows: A. alabamense, P. dupontii, R. emersonii, T. aurantiacus and T. crustaceus had higher relative abundance in the air samples, while C. thermophilum, M. cinnamomea, M. thermophila, P. variotii and T. lanuginosus showed higher relative abundance in the compost reservoirs. Further, two species M. albomyces and S. thermophilum could not be detected in the air samples (Table 3). The higher relative abundance in air can be explained with rich propagule-producing ability of Acremonium, Penicillium, Rasamsonia and Rhizomucor species.
References
Anastasi, A., Varese, G. C., & Marchisio, V. F. (2005). Isolation and identification of fungal communities in compost and vermicompost. Mycologia, 97(1), 33–44.
Andersen, A. A. (1958). New sampler for the collection, sizing, and enumeration of viable airborne particles. Journal of Bacteriology, 76, 471–484.
Azad, K., Hossain, F., & Halim, M. A. (2013). Screening of cellulase, pectinase and xylanase activities and optimization of radial mycelial growth of two thermophilic fungi. Bangladesh Journal of Botany, 42(2), 207–213.
Bellemain, E., Carlsen, T., Brochmann, C., Coissac, E., Taberlet, P., & Kauserud, H. (2010). ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiology, 10, 189. doi:10.1186/1471-2180-10-189.
Breitenbach, M., Crameri, R., & Lehrer, S. B. (Eds.). (2002). Fungal allergy and pathogenicity. Basel: Karger.
Bru-Adan, V., Wéry, N., Moletta-Denat, M., Boiron, P., Delgenes, J. P., & Godon, J. J. (2009). Diversity of bacteria and fungi in aerosols during screening in a green waste composting plant. Current Microbiology, 59, 326–335. doi:10.1007/s00284-009-9438-3.
Cole, G. T., & Samson, R. A. (1979). Patterns of development in conidial fungi. London: Pitman.
Cooney, D. G., & Emerson, R. (1964). Thermophilic fungi: An account of their biology, activities, and classification. San Francisco: W. H. Freeman and Co.
Cox, C. S. (1987). The aerobiological pathway of microorganisms. New York: Wiley Interscience.
Crisan, E. V. (1964). Isolation and culture of thermophilic fungi. Contributions from Boyce Thompson Institute of Plant Research, 22, 291–301.
Evans, H. C. (1972). Thermophilous fungi isolated from the air. Transactions of the British Mycological Society, 59, 516–519.
Farina, C., Gamba, A., Tambini, R., Beguin, H., & Trouillet, J. L. (1998). Fatal aortic Myceliophthora thermophila infection in a patient affected by cystic medial necrosis. Medical Mycology, 36(2), 113–118.
Feller, W. (1950). An introduction to the probability theory and its application. New York: Wiley.
Fischer, G., Muller, T., Ostrowski, R., & Dott, W. (1999). Mycotoxins of Aspergillus fumigatus in pure culture and in native bioaerosols from compost facilities. Chemosphere, 38, 1745–1755.
Gregory, P. H. (1973). The microbiology of the atmosphere. Aylesbury, Bucks: Leonard Hill Books.
Grinn-Gofroń, A., & Strzelczak, A. (2013). Changes in concentration of Alternaria and Cladosporium spores during summer storms. International Journal of Biometeorology, 57, 759–768.
Hadaschik, E., Koschny, R., Willinger, B., Hallscheidt, P., Enk, A., & Hartschuh, W. (2012). Pulmonary, rhino-orbital and cutaneous mucormycosis caused by Rhizomucor pusillus in an immunocompromised patient. Clinical Experimental Dermatology, 37(4), 355–357. doi:10.1111/j.1365-2230.2011.04235.x.
Hryhorczuk, D., Curtis, L., Scheff, P., Chung, J., Rizzo, M., Lewis, C., et al. (2001). Bioaerosol emissions from a suburban yard waste composting facility. Annals of Agricultural and Environmental Medicine, 8, 177–185.
Ingold, C. T. (1971). Fungal spores, their liberation and dispersal. Oxford: Clarendon Press.
Jones, A. M., & Harrison, R. M. (2004). The effects of meteorological factors on atmospheric bioaerosol concentrations—a review. The Science of the Total Environment, 326, 151–180.
Kampfer, P., Jureit, C., Albrecht, A., & Neef, A. (2002). Imission of microorganisms from composting facilities. In S. Klammer (Ed.), Microbiology of composting (pp. 571–584). Berlin: Springer.
Kimura, M., Udagawa, S., Makimura, K., Satoh, K., Toyazaki, N., & Ito, H. (2009). Isolation and identification of Rhizomucor pusillus from pleural zygomycosis an immunocompetent patient. Medical Mycology, 47(8), 869–873. doi:10.3109/13693780903059485.
Lacey, J. (1975). Airborne spores in pastures. Transactions of the British Mycological Society, 64(2), 265–281. doi:10.1016/S0007-1536(75)80112-4.
Lacey, J. (1979). Aerial dispersal and the development of microbial communities. In J. M. Lynch & N. J. Poole (Eds.), Microbial ecology (pp. 140–170). Oxford: Blackwell.
Lacey, M. E., & West, J. S. (2006). The air spora. A manual for catching and identifying airborne biological particles. Dordrecht: Springer.
Langarica-Fuentes, A., Handley, P. S., Houlden, A., Fox, G., & Robson, G. D. (2014). An investigation of the biodiversity of thermophilic and thermotolerant fungal species in composts using culture-based and molecular techniques. Fungal Ecology, 11, 132–144. doi:10.1016/j.funeco.2014.05.007.
LeGoff, O., Bru-Adan, V., Bacheley, H., Godon, J. J., & Wery, N. (2010). The microbial signature of aerosols produced during the thermophilic phase of composting. Journal of Applied Microbiology, 108, 325–340. doi:10.1111/j.1365-2672.2009.04427.x.
Li, A. N., Yu, K., Liu, H. Q., Zhang, J., Li, H., & Li, D. C. (2010). Two novel thermostable chitinase genes from thermophilic fungi: Cloning, expression and characterization. Bioresource Technology, 101(14), 5546–5551. doi:10.1016/j.biortech.2010.02.058.
Magyar, D. (2007). Aeromycological aspects of mycotechnology. In M. K. Rai (Ed.), Mycotechnology: Current trends and future prospects (pp. 226–263). New Delhi: I.K. International Publishing House.
Martin, J. P. (1950). Use of acid, rose bengal, and streptomycin in the plate method for estimating soil fungi. Soil Science, 69, 215–232.
McClendon, S. D., Batth, T., Petzold, C. J., Adams, P. D., Simmons, B. A., & Singer, S. W. (2012). Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnology for Biofuels, 5, 54. doi:10.1186/1754-6834-5-54.
Millner, P. D., Bassett, D. A., & Marsh, P. B. (1980). Dispersal of Aspergillus fumigatus from sewage sludge compost piles subjected to mechanical agitation in open air. Applied and Environmental Microbiology, 39, 1000–1009.
Morgenstern, I., Powlowski, J., Ishmael, N., Darmond, C., Marqueteau, S., Moisan, M. C., et al. (2012). A molecular phylogeny of thermophilic fungi. Fungal Biology, 116(4), 489–502.
Morio, F., Fraissinet, F., Gastinne, T., Le Pape, P., Delaunay, J., Sigler, L., et al. (2011). Invasive M. thermophila infection mimicking invasive aspergillosis in a neutropenic patient: A new cause of cross-reactivity with the Aspergillus galactomannan serum antigen assay. Medical Mycology, 49(8), 883–886.
Mouchacca, J. (1997). Thermophilic fungi: Biodiversity and taxonomic status. Cryptogamie Mycologie, 18, 19–69.
Mouchacca, J. (2000). Thermophilic fungi and applied research: A synopsis of name changes and synonymies. World Journal of Microbiology & Biotechnology, 16, 881–888.
Pyrri, I., & Kapsanaki-Gotsi, E. (2007). A comparative study on the airborne fungi in Athens, Greece, by viable and non-viable sampling methods. Aerobiologia, 23, 3–15.
Reiss, E., Shadomy, H. J., & Lyon, G. M. (2011). Fundamental medical mycology. Hoboken, NJ: Wiley-Blackwell.
Ryckeboer, J., Mergaert, J., Vaes, K., Klammer, S., De Clercq, D., Coosemans, J., et al. (2003). A survey of bacteria and fungi occurring during composting and self-heating processes. Annals of Microbiology, 53, 349–410.
Salar, R. K., & Aneja, K. R. (2007). Significance of thermophilic fungi in mushroom compost preparation: Effect on growth and yield of Agaricus bisporus (Lange) Sing. Journal of Agricultural Technology, 3(2), 241–253.
Sandhu, D. K., & Singh, S. (1985). Airborne thermophilous fungi at Amritsar, India. Transactions of the British Mycological Society, 84(1), 41–45.
Shannon, C. E., & Weaver, W. (1963). The mathematical theory of communication. Urbana: University of Illinois Press.
Simpson, E. H. (1949). Measurement of diversity. Nature, 163, 688.
Taha, M. P. M., Drew, G. H., Tamer, A., Hewings, G., Jordinson, G. M., Longhurst, P. J., & Pollard, S. J. T. (2007). Improving bioaerosol exposure assessments of composting facilities—comparative modelling of emissions from different compost ages and processing activities. Atmospheric Environment, 41(21), 4504–4519.
Thakur, S. B. (1977). Occurence of spores of thermophilic fungi in the air at Bombay. Mycologia, 69, 197–199.
Tilak, S. T., & Pande, B. N. (2005). Current trends in aeromycological research. In M. K. Rai & S. K. Deshmukh (Eds.), Fungi: Diversity and biotechnology (pp. 281–510). Jogpur: Scientific Publishers.
Vestlund, A. T., Al-Ashaab, R., Tyrrel, S. F., Longhurst, P. J., Pollard, S. J. T., & Drew, G. H. (2014). Morphological classification of bioaerosols from composting using scanning electron microscopy. Waste Management, 34, 1101–1108.
Wéry, N. (2014). Bioaerosols from composting facilities—a review. Frontiers in Cellular and Infection Microbiology, 4. doi:10.3389/fcimb.2014.00042.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: A guide to methods and applications (pp. 315–322). New York: Academic Press Inc.
Acknowledgments
This work was supported by Grant Research Centre of Excellence-8526-5/2014/TUDPOL. We thank Brigitta Edőcs for her excellent technical assistance, and Zoltán Nagy for his help in the correction of the English text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sebők, F., Dobolyi, C., Bobvos, J. et al. Thermophilic fungi in air samples in surroundings of compost piles of municipal, agricultural and horticultural origin. Aerobiologia 32, 255–263 (2016). https://doi.org/10.1007/s10453-015-9396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10453-015-9396-0