Abstract
An appraisal of the snail (Mollusca: Gastropoda) assemblage of the Sundarbans mangrove region, West Bengal, India, was made through consistent monitoring of three selected sites. In a span of 3 years, 18 species of snails under 11 families, and 9 orders were collected in 304 samples with the Shanon-Weiner diversity index (H’) ranging between 0.8445 and 1.6909. In terms of numerical abundance, Stenothyra deltae (34.07 ± 5.71SE) and Cerithidia cingulata (26.01 ± 1.33SE) were dominant, while Nassarius stolatus (0.01 ± 0.01 SE) qualified as a rare species in the three sites. Significant differences (p < 0.05) in abundance in the sites were observed for Cerithidia cingulata, C. alata, Gangetica miliacea, and Stenothyra deltae. A canonical correspondence analysis reflected that the abundance of G. miliacea is shaped by the conductivity, C. cingulata is linked with temperature and the phosphate concentration is a better predictor of the abundance of S. deltae. The pattern of the diversity of the snails may probably reflect the variations in the environmental quality including the food resources. Thus, the snail assemblage pattern may be used as a basis for the biological monitoring of the Sundarbans mangroves and other similar sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The estuarine ecosystem represents a transitional condition between saltwater and freshwater habitats, with greater extent of diversity of the organisms (Borges et al. 2016; Leonardi et al. 2020; Isroni et al. 2023). Owing to the amalgamation of saline and non-saline water, representative organisms of both the habitats are encountered in the estuarine condition. As a result, the estuarine system represents as ecotone to study the ecological effects and environmental impacts on the wetland including intertidal fauna (Sousa et al., 2005). With reference to the estuaries in the tropics, a large number of euryhaline and stenohaline taxa from diverse assemblages governed by wide range of environmental factors. Among the macroinvertebrates, the snails (Mollusca: Gastropoda) are both taxonomically and numerically abundant in the mangrove region of estuaries (Premcharoen et al. 2016; Ravinesh et al. 2021). For instance, Bang Taboon mangrove estuary of Thailand is dominantly inhabited by 18 species of mollusca (Premchareon et al., 2016) and in India, mangrove region of Astamudi estuary is predominantly diversified by the gastropod assemblage of 69 species (Ravinesh et al. 2021). The estuaries provide ample space for the exploitation of the snails, more suitable with their slow dispersal ability (Byers 2000). Apart from restructuring the soil and the associated degrading substances, the movement and activities of the snails are important for the nutrient dynamics in the estuarine systems. In course of the grazing and scraping activities, the snails consume a huge quantity of the periphytons and detritus, which are highly linked with the chemical and the physical features of the water and sediment of the habitat concerned.
The studies from different regions of the world indicate the variations in the molluscan assemblage as a function of the environmental factors particularly the salinity and the detritus that are deposited in the estuarine tidal mudflat. In Texas, USA, the estuaries are influenced by the latitudinal ecotone and salinity regulation through the freshwater inflow influenced the abundance of the 14 intertidal molluscs (Montagna and Kalke 1995). In the tidal mudflats and the sandflats of Scheldt River estuary in Belgium-Netherlands, the salinity appeared to be a major factor determining the distribution of the 31 mollusc taxa (Bruyndoncx et al. 2002). The organic matter content of the sediment, the salinity and the dissolved oxygen influenced the gastropod diversity in the Guanabara Bay, Brazil (Neves, et al. 2013). Generally, the suspension and the deposit feeder molluscs dominate the coastal area of the polluted Sarno River estuary, Italy, featured with continuous organic deposition in the river mouth (Donnarumma et al. 2020). Among the 23 mollusc species collected from the tidal flat of the Nicoya estuary, Pacific region, Costa Rica, the fluctuation in the abundance varied with the rainy season reflecting the role of the salinity in defining the snail assemblage in the concerned region (Vargas-Zamora and Shibaja-Cordero, 2011). All the 24 species of mollusc recorded in the coastal lagoons of Gulf of Mexico were significantly correlated with salinity (Carrillo et al. 2021). Similarly, in the subtropical Brazilian mangrove of Sao Paulo state ranging between four mangrove areas which cover 52% of the shoreline, a total of 15 snail species were observed, which varied in the abundance based on the extent of the urbanization as reflected through the soil organic matter content (Saad et al. 2019). In freshwater tidal area linked with the River Minho, northwest of Iberian Peninsula, 10 mollusc species were recorded, which varied in abundance based on the salinity condition (Sousa et al., 2005). Successional assemblages of mollusc and their functional adaptation to the transitional area over time act as the key descriptor of environmental and geomorphological changes of that area (Leonardi et al. 2020 and Carrillo et al. 2021). A correspondence of the molluscan assemblages to the geomorphological and environmental parameters was observed in Tyrrhenian coastal area of Sicily, Italy (Leonardi et al. 2020). Besides, the molluscan assemblages of the estuaries are influenced by the urbanization and the anthopogenic activities (Elgharsalli et al. 2015; Saad et al. 2019). Habitat-wise distribution and comparative assemblage of snail in Tyrrhenian Sea reflected that the environment friendly habitat increases the abundance of faunal composition (Casoli et al. 2019).
Molluscan fauna inhabits estuarine region ubiquitously and serves as the key indicator for the characterisation of that ecoregion. Analysis of mollusc assemblage record of a particular region can also predict the past and present ecological status (Dietl et al. 2016) as well as the environmental quality of the present habitats as observed in the European estuarine and coastal region (Donnarumma et al. 2020; Borja et al. 2000). The correspondence of the environmental factors and the freshwater snail faunal assemblages in estuarine environment (Feebarani et al. 2016, Sarkar et al., 2017), and the use of the benthic fauna as the key factor for energy transfer from the detritus in highly vegetated habitats (Satheeskumar and Khan, 2012) have been observed from Indian context. Thus the focus of the present study was to highlight the taxonomic identity of the species of snails forming the key species in the benthic region. In addition, the combination of the species and the functional roles of each will help understand the extent of ecosystem services provided by them (Kantharajan et al. 2017; Ravinesh et al. 2021). Using data obtained through remote sensing a study in the Kannada coast of Karanataka that molluscan diversity is directly related to intertidal habitat and environmental changes (D’Souza et al. 2022). As an ecological driver of the estuarine conditions and the regulator of the nutrient release in the ecosystem, elucidation of the role of the snail is pertinent and important. In Indian context, the structural and the functional correspondences of the snails, mussels and oysters of the estuaries have been accomplished with considerable details (Sarkar et al., 2017, Yadav et al. 2019; Chattopadhyay et al. 2021), but the roles of the snails are yet to be deciphered with greater taxonomic and ecological details. Thus an attempt to elaborate the snail species assemblage in the estuarine water was made to elucidate the pattern of their community composition, identifying possible key species as ecological driver of Matla estuary and effect of environmental gradients on their community.
In the present study an attempt was made to procure data on the mollusc assemblage considering it as ecological key determinants of environmental changes in the Hugli-Matla estuary of the Sundarbans, West Bengal, India. The results of the study will be helpful in understanding the pattern of the aquatic snail species availability against the environmental factors in the estuarine system, thereby aiding in the environmental management of the coastal region of India. An extrapolation of the present findings of the study will be helpful in deducing the population parameters and the utility of the estuarine soil in India.
Materials and methods
Study sites
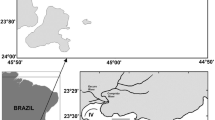
The Hugli-Matla estuary is one of the major distributaries of Ganga-Bhagirathi River system that opens into the Bay of Bengal at Sagar Island of Indian Sundarban. Hugli-Matla estuarine system spreads over 0.8 million ha of southern West Bengal covering a network of world’s largest estuarine distributaries and creeks along with the Sundarban mangrove ecosystem. This estuarine region (Pantalu, 1966) is unique in ecological, geomorphologic and physicochemical characteristics (Zanardi-Lamardo et al. 2019) due to faunal composition, diurnal tides and huge sedimentological discharge. Due to significant wetland faunal composition, local fishery impoundments commonly known as ‘bheries’, have been established in huge numbers in this deltaic region. The Hugli estuary which receives water from almost eight rivers along with Matla river, stretches 290 km in length and are divided into three zones according to salinity level viz., Zone I, extending from Nabadwip to Kolkata as freshwater, Zone II from Kolkata to Diamond Harbour as mesohaline and Zone III euryhaline stretching entire Sundarbans (Dutta et al., 1973). In this investigation, three sites (Table 1) from the bank of Matla estuary of Canning town (22°30' latitude and 88°70' longitude), Sundarbans region of West Bengal, India covering under mesohaline zone were selected for fortnightly survey during April, 2004–March, 2006 which are as follows:
Matla Char (Char) [22°17.838′ latitude and 088°40.700′ longitude]-This is a natural estuarine mudflat located between the latitude of 22°17.838′ north and longitude of 088°40.700′ east in the Matla riverbed at Canning, South 24-Parganas district, West Bengal. This station is almost undisturbed and unpolluted, with negligible mangrove trees but considerable coverage of Porterasia coarcata vegetation at the littoral zone from where fortnightly samples were collected.
Viswabank Bheri (CB1) [22°18.049′ latitude and 088˚40.606′ longitude] -This is a man-made, shallow, semi derelict fishery system or impoundment of about 22 ha in area, used for fish and shellfish culture, located between the latitude 22°18.049′ to 22°17.843′ north and longitude 088°40.606′ to 088°40.627′ east near river Matla at Canning and fed by salt water from river Matla. It is almost undisturbed, unmanaged, left uncultivated for last 2 – 3 years (2000–2003), and partly covered with grasses and algal vegetation.
Nikarighata Bheri (CB2) [22°17.691′ latitude and 088°40.517′ longitude]—Nikarighata Bheri is a man-made, privately owned well managed shallow semi-intensive brackishwater shrimp farm of about 45 ha in area. It is located in between the latitude 22°17.691′ N and longitude 088°40.517′ E and fed by saltwater from river Matla through an inlet and mostly covered with submerged algal mass suitable for shrimp farming.
The selection of the sampling sites was made following preliminary observations on the tidal flood plain and the differences in the water height in the respective banks of the rivers of the concerned area. Owing to the variations in the tidal waves, the flood plain varies in terms of the soil quality and the deposition of the alluvial soil from the upstream. Because of the water movement due to the tides, the nutrient content of the flowing water changes resulting in variations in the quality of the habitat conditions available for the exploitation by the different benthic species. A substantial variation in the tidal waves and the pattern of the deposition formed a consistent level of heterogeneity over the entire study sites. These features provide the foundation to assume that the selected species will vary in abundance and create heterogeneity in the species combinations. Thus, multiple sites were selected and sampled over the time period to decipher the information on the snail’s sufficiency and population in the estuary. The map of the sampling region is presented in Fig. 1 (Supplementary file 1).
The species richness (S), relative abundance (N) and abundance/species (N/S) in the samples collected from the three different estuarine wetlands of Canning, South 24 Parganas, West Bengal, India, Site 1, CB1; Site 2, CB2; and Site 3, Char. The x- axis in the graphical presentations corresponds to the sample number (n = 324 samples)
Sampling procedure
Quantitative samplings from the three study sites on Matla Estuary at Canning where diurnal tides rippled the mud flat regularly were investigated fortnightly at low tide throughout the year from April 2004–March 2006. Sampling was done by the box type sampler having an area of 15 cm × 15 cm and capable of penetrating the soft bottom to its maximum depth of 10 cm (Paul and Nandi 2003). Nine samples from each of the three sites at Canning were collected each time and were washed thoroughly in a standard sieve of 0.5 mm mesh size (Jonasson 1955; Havgaard 1973). The sampling, collection, preservation and identification of molluscs were made following standard books and methods (APHA, 1998). The snails were identified using suitable references (Subba Rao, 1993) and from concerned taxonomists of Zoological Survey of India. Most of the collected snails were captured, counted and released and few were kept in the laboratory for identification and analysis. In course of the collection, the population density of the snails was determined, which represented the relative abundance of the species in the concerned sample. The snails obtained in the sampler (15 cm × 15 cm) were expressed as number of organisms per Charter square using the following formula [Eq. (1)] as outlined by Welch (1948):
where, n = number of organisms/m2, O = number of organisms counted, a = area of the sampler and s = number of replicates taken.
In the present investigation the months from March to June were considered as hot premonsoon season with occasional rain, July to October as wet monsoon season with maximum rainfall and November to February as cold and dry postmonsoon season with negligible rain. Water samples were collected in clean glass bottles from different sites of each wetland at a depth of 0.4 m from the water surface. Some water quality parameters were determined in the fields and the remaining in the laboratory. Sediment samples were collected at each site by a box type sampler (15 cm × 15 cm). The sediment samples are kept in clean polythene bags and brought to the laboratory for analysis. Nine physico–chemical parameters of water, viz., temperature, pH, salinity, conductivity, total alkalinity, hardness, dissolved oxygen and biological oxygen demand (BOD) were estimated fortnightly. In case of sediment, temperature, pH, salinity, percentage of organic carbon and organic matter were determined. All the parameters were analyzed following standard methods (APHA, 1998, Jackson 1973, and Piper 1966) and / or using photometer SQ 118 (Merck, Germany). The salinity was estimated using Refractometer (RF10).
Data analysis
The data on the relative abundance of the snails in the sites were subjected to the diversity analysis (Buzas and Hayek 1998) using the PAST software (Hammer et al. 2001) highlighting the species richness, abundance and evenness components for the three sampling sites. A species specific comparison of the abundance of the snails in the three sites was made employing the ANOVA (Zar, 1999) and post hoc Tukey test. Using the data on the relative abundance of the snails in all the sites, an agglomerative hierarchical cluster analysis was carried out to represent the similarity in the relative abundance of the snails (Manly 1994) along with SHE (Buzas and Hayek 1998). Further, a non-metric multidimensional (nMDS) scaling was made to portray the ordination of the snails based on the proximity matrix derived on the basis of similarity in abundance. In order to assess the abundance of the snails against the water quality parameter, canonical correspondence analysis (terBraak 1987; terBraak and Verdonschot 1995; terBraak and Simulaer 2002) was conducted. As an ordination technique, the CCA extracts the synthetic environmental gradients from the original data sets to predict the preferences of the different species represented through an ordination pattern (terBraak, and Verdonschot, 1995). Considering CCA as an equivalent to a weighted principal component analysis, in the present instance, the snail environmental data matrix was arranged in a way where the snails were given weight relative to their total abundance and the environmental variables are being weighted inversely with their covariance matrix (terBraak 1987). A biplot (ordination of the species and the environmental variables) diagram portrays the environmental variables (as arrows) and the species as scattered in the ordination space. In the diagram the length of the arrow (environmental variable) is relative to the strength of the correlations with the ordination axes. Using the environmental arrow as the axis, the snails were oriented in accordance to the weighted averages with reference to the concerned environmental variable (terBraak, 1987; terBraak and Verdonschot, 1995). As a result, the orientation of the species in the space with reference to the environmental variables are reflected in the biplot, which helps to infer about the prospective role of the environmental factors in the distribution of the snails in the community.
Results
The continuous survey of the wetlands in the Matla estuarine system revealed the presence of 18 species of aquatic snails under 11 families and nine orders, with varying abundance in the different time period of collection (selected species are shown in Fig. 2 of the Supplementary File 1). In each of the samples in the three wetlands, the species richness and the relative abundance of the snails varied considerably (Fig. 1) resulting in the differences in the relative abundance of each species in the samples. On a comparative scale, the number of species observes in CB1(site1) was 0 to 8 (mean 3.17 ± 0.17) and CB2 (site2) was 1 to 7 (mean 2.4 ± 0.14), while for the Matla char (site 3) the number of species observed in individual samples ranged between 0 and 7 (mean 2.48 ± 0.16SE). The corresponding relative abundance of the snails was highest in CB1 (0 – 480, 75.65 ± 8.26 SE per sample) followed by CB2 (4—204, mean 40.04 ± 2.78 SE per sample) and Char (0 – 117, mean 16.39 ± 1.52 SE per sample). When assessed for the relative abundance per species (N/S) of the snails in the samples, the highest value was observed for CB1 (25.23). The diversity indices (Fig. 2) show corresponding differences in species richness and abundance of the three sites.
The SHE (Species richness, S, Shannon-Weiner diversity index H’, and Evenness index E) for the estuarine snails observed in the three different sites in Canning, West Bengal, India (a) Site 1, CB1 (n = 104) (b) Site 2, CB2 (n = 101 observations) and (c) Site 3, Char (n = 100 observations) (at least 19 samples from the sites were not considered due to absence of any snails)
From the taxonomic point of view, the snails belonged to 11 families and the abundance remained variant with reference to the habitat concerned. Among the different species of the snails, Stenothyra deltae (SDE) followed by Cerithidia cingulata (CCI) was most abundant in terms of numerical abundance in all the studied sites while Cerithidia alata was abundant in the littoral zone of Matla river bed only. In all instances, the abundance and the availability of the snail species and the number of the snails differed with reference to the physical and the chemical conditions of the soil. It may be due to the availability of the congenial condition for the growth and expansion of the snails, the assemblages were more or less dominated by Stenothyra deltae (SDE) with the population density of 34.07 ± 5.71 in CB1, 7.87 ± 1.88 in CB2 and 0.51 ± 0.19 in Char followed by Cerithidia cingulata (CCI) with the numerical abundance as 25.67 ± 1.82 in CB1, 26.01 ± 1.33 in CB2 and 3.25 ± 0.39 in Char. The intermittent flow of water or the moist condition favoured the residence of huge number of snails capable of transforming the soil features for facilitating the growth of other biota too. On the basis of the results of the ANOVA carried out on the relative abundance of the snails in the three different wetlands, it was observed that the differences in the relative abundance were significant for the snail Stenothyra deltae followed by Cerithidia cingulata (CCI), Gangetia miliacea (GMI) and Cerithidia alata (CAL). But for the rest of the snails, the differences were not significant (Tables 2 and 3).
On the basis of the relative abundance of the snails in the samples, an agglomerative hierarchical cluster analysis was carried out. The dendrogram (Fig. 3a) represents the relationship among the 18 snails based on the similarity of their abundance in the samples. Three major classes could be resolved in the cluster analysis comprising of 9, 5 and 4 species of the snails respectively, comprising of the objects of the classes. Using the proximity matrix based on the Pearson’s product moment correlation coefficient values among the snails, a non-metric multidimensional scaling (NMDS) was also constructed to show the differences in the ordination of the snails resulting from the similarity in the relative abundances in the samples concerned (Fig. 3b). A Kruskal’s stress value of 0.310 suggested the application of the NMDS on the data obtained on the snails. Thus the results indicate that the three wetlands, part of the Sundarbans, India, remained considerably diverse in terms of the snails present therein.
a the dendrogram deduced through the cluster analysis based on the similarity in relative abundance of the estuarine snails in the samples. b The biplot showing the ordination of the snails obtained through the NMDS carried out on the basis of the proximity matrix constructed on the basis of the relative abundance of the snails in the samples concerned
The results of the CCA represented the relation of the environmental variables and the snail species observed in the study area. The inertia of constrained and unconstrained variables (Fig. 4) were 0.332 and 1.810, respectively, explaining 15.48% and 84.52% of dependent variable (species occurrence). The four canonical axes explained 97.87% of variations in the species environment relation. The total inertia was 2.141 and the sum of all canonical eigen values was 0.332. A test of significance of first canonical axis with eigen value 0.2 was significant at P < 0.05 level (F = 0.183, P = 0.006). The first canonical eigen value (λ1 = 0.21) was 2.76 times the second one (λ2 = 0.073) indicating a clear gradient. The first two canonical axes explain 82.31% (λ1 = 60.43% and λ2 = 21.87%) of variance in the weighted averages of the species with respect to each environmental variable. The ordination of the environmental and species variables is exhibited through the biplot (Fig. 4). From the length of the environmental arrows, it appears that pH, Secchi disk visibility (SD) and alkalinity (A) bear stronger correlation with the ordination axes. The species point projection along the direction (axis) of the environmental arrow indicates that the abundance of Gangetica miliacea (GMI) is shaped by the conductivity (C), while the abundance of Cerithidia cingulata (CCI) is defined by the temperature. The phosphate concentration is a better predictor of the abundance of Stenothyra deltae SDE. The environmental requirements of Assiminea beddomeana (ABE), C. alata (CAL), Telescopium (T.) telescopium (TTE) appeared to be highly contrasting with GMI, CCI, SDE and Assiminea brevicula (ABR). Perhaps, the ordination of ABE, CAL, and TTE reflects the generalist nature of the snail species in the concerned estuarine ecosystem.
The biplot representing the relationship between the environmental variables filled circles with the lines) and the snail species (filled squares) observed in the estuarine ecosystem of the Sunderbans, West Bengal, India. A total of 105 samples were considered from three different sites for the CCA analysis and selected environmental parameters (T-Temperature, C-Conductivity, pH, A–Alkalinity, SD-Secchi disk visibility, P–Phosphate), and snail species (ABE–Assiminea beddomeana, ABR – A. brevicula, CAL – Cerithidia alata, CCI – C. cingulata, GMI—Gangetica miliacea, SDE – Stenothyra deltae, and TTE – Telescopium (T.) telescopium)
Discussion
From the results it is apparent that the estuarine wetlands of the Sundarbans, West Bengal, India, host a significant number of snails that differ in the relative abundance in the concerned sampling sites. As observed in earlier works, the estuarine ecosystems of the Sundarbans, are characteristically inhabited by a wide range of macroinvertebrates including the snails. A previous study recorded 13 species of snails from Taldi, Canning, South 24 Parganas, India (Datta and Sarangi 1980). However, the relative abundance of the snails varied between 423 to 9740 per unit area (m2) which appeared to be similar to the observations made elsewhere, like in the Brazilian mangrove (Saad et al. 2019), Iberian peninsula (Sousa et al., 2005), Gulf of Nicowa, Costa Rica (Vargas-Zamora and Sibaja-Codero, 2011), Texas estuary (Montagna and Kalke 1995), Moroccan Atlantic coast (Boutoumit et al. 2021), and estuary of Belgium and Netherlands (Bruyndoncx et al. 2002), where comparable level of the abundance of the snails were observed. In Indian context, 21 species of estuarine snails recorded from a study in the coastal region of Puducherry (Pondichery) (Satheeshkumar and Khan 2012). However, in the 70 rivers of Kyushu, Japan, the species richness was 82 under 37 families, which appears to be quite diverse compared to the present observations (Itsukushima et al. 2018). The species richness of the estuarine snails was estuarine regions of Belgium and the Netherlands (Bruyndoncx et al. 2002), in Guanabara Bay, Brazil (Neves et al. 2013), and Sarno-river estuary, Italy (Donnarumma et al. 2020), remained considerably high in comparison to the present observation. As a prominent factor, the environmental conditions like the salinity, temperature and the nutrient content of the water influence the species diversity of the estuarine snails in multiple ways. Observations in the present instance also indicate that the water quality parameters may contribute to the differences in the assemblages of the snails. Since, the nutrient availability in the concerned estuary and sea water influence the productivity and the macroinvertebrate assemblages, which are directly or indirectly linked with the availability of the resources for consumption by the estuarine snails. As a consequence, any factor that regulates the detritus production or the productivity of the concerned habitat will be influencing the aggregation of the estuarine snails considerably. This may be reflected in the differences in the relative abundance of the snails in the different sampling sites. For instance, the snail density was 1069 – 2015 no/m2 in CB1, 732 – 9748 no/m2 in CB2 and 424 – 1030 no/m2 in Char, which may be linked with the high organic content of the sediment in the monsoon season. The brackishwater gastropod species Stenothyra deltae (SDE) was more or less abundant in every month in both the bheries. The maximum population was observed in February in both CB1 (2575 no/m2) and CB2 (1465 no/m2) and in June in Char (178 no/m2) which might be due to juvenile count. Lowest density was recorded in October in CB1 (15 no/m2) and in November in CB2 (25 no/m2) whereas it was absent from premonsoon to monsoon (February to September) in Char which might be due to their sensitivity to high and low salinity. Cerithidea (Cerithideopsilla) cingulata (CCI) belonging to Potamididae family was the dominant species in the bheries (CB1 and CB2) and common in estuarine mudflat (Char) during the study period. Higher population density of this species was noticed in monsoon season (July—October). It reached its highest peak in the month of July (965 no/m2) in CB1 followed by CB2 in August (790 no/m2) and Char in August and September (178 no/m2). Cerithidea (Cerithidea) alata (CAL) was recorded only in the littoral region of Char throughout the year but not in brackishwater bheries (CB1 and CB2). This predominant gastropod showed its highest population density (2200 no/m2) in August which tended to decrease after monsoon season. The density of Telescopium (T.) telescopium (TTE) showed one distinct peak in the month of May in the bheries and in June in Char. Stenothyra blanfordiana (SDE) was common in both the bheries. Density of this species was maximum in May in both the bheries (785 no/m2 in CB1 and 67 no/m2 in CB2), absent in monsoon in CB1 and monsoon to postmonsoon season in CB2. The highest population of Assiminea brevicula (ABR) was recorded higher in CB1, followed by CB2 and Char. This species reached its peak in population density in April in both the bheries (730 no/m2 in CB1 and 130 no/m2 in CB2) and in January in Char (933 no/m2) which might be related to their breeding activity. Low population of this species in Matla Estuary was associated with habitat preference. Assiminea beddomeana (ABE) was quite common in CB1 than other two sampling sites. This was due to their high tide level habitat preference in the mudflat. This species showed a peak in September in CB1 (30 no/m2) and in March in CB2 (22 no/m2). Gangetia miliacea (GMI) was well represented in the bheries throughout the study period. The month-wise trend of this species showed maximum values in May (1067 no/m2 in CB1) and June (319 no/m2 in CB2). Neritina (Dostia) violacea (NVI) species was abundant in CB1 and common in CB2. The population fluctuation showed a peak in July in CB1 (30 no/m2) but with no such fluctuation in CB2 (7 no/m2). Thiara (Mainwaringia) paludomoidea (TPA) was common in the unmanaged CB1 and rare in CB2 which might be due to anthropogenic activities. One species, Tornatina estriata (TES), formed small local population in CB1 during premonsoon season only, while it was less common in CB2 in that season and not recorded from the estuarine mudflat of Matla river. Maximum density of this species was noticed in April in CB1 (111 no/m2). In addition, the tidal flux in the estuarine littoral regions offer diverse niches for exploitation by several macrozoobenthic invertebrates including snails. On the whole the physical and the chemical features of the esturarine zones in the coastal regions provide ample space for the assemblages of macroinvertebrates to thrive and execute diverse functional roles. An appraisal of the macroinvertebrate faunal assemblages associated of the estuary indicates about the physical and the chemical conditions of the estuary. Salinity and organic carbon of the sediment among the studied fourteen parameters of estuarine mudflat in Sundarban affected mostly the diversity and density of snails which is in accordance with the findings of Bruyndoncx et al., (2002), Neves et al., (2013), Leonardi et al., (2020), Donnarumma et al., (2020), Boutoumit et al., (2021) and Carrillo et al., (2021). Likewise, D'Souza et al., (2022) recorded that the water temperature played significant role for the changes in snail population density and diversity whereas Satheeskumar and Khan, (2012) emphasized on the environmental factors like organic matter, DO and salinity as the significant ecological drivers of molluscan density variation.
Many of the studies on the estuarine and coastal ecosystems indicate that the snail assemblages can be considered as the ecological drivers in the estuarine wetlands (Sousa et al., 2005, Neves et al. 2013, Itsukushima et al. 2018, Boutoumit et. al., 2021, Wells et al. 2022). Sediment texture, salinity, temperature, pH and habitat type played a vital role in the distribution of malacological fauna in Moroccan Atlantic coast (Boutoumit et al. 2021). Habitat destruction and reduction in microalgae blooms affected the molluscan population density in the Peel-Harvey estuary of Australia from the year 1978 to 2020 (Wells et al. 2022). In the Sundarbans estuary representing one of the unique mangrove regions in India, the biodiversity and environmental condition has been explored by several authors (Chakraborty, 2011, Gopal and Chauhan 2006, Manna et al., 2010). But there is considerable number of redundancies in ecological data assessment through molluscan faunal diversity analysis in the Sundarbans estuarine region.
Considering the Sundarbans as the unique ecosystem with diverse macroinvertebrates linked with the varied functions that sustain the productivity and the stability of the system. In order to continue with the benefits and the ecosystem services derived from the Sundarbans, the documentation of the diversity of the dominant groups is a pre-requisite. As observed in the present instance, the snails are quite diverse in the estuarine system, and possibly linked with multiple functional roles in the detritus processing and productivity in the concerned system. The snails are consumers regulating the periphyton, algae and the decaying materials, besides being the prey to a number of species, thereby contributing to the sustenance of the food web of the marine ecosystem. A species specific contribution to the functions will be important from the conservation perspective of the estuarine snails. Among the described macroinvertebrates of the estuarine system, the snails are considerably diverse in the morphological features as well as in the dominance among other species. Thus species specific information on the estuarine snails are required for further elaboration of the functions and possible conservation efforts to sustain the functions attributable to the snails. While the present study is restricted to the documentation of the diversity of the snails and selected environmental parameters, further studies are required to highlight the species specific roles for understanding the precise functions and framing conservation strategies.
Conclusion
An exploration of the estuarine snails in the coastal region of the Sundarbans in southern West Bengal, India was carried out emphasizing the assemblage pattern and the environmental quality. In course of sampling from three different sites, 18 species of snails were recorded with varying abundances in the sites. Among the snails, Assiminea brevicula, A. beddomeana, Cerithidia cingulata, C. (Cerithidea) alata, Gangetia miliacea, Neritina (Dostia) violacea, Stenothyra deltae, S. blanfordiana, and Telescopium (T.) telescopium, were common to abundant in the samples. The relationship between the abundance of the snails and the environmental conditions were observed throughout the sampling period. Many of these snail species are linked with food security and the livelihood of the local people. However, the snails appear to be useful in monitoring the environmental quality owing to the correspondences with the environmental quality.
Data availability
The data concerning experiments of the present study can be shared upon authentic and reasonable request.
References
APHA (1998) Standard Methods for the examination of water and waste water American Public Health Association
Borges L, Hollatz C, Lobo J, Cunha AM, Vilela AP, Calado G, Coelho R, Costa AC, Ferreira MSG, Costa MH, Costa FO (2016) With a little help from DNA barcoding: investigating the diversity of Gastropoda from the Portuguese coast. Sci Rep 6:20226. https://doi.org/10.1038/srep20226
Borja A, Franco J, Perez V (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Mar Pollut Bull 40(12):1100–1114
Boutoumit S, Bououarour O, Kamcha RE, Pouzet P, Zourarah B, Benhoussa A, Maanan M, Bazairi H (2021) Spatial patterns of macrozoobenthos assemblages in a sentinel coastal lagoon: biodiversity and Environmental Drivers. J Mar Sci Eng 9(5):461. https://doi.org/10.3390/jmse9050461
Bruyndoncx L, Jordaens K, Ysebaert T, Meire P, Backeljau T (2002) Molluscan diversity in tidal marshes along the Scheldt estuary (The Netherlands, Belgium). Hydrobiologia 474:189–196
Buzas MA, Hayek L-AC (1998) SHE analysis for biofacies identification. J Foraminiferal Res 28:233–239
Byers JE (2000) Effects of body size and resource availability on dispersal in a native and a non-native estuarine snail. J Exp Mar Biol Ecol 248:133–150
Carrillo RMJ, Ocaña FA, Macías EB (2021) Functional variations of mollusks along an environmental gradient in a coastal lagoon of the Southern Gulf of Mexico. Reg Stud Mar Sci 44:101723
Casoli E, Bonafazi A, Ardizzone G, Gravina MF, Giovanni FR, Sandulli R, Donnarumma L (2019) Comparative analysis of mollusc assemblages from different hard bottom habitats in the central Tyrrhenian Sea. Diversity 11(5):74. https://doi.org/10.3390/d11050074
Chakraborty SK (2011) Mangrove Ecosystem of Sundarbans, India: Biodiversity, Ecology, Threats and Conservation. Mangroves: Ecology, Biology and Taxonomy. Ed. Metras JN: NOVA publisher, USA, 83–112
Chattopadhyay D, Sarkar D, Bhattacherjee M (2021) The distribution pattern of marine bivalve death assemblage from the western margin of Bay of Bengal and its oceanographic determinants. Front Mar Sci 8:675344
D’Souza SL, Bhat HG, Shenoy KB (2022) Study of intertidal molluscan diversity of the Dakshina Kannada coast, India using remote sensing and GIS techniques. Curr Sci 122(12):1426–1440
Datta NC, Sarangi N (1980) Preliminary studies on the macrozoobenthos in a brackishwater Bheri at Taldi, West Bengal. J Inland Fish Soc Ind 12(2):81–88
Dietl GP, Durham SR, Smith JA, Tweitmann A (2016) Mollusk assemblages as records of past and present ecological status. Front Mar Sci 3:169. https://doi.org/10.3389/fmars.2016.00169
Donnarumma L, Sandulli R, Appolloni L, Ferrigno F, Rendina F, Di Stefano F, Russo GF (2020) Bathymetrical and temporal variations in soft-bottom molluscan assemblages in the coastal area facing the Sarno River mouth (Mediterranean Sea, Gulf of Naples). Ecol Ques 31(4):53–65. https://doi.org/10.12775/EQ.2020.028
Dutta P, Laha GC, Mitra PM, De DK, Chaudhury A, Pandit PK, Chaudhury AR, De RN, Saha BK, Mazumdar HS, Sarkar ND, Mandal NC, Bhattacharya GP, Namasudra AK, Ghosh SP, Paul AR (1973) Fishery resources of the Hooghly–Matlah estuarine system. Bull No. Barrackpore, West Bengal
Elgharsalli R, Rabaoui L, Aloui-Bejaoui N (2015) Community structure of a molluscan assemblage in an anthropized environment, Hammamet marina, north-eastern Tunisia. Turkish J Fish Aquat Sci 15(3):751–760. https://doi.org/10.4194/1303-2712-v15_3_20
Feebarani J, Joydas TV, Damodaran R, Borja A (2016) Benthic quality assessment in a naturally- and human-stressed tropical estuary. Ecol Ind 67:380–390
Gopal B, Chauhan M (2006) Biodiversity and its conservation in the Sundarban Mangrove Ecosystem. Aquat Sci 68:338–354
Hammer Ř, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeont Electr 4:1–9
Havgaard P (1973) A new system of sieves for benthic samples. Sarsia 53:15–18
Isroni W, Sari PDW, Sari LA, Daniel K, South J, Islamy RA, Wirabuana PYAP, Hasan V (2023) Checklist of mangrove snails (Gastropoda: Mollusca) on the coast of Lamongan District, East Java. Indonesia Biodiversitas 24(3):1676–1685. https://doi.org/10.13057/biodiv/d240341
Itsukushima R, Yoshikawa H, Morita K (2018) A dataset of molluscan fauna sampled in river estuaries of medium and small size river in Kyushu island, Japan. Biodivers Data J 11(6):e26101. https://doi.org/10.3897/BDJ.6.e26101
Jackson ML (1973) Soil Chemical Analysis. Prentice Hall of India Pvt. Ltd., New Delhi
Jonasson PM (1955) The efficiency of sieving techniques for sampling fresh water bottom fauna. Oikos 6:183–207
Kantharajan G, Pandey PK, Krishnan P, Samuel VD, Bharti VS, Purvaja R (2017) Molluscan diversity in the mangrove ecosystem of Mumbai, west coast of India. Reg Stud Mar Sci 14:102–111
Leonardi M, Bergamasco A, Giacobbe S, Azzaro F, Cosentino A, Crupi A, Lanza S, Randazzo G, Crisafi E (2020) A four decades multiparametric investigation in a Mediterranean dynamic ecosystem: Mollusc assemblages answer to the environmental changes. Estuar Coast Shelf Sci 234:106625
Manly BFJ (1994) Multivariate statistical methods: a primer. Chapman and Hall, London, UK
Manna S, Chaudhuri K, Bhattacharyya S, Bhattacharyya M (2010) Dynamics of Sundarban estuarine ecosystem: eutrophication induced threat to mangroves. Sal Sys 6:8. https://doi.org/10.1186/1746-1448-6-8
Montagna PA, Kalke RD (1995) Ecology of infaunal mollusca in south Texas estuaries. Am Malacol Bull 11(2):163–175
Neves RAF, Echeverria CA, Pessoa LA, Paiva PC, Paranhos R, Valentin JL (2013) Factors influencing spatial patterns of molluscs in a eutrophic tropical bay. J Mar Biol Assoc UK 93(3):577–589
Pantalu VR (1966) Contribution to the study of biology and fishery of some estuarine fishes. thesis. Calcutta University
Paul S, Nandi NC (2003) Studies on intertidal macrozoobenthos of Hugli river in and around Calcutta in relation to water and soil conditions. Rec Zool Sur India Occ. Paper No 213:1–135
Piper CS (1966) Soil and Plant Analysis. Publishers, Bombay, Hans
Premcharoen S, Witirawat S, Tharapoom P (2016) Molluscan fauna in Bang Taboon Mangrove estuary, inner Gulf of Thailand: Implications for conservation and sustainable use of coastal resources. MATEC Web Confer 60:02003
Ravinesh R, Kumar AB, Anjana VL (2021) Diversity and distribution of molluscan fauna of Asthamudi estuary, Kerala, India. Wet Ecol Manag 29:745–765
Saad LdO, Cunha CM, Colpo KD (2019) How mollusk assemblages respond to different urbanization levels: characterization of the malacofauna in subtropical Brazilian mangroves. Mar Biodiver 49:989–999
Sarkar D, Bhattacherjee M, Chattopadhyay D (2017) Influence of regional environment in guiding the spatial distribution of marine bivalves along the Indian coast. J Mar Biol Assoc UK
Satheeshkumar P, Khan AB (2012) Influence of environmental parameters on the distribution and diversity of molluscan composition in Pondicherry mangroves, southeast coast of India. Ocean Sci J 47(1):61–71
Sousa R, Guilhermino L, Antunes C (2005) Molluscan fauna in the freshwater tidal area of the River Minho estuary, NW of Iberian Peninsula. Ann Limnol Int J Limnol. 41(2):141–147. https://doi.org/10.1051/limn/2005009
Subba Rao NV (1993) Freshwater Molluscs of India. In: K. S. Rao (ed.), Recent Advances in Freshwater Biology
ter Braak CJF (1987) The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 69:69–77
ter Braak CFJ, Verdonschot PEM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57:255–289
ter Braak CFJ, Šmilauer P (2002) CANOCO reference manual and canodraw for windows user’s guide: software for canonical community ordination (version 4.5). Ithaca, NY, USA: Microcomputer Power CANOCO for Windows version 4.5
Vargas-Zamora JA, Sibaja-Cordero JA (2011) Molluscan assemblage from a tropical intertidal estuarine sand-mud flat, Gulf of Nicoya, Pacific, Costa Rica (1984–1987). Int J Trop Biol Conserv 59(3):1135–1148
Welch PS (1948) Limnological Methods. Blakiston Co., Philadelphia, p 381
Wells FE, Gagnon MM, Spilsbury F, Whisson C (2022) Stability and change in a changing environment: soft-bottom benthic molluscs in the Peel-Harvey estuary over 42 years. Mar Freshwat Res 73(6):792–802
Yadav R, Malla PK, Dash D, Bhoi G, Patro S, Mohapatra A (2019) Diversity of gastropods and bivalves in the mangrove ecosystem of Paradeep, east coast of India: a comparative study with other Indian mangrove ecosystems. Moll Res 39:325–333. https://doi.org/10.1080/13235818.2019.1644701
Zanardi-Lamardo E, Mitra S, Vieira-Campos AA, Cabral CB, Yoguia GT, Sarkar SK, Biswas JK, Godhantaraman N (2019) Distribution and sources of organic contaminants in surface sediments of Hooghly river estuary and Sundarban mangrove, eastern coast of India. Mar Pollut Bull 146:39–49
Zar JH (1999) Biostatistical analysis. IV edn. Singapore: Pearson Education (Singapore) (P) Ltd., New Delhi (Indian Branch).
Acknowledgements
We thankfully acknowledge the critical comments of the two esteemed reviewers that enabled enhancement of the manuscript in the present form. The authors acknowledge the Director, Zoological Survey of India for providing the necessary facilities in executing the research work. MR and GA acknowledge the Head, Department of Zoology, University of Calcutta, for the facilities provided. MR acknowledges the Principal, Kishore Bharati Bhagini Nivedita College (Co-Ed), Kolkata, India.
Funding
The first author MR acknowledges Zoological Survey of India for the fellowship provided in carrying out the research work.
Author information
Authors and Affiliations
Contributions
NCN conceived and supervised the research work; MR conceived, executed the survey and data collection, carried out the data compilation, analysis and first draft; GA carried out the analysis, compilation and drafting the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
As authors of this article we declare no competing interest.
Ethical Approval
Not applicable.
Consent for publication
All the authors have consent for the publication of this article.
Additional information
Handling Editor: S.S.S. Sarma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roy, M., Aditya, G. & Nandi, N.C. Drivers of the diversity and spatial heterogeneity of aquatic snails in estuarine habitats: evidence from West Bengal in India. Aquat Ecol (2024). https://doi.org/10.1007/s10452-024-10135-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10452-024-10135-0