Abstract
Deforestation of riparian areas is a major driver of biodiversity loss in aquatic ecosystems. Thus, we investigated the influence of forest cover and physical and chemical characteristics of streams on zooplankton communities in the southeastern Amazon. We addressed the following questions: (1) Are environmental factors (water physical and chemical characteristics and landscape variables) and dispersive processes (reflected in the spatial structure among sampling sites) efficient predictors of zooplankton communities in different hydrologic seasons? (2) Can zooplankton species be indicators of watersheds’ forest-cover levels? We sampled 15 streams located in nine rural settlements in northern Mato Grosso, Brazil, in the dry (August) and rainy (March) seasons of 2017. The forest-cover level had a significant effect on the physical and chemical characteristics (conductivity, dissolved oxygen, and temperature) of streams and also on the structure and composition of zooplankton communities, mainly of rotifers and testate amoebae. Areas with low vegetation cover had seasonal changes in species richness, individuals density, and zooplankton community structure. Environmental and spatial variables had no significant effect on the structure of zooplankton communities, which may indicate the strong influence of stochastic factors. Species from three zooplankton groups (rotifers, microcrustaceans, and testate amoebae) were indicators of forest-cover classes. This study provided valuable contributions to the conservation of riparian ecosystems and the use of biological indicators in environmental monitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous human activities affect neotropical aquatic ecosystems (Bailly et al. 2016; Ríos-Touma and Ramírez 2019). Deforestation is one of the main drivers of biodiversity loss (Rockström et al. 2009; Steffen et al. 2015), particularly in regions with endemic species, such as the Amazon (Reyer et al. 2015; Trumbore et al. 2015; Lathuillière et al. 2016). Along with large-scale agropastoral activities, rural settlements have contributed to intense deforestation and forest cover loss, especially in southeastern Amazon (Alencar et al. 2015; Mullan et al. 2018; Roriz et al. 2017). In this sense, it is necessary to understand how multiple anthropic disturbances systematically interact with environmental, local, and spatial variables in order to holistically assess human impacts on the functioning of aquatic ecosystems (Heugens et al. 2001; Bozelli et al. 2009; Nobre et al. 2020).
Anthropogenic disturbance gradients, particularly in riparian zones, have affected the structures and environmental conditions of Amazonian streams (Castello and Macedo 2016; Zimbres et al. 2018a), directly influencing aquatic biological communities (Kozlowski et al. 2016; Betts et al. 2017), such as phytoplankton (Bleich et al. 2015), zooplankton (Brasil et al. 2019), macroinvertebrates (Brito et al. 2020), and fish (Leão et al. 2020). Among these communities, zooplankton plays important roles in different aspects. For example, these organisms act as primary and secondary consumers that are subsequently consumed by larger organisms, such as fish, exercising a fundamental role in matter and energy transfer in the aquatic food web (Caroni and Irvine 2010; Du et al. 2015; García-Chicote et al. 2018).
Due to the relatively short life cycles of zooplankton organisms that can vary from a few days (testate amoebae and rotifers) to a few months (microcrustaceans) (Lampert and Sommer 2007), zooplankton communities respond quickly to environmental changes of both natural origin, e.g., hydrological changes (Gomes et al. 2020) and anthropogenic (Attayde and Bozelli 1998; Xiong et al. 2019). As a result, some zooplankton species can be indicative of riparian vegetation cover levels, also known as indicator species (Medeiros et al. 2019). After the indicator species delimitation, the detected organisms can be used in biological monitoring and conservation programs (Carignan and Villard 2002).
In this context, research on the responses of zooplankton communities to environmental patterns (natural and anthropogenic), spatial, and seasonal variations (rain and drought), from a local and regional perspective, may allow the assessment of environmental degradation impacts on this community. Thus, to evaluate the influence of forest cover and physical and chemical characteristics of streams on the zooplankton communities located in rural settlements in southeastern Amazon, we investigated the following questions: (1) Are environmental factors (water physical and chemical characteristics and landscape variables) and dispersive processes (reflected in the spatial structure among sampling sites) efficient predictors of zooplankton communities in Amazonian streams in different hydrological periods (rainy and dry)? (2) Can zooplankton species be indicators of watersheds' forest-cover levels?
We expected greater discharge of sediment from terrestrial areas to water bodies in deforested areas with low forest cover than in forested areas (Thomas et al. 2004), which means that deforested areas are expected to have lower soil water infiltration and, consequently, increase discharge during rainy events (Zhang et al. 2017; Nóbrega et al. 2018). Also, as the soil is bare due to deforestation, there will be no water infiltration in the riparian zone, which facilitates soil erosion, carrying more sediments to the aquatic system. Therefore, we expected a significant variation in zooplankton communities structure in streams according to the forest-cover level and local environmental characteristics, since the deforestation level should favor the establishment of specific taxa since the increase in sediment deposition due to discharge in the rainy season suffocates the respiratory organs of sensitive taxa (Buendia et al. 2013; Hauer et al. 2018).
Materials and methods
Study area
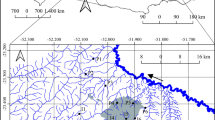
We conducted the study in 15 streams (sample units) of the Brazilian Amazon Basin, located in nine rural settlements in the north of Mato Grosso (Fig. 1): ETA, Bonjaguá, Cachimbo II, Alto Paraíso, Pinheiro Velho, Cotrel, São Cristóvão, Carlinda, and Cachoeira da União. We selected the sampling units seeking to encompass as much forest-cover gradient as possible in the areas where the settlements' streams were.
The climate in Mato Grosso, Brazil, is predominantly super-humid tropical monsoon, with a mean temperature of 24 °C and a maximum of 40 °C, and mean annual precipitation above 1500 mm (Nimer and Brandão 1989; Marcuzzo et al. 2011). We collected the samples in the rainy (March) and dry (August) seasons of 2017.
Environmental variables
We measured the limnological environmental variables once per sample unit. For this, we measured the aquatic variables, temperature (°C), electric conductivity (μS cm−1), oxy-reduction potential (mV), pH, turbidity (NTU), and dissolved oxygen (mg L−1), using a multiparameter probe (U-50, HORIBA Advanced Techno Co. Ltd., Kyoto, Japan). Additionally, we collected 500 mL water samples in the water column (≅ 50 cm) and froze them for laboratory analysis. We used the water samples to determine the concentrations of ammonia (mg L−1), total phosphorus (mg L−1), nitrate (mg L−1), nitrite (mg L−1), and TKN (total Kjeldahl nitrogen) (mg L−1). To perform the analyses, we followed the American Water and Waste Association's Standard Method for the Examination of Water and Waste-Water (APHA 2005), with adaptations.
Zooplankton communities
To relate the limnological environmental variables with the zooplankton communities, we collected the organisms and measured the environmental variables in the same places. For each sample unit, we collected one water sample from the water column. So, we collected 300 L of water and filtered it through a 20-μm mesh opening plankton net using a bucket. Subsequently, we put the filtrate in 200-mL polyethylene bottles, fixed with a 4% formaldehyde solution, buffered with sodium tetraborate. In the laboratory, the samples were concentrated to a known volume, between 10 and 20 mL, for identification.
The organisms' identification had quantitative and qualitative steps. For quantitative identification, we sub-sampled the concentrated sample with a Hensen-Stempel pipette and inserted it in a Sedgewick-Rafter counting chamber. After this procedure, we evaluated the samples qualitatively to check if there were new species that were not identified in the quantitative samples. For this, we collected the material sedimented in the samples using a Pasteur pipette and also inserted it in the Sedgewick-Rafter counting chamber. We followed these steps until new species were not registered.
Samples that did not have 200 individuals after we identified 10% of the concentrated volume in the quantitative analysis were identified throughout. Therefore, we gradually collected the entire contents of these samples using a Pasteur pipette and inserted it into the counting chamber until the sample was empty. We counted and identified the individuals held in this chamber to the lowest possible taxonomic level. After the identification, we estimated the proportion of individuals per m3 (Bottrell et al. 1976). We identified the organisms to the lowest possible taxonomic level, based on their morphological characteristics, checking identification keys for each group: testate amoebae (Ogden and Hedley 1980), cladocerans (Elmoor-Loureiro 1997), copepods (Silva 2003; Neves 2011), and rotifers (Koste 1978; Joko 2011).
Pfafstetter coding system and forest cover
We used the hydrographic base made by the Brazilian National Water Agency (ANA), based on Otto Pfafstetter's coding method of hydrographic basins (Ottobasins) (Pfafstetter 1989; ANA 2006). This method is a hierarchical and multiscale coding logic for watersheds, in which vector data are extracted from digital elevation models (Guenther 2001; El-Sheimy et al. 2005) derived from the Shuttle Radar Topography Mission (Farr and Kobrick 2000; Farr et al. 2007) with a spatial resolution of 90 m, allowing a more realistic representation of the watershed ridgelines. In ANA's coding system, Ottobasins vary from levels 1 to 6, according to their aggregation level. We used level 6 because it encompasses the smallest number of sampling units. For each Ottobasin, we used the following hydrographic parameters: total area (km2), altitude (mean and standard deviation), slope (mean and standard deviation), the sum of drainage length (SDL) (km), and the ratio between SDL and Ottobasin area (SDLAR).
For each Ottobasin, we determined the following environmental data associated with forest cover, using the SAGA software (Conrad et al. 2015): forest cover (% and km2), total edge (perimeter) of forest fragments (TE), relative amount of edge and landscape area (ED), mean edge per forest fragment (MPE), mean forest-fragment size per class (MPS), number of forest fragments of one class (NumP), median of forest fragments' size (MedPS), standard deviation of fragments' size (PSSD), the sum of the ratio between forest-fragment perimeter and area divided by the number of forest fragments (MPAR).
Data analysis
We performed all statistical analyses in the statistical program R (Team 2018). We evaluated the effect of seasonality on zooplankton communities' structure with Permutational Multivariate Analysis of Variance Using Distance Matrices (PERMANOVA). Before the PERMANOVA, we standardized the zooplankton and zooplankton groups' data using the Hellinger method. Then, we used those data for the construction of Euclidean distances matrix. We performed this analysis using the adonis 2 function of the vegan package (Oksanen et al. 2013).
To verify and compare the influences of the environmental and spatial predictors on the zooplankton community, we performed a partial redundancy analysis (pRDA), but only in cases where global variable selection tests showed significant values, as described below. To select the environmental variables used in the global model, we performed a variance inflation factor (VIF) analysis and excluded those with values above 20 (Borcard et al. 2018). Then, we selected the most important environmental and spatial variables determining the structure of the zooplankton communities using the forward selection (Blanchet et al. 2008) with the ordistep function from the vegan package. For this, first, we performed a global test with the sets of variables and each of the zooplanktonic groups and then proceeded to ordistep only in case the global redundancy analysis (RDA) presented significant results (P < 0.05).
For the spatial variables, we initially converted the coordinate values of the sample units to the Cartesian plane through the geoXY function of the SoDA package. Afterward, we submitted the coordinates to an ordering of distance-based Moran's eigenvector maps (dbMEM) with the dbmem function of adespatial package (Dray et al. 2017). We then performed another global analysis of the spatial predictors of the zooplankton community and proceeded only in case the analysis of variance (ANOVA) RDA test showed significant values (P < 0.05).
After these steps, and only in case the global values of the environmental and spatial variables were significant, we performed a partial redundancy analysis (pRDA) (Legendre and Legendre 2012) to evaluate the effect of local environmental characteristics and the spatial structuring as predictors of the zooplankton communities. In the pRDA, the predictors comprised the environmental and spatial variables selected by the forward selection. We tested the significance of each component with ANOVA (Borcard et al. 2018). We performed the same analyses (pRDA) with the occurrence (presence/absence) values of the species. For these analyses, we converted the biological variables into binary values.
We divided sampling units into three classes, based on forest-cover ratios of their Ottobasins, using the k-means method (Legendre and Legendre 2012): low (1 to 15%), medium (16 to 30%), and high (31–50%). We performed dependent sample t-tests to evaluate the effect of seasonality (rainy and dry seasons) per forest-cover class on the environmental characteristics of water bodies and their influence on total species richness and total density of individuals in zooplankton communities.
We performed an indicator-species analysis, indicator-value index analysis (indval), to evaluate whether zooplankton species can indicate forest-cover levels in the Ottobasins (Legendre and Legendre 2012).
Results
We selected twelve factors as predictive variables with VIF method: temperature, turbidity, total dissolved solids, conductivity, total phosphorus, sum of the perimeter/area ratio divided by the number of fragments (MPAR), median fragment size (MedPS), dissolved oxygen, depth, TKN, total number of fragments of a class (Nump), and Ottobasin area. The divisions of the sampling units according to the level of forest cover were low (1 to 15%)—6 sampling units: 1, 2, 4, 7, 10 and 11; medium (16 to 30%)—4 sample units: 3,9,13 and 14; and high (31–50%)—5 sample units: 5, 6, 8, 12 and 15 (Table SM.1).
In areas with low forest cover, water temperature and electrical conductivity were higher in the rainy and dry seasons, respectively. In areas with medium forest cover, the pH was higher in the dry season (Table 1). Sampling units in areas with high forest cover had higher water temperature and oxidation potential in the rainy season, while total phosphorus concentrations were higher in the dry season (Table 1).
A total of 206 taxa and 674,240 ind m−3 were identified, considering both seasons. Testate amoebae, rotifers, and microcrustaceans had 98, 72, and 36 taxa and 661,374, 3889, and 8977 individuals, respectively (Table SM.2). The class with low forest cover was the only one with seasonal differences in species richness, individuals density, and zooplankton community structure (Table 2). Rotifers had higher species richness in the rainy season (mean of 10.5 species) than in the dry season (mean of 4 species). Total zooplankton and testate amoebae had higher densities in the dry season (mean of 36,985 and 36,468 individuals, respectively) than in the rainy season (mean of 15,135 and 14,920 individuals, respectively). On the other hand, rotifers presented higher densities in the rainy season (mean of 160 individuals) than in the dry season (mean of 42 individuals). Regarding community structure, total zooplankton and testate amoebae presented significant differences between seasons for low forest-cover class (Table 2).
In general, environmental and spatial variables were not important predictors of the zooplankton community, for both density and species occurrence data (Table 3). In the rainy season, environmental variables explained only the structure of total zooplankton (density) and testate amoebae (density). The spatial variables explained only zooplankton density in the rainy season. In the dry season, environmental variables explained only the community structure of rotifers (density).
The indicator species analysis suggests that in the rainy season, harpacticoid copepods (indicator value, IV, = 0.67, P = 0.032) and Lepadella patella (IV = 0.60, P = 0.030) were indicative of low forest-cover areas, Centropyxis gibba (IV = 0.74, P = 0.030) were indicative of medium forest-cover areas, and calanoid copepodites (IV = 0.50, P = 0.047) were indicative of high forest-cover areas. In the dry season, Arcella discoides (IV = 0.60, P = 0.035) was indicative of low forest-cover areas, Lecane crepida (IV = 0.79, P = 0.019) indicated mean values, and Lesquereusia epistomium (IV = 0.50, P = 0.049) indicated high forest-cover areas.
Discussion
Here, electrical conductivity, water temperature, pH, total phosphorus concentrations, and oxidation potential presented seasonal variations according to the forest-cover class. Indeed, different seasons have different dynamics, with greater substrate stability in the dry season and a lower drag effect due to the slower water flow velocity and lower water volume (Bispo et al. 2001), whereas in the rainy season, there is greater availability of dissolved oxygen and increased water flow, with direct effects on organisms (Statzner et al. 1988; Callisto et al. 2001).
Environmental variations in streams are common over both time and space. Greater inputs of allochthonous material can be expected at the beginning of the rainy season, as precipitation favors the transport of material from riparian forests or zones of influence of the banks to water bodies from the catchment (Bambi et al. 2017). Studies in tropical regions show that there is a seasonal behavior in peak inputs of allochthonous organic matter into streams during periods of drought or associated with the beginning of the rainy season (Gonçalves Jr et al. 2006; França et al. 2009) as a function of temporal variability (Nobre et al. 2020). Furthermore, deforestation of riparian vegetation is a major source of environmental variation, associated with the organic matter processing speed, nutrient absorption, reduction in oxygen concentrations, and increase in water temperature and electrical conductivity (Sweeney et al. 2004; Bleich et al. 2014; Prudente et al. 2017).
Seasonal changes in species richness, individuals density, and zooplankton community structure were observed only in the low forest-cover class. That can be a consequence of sediment deposition increase during the rainy season in areas affected by anthropic actions, since deforested areas have lower soil water infiltration rates, immediately generating runoff (Alaoui et al. 2018), which significantly contributes to the decline in aquatic organism populations (Richter et al. 1996). Some zooplankton species feed on primary producers, and these are especially susceptible to physical and chemical variations caused by hydrological changes due to the decrease in food availability between trophic levels. Also, suspended solids levels can cause reductions in zooplankton density (Chará-Serna et al. 2019), leading to changes in the local community composition in addition to the cascade negative effects, decreasing the species fitness and limiting the habitat (Richter et al. 1996; Wood and Armitage 1997; Henley et al. 2000).
The increase in stream velocity due to rainfall increase provides greater suspension of microorganisms in the water column that use benthic and littoral compartments (including fauna associated with the surface of aquatic macrophytes) (Bonecker et al. 1996; Fulone et al. 2008). Therefore, the pluvial regime in streams with low forest cover had a greater influence on communities' structural variation, mainly on rotifers' species richness and individuals density of rotifers, testate amoebae, and total zooplankton. Also, the zooplankton community can be indirectly affected by changes in riparian vegetation, which, in the case of low forest cover, offers lower barriers for the entry of allochthonous material. That factor may also have been responsible for the variations in the testate amoebae and rotifer densities in the low forest-cover classes (Medeiros et al. 2019).
We suggest that the greater influx of allochthonous material in the rainy season due to the low amount of barriers in areas with low forest cover may have altered the zooplankton community density (Arimoro and Oganah 2010). Even though we observed greater variation in the low forest-cover class in the rainy season, testate amoebae density increased in the dry season in streams with low forest cover. This community is also frequently found in lotic environments (Bonecker et al. 1996) because they have gaseous vacuoles that facilitate water column fluctuation (Štěpánek and Jiří 1958; Ogden 1991), and present flattened shells (Mitchell et al. 2008; Fournier et al. 2016; Schwind et al. 2016) that make them less susceptible to water flow transport (Velho et al. 2003).
Here, environmental and spatial variables had no significant effect on a specific period for some groups of zooplankton communities. The lack of local and spatial predictors may indicate the strong influence of stochastic factors (e.g., birth, mortality, colonization, and extinction) on the structure of the zooplankton communities in these streams (Chase 2007). The fast and directional (downstream) streamflow associated with the low swimming capacity of zooplankton organisms probably influenced the weak relation the measured local environmental predictors and spatial predictors (migratory processes) with the zooplankton communities in these environments (Astorga et al. 2012; De Bie et al. 2012).
Species from three zooplankton groups (rotifers, microcrustaceans, and testate amoebae) were indicators of forest-cover classes. High species diversity, rapid reproductive rates, and large amplitude of adaptive responses to environmental dynamics (such as hydrological, physical, and chemical variations) are important factors that make these organisms good indicators of certain natural and anthropogenic conditions and/or disturbances (Stoch et al. 2009; Schuler et al. 2017; Strecker and Brittain 2017). Moreover, the broad behavioral plasticity, which probably provides ample diversification of trophic and spatial niche, may also favor zooplankton organisms to become more resilient to anthropogenic pressures and excel in more degraded environments (Kuczyńska-Kippen and Basińska 2014; Zhai et al. 2015). The sensitivity of this group's taxa to local and landscape changes is due to differences in the species life cycle, morphology, physiology, and behavior (Bonada et al. 2006).
Finally, understanding how various anthropic disturbances interact systematically with environmental, local, and spatial variables is fundamental for the assessment of human impacts and the functioning of aquatic ecosystems (Heugens et al. 2001; Bozelli et al. 2009), especially in the current scenario where neotropical aquatic ecosystems are highly threatened (Bailly et al. 2016; Ríos-Touma and Ramírez 2019).
Conclusions
Quantifying the effects of deforestation on the physical and chemical characteristics of aquatic environments and their biological communities is a major challenge. Understanding how environmental, hydrological, and spatial variables influence zooplankton requires further investigation, requiring further studies to support this connection to understand the individual responses of these organisms better.
This study provided valuable contributions to the conservation of riparian ecosystems and the use of biological indicators in environmental monitoring programs. Considering the current trends of increasing human impacts in the Amazon region (e.g., expansion of human occupation, intensification of agriculture, and increasing deforestation) and decreasing investments in scientific development in Brazil, our results present significant inputs toward the creation/adaptation of national land-use and environmental policies for the conservation and restoration of riparian habitats. Corroborating many other studies addressing different biological groups, e.g., bacterioplankton (Câmara dos Reis et al. 2019), fish (Brejão et al. 2018; Jézéquel et al. 2020), macroinvertebrate (Brito et al. 2020), macrophyte (Fares et al. 2020), mammals (Zimbres et al. 2018b), phytoplankton (Cardoso et al. 2017), our results show that deforestation in riparian zones can influence the structure of zooplankton communities.
For this reason, the use of a microbasin scale approach can contribute more effectively to the effects of changes in vegetation on aquatic environments than an assessment of variables only on riparian vegetation (Nobre et al. 2020). Our results indicate that the use of species indicative of deforestation classes should take into account two aspects: (i) the seasonality, since the indicator species are different in the dry and rainy seasons, and (ii) the "indication strength" (indicator value), since the values varied between 0.50 and 0.79. We suggest that for a better understanding of the environmental influences on the zooplankton community, future studies in streams should encompass flow assessments and soil sampling along the basin.
References
Alaoui A, Rogger M, Peth S, Blöschl G (2018) Does soil compaction increase floods? A review. J Hydrol 557:631–642
Alencar A, Pereira C, Castro I et al (2015) Desmatamento nos Assentamentos da Amazônia: histórico, tendências e oportunidades. IPAM, Brasília, DF
ANA (2006) Topologia hídrica: método de construção e modelagem da base hidrográfica para suporte à gestão de recursos hídricos: versão 1.11
APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington DC
Arimoro FO, Oganah AO (2010) Zooplankton community responses in a perturbed tropical stream in the Niger Delta, Nigeria. Open Environ Biol Monit J 3(1):1–11. http://dx.doi.org/%2010.2174/1875040001003010001
Astorga A, Oksanen J, Luoto M et al (2012) Distance decay of similarity in freshwater communities: do macro-and microorganisms follow the same rules? Glob Ecol Biogeogr 21:365–375
Attayde JL, Bozelli RL (1998) Assessing the indicator properties of zooplankton assemblages to disturbance gradients by canonical correspondence analysis. Can J Fish Aquat Sci 55:1789–1797
Bailly D, Cassemiro FAS, Winemiller KO et al (2016) Diversity gradients of Neotropical freshwater fish: evidence of multiple underlying factors in human-modified systems. J Biogeogr 43:1679–1689
Bambi P, de Souza RR, Feio MJ et al (2017) Temporal and spatial patterns in inputs and stock of organic matter in savannah streams of Central Brazil. Ecosystems 20:757–768
Betts MG, Wolf C, Ripple WJ et al (2017) Global forest loss disproportionately erodes biodiversity in intact landscapes. Nature 547:441
Blanchet FG, Legendre P, Borcard D (2008) Forward selection of explanatory variables. Ecology 89:2623–2632
Bleich ME, Mortati AF, André T, Piedade MTF (2014) Riparian deforestation affects the structural dynamics of headwater streams in Southern Brazilian Amazonia. Trop Conserv Sci 7:657–676
Bleich ME, Piedade MTF, Mortati AF, André T (2015) Autochthonous primary production in southern Amazon headwater streams: novel indicators of altered environmental integrity. Ecol Indic 53:154–161
Bonada N, Prat N, Resh VH, Statzner B (2006) Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annu Rev Entomol 51:495–523. https://doi.org/10.1146/annurev.ento.51.110104.151124
Bonecker CC, Bonecker SLC, Bozelli RL et al (1996) Zooplankton composition under the influence of liquid wastes from a pulp mill in middle Doce River(Belo Oriente, MG, Brazil). Arq Biol e Tecnol 39:893–901
Borcard D, Gillet F, Legendre P (2018) Numerical ecology with R. Springer, Berlin
Bottrell HH, Duncan A, Gliwicz ZM, et al (1976) A review of some problems in zooplankton production studies. Norw J 419–456
Bozelli RL, Caliman A, Guariento RD et al (2009) Interactive effects of environmental variability and human impacts on the long-term dynamics of an Amazonian floodplain lake and a South Atlantic coastal lagoon. Limnologica 39:306–313
Brasil LS, Luiza-Andrade A, Kisaka TB et al (2019) Cladocera distribution along an environmental gradient on the Cerrado-Amazon ecotone: a preliminary study. Acta Limnol Bras. https://doi.org/10.1590/s2179-975x2919
Brejão GL, Hoeinghaus DJ, Pérez-Mayorga MA et al (2018) Threshold responses of Amazonian stream fishes to timing and extent of deforestation. Conserv Biol 32:860–871
Brito JG, Roque FO, Martins RT et al (2020) Small forest losses degrade stream macroinvertebrate assemblages in the eastern Brazilian Amazon. Biol Conserv 241:108263
Buendia C, Gibbins CN, Vericat D et al (2013) Detecting the structural and functional impacts of fine sediment on stream invertebrates. Ecol Indic 25:184–196
Callisto M, Moreno P, Barbosa FAR (2001) Habitat diversity and benthic functional trophic groups at Serra do Cipó, Southeast Brazil. Rev Bras Biol 61:259–266
Câmara dos Reis M, Lacativa Bagatini I, de Oliveira VL et al (2019) Spatial heterogeneity and hydrological fluctuations drive bacterioplankton community composition in an Amazon floodplain system. PLoS ONE 14:e0220695
Cardoso SJ, Nabout JC, Farjalla VF et al (2017) Environmental factors driving phytoplankton taxonomic and functional diversity in Amazonian floodplain lakes. Hydrobiologia 802:115–130
Carignan V, Villard MA (2002) Selecting indicator species to monitor ecological integrity: a review. Environ Monit Assess 78:45–61. https://doi.org/10.1023/A:1016136723584
Caroni R, Irvine K (2010) The potential of zooplankton communities for ecological assessment of lakes: redundant concept or political oversight? In: Biology and environment: proceedings of the Royal Irish Academy. JSTOR, pp 35–53
Castello L, Macedo MN (2016) Large-scale degradation of Amazonian freshwater ecosystems. Glob Chang Biol 22:990–1007
Chará-Serna AM, Epele LB, Morrissey CA, Richardson JS (2019) Nutrients and sediment modify the impacts of a neonicotinoid insecticide on freshwater community structure and ecosystem functioning. Sci Total Environ 692:1291–1303
Chase JM (2007) Drought mediates the importance of stochastic community assembly. Proc Natl Acad Sci 104:17430–17434
Conrad O, Bechtel B, Bock M et al (2015) System for automated geoscientific analyses (SAGA) v. 2.1.4. Geosci Model Dev 8:1991
da Bispo PC, Oliveira LG, Crisci VL, Silva MM (2001) A pluviosidade como fator de alteração da entomofauna bentônica (Ephemeroptera, Plecoptera e Trichoptera) em córregos do Planalto Central do Brasil. Acta Limnol Bras 13:1–9
da Silva WM (2003) Diversidade dos Cyclopoida (Copepoda, Crustácea) de água doce do estado de São Paulo: taxonomia, ecologia e genética
De Bie T, De Meester L, Brendonck L et al (2012) Body size and dispersal mode as key traits determining metacommunity structure of aquatic organisms. Ecol Lett 15:740–747
Dray S, Blanchet G, Borcard D et al (2017) Adespatial: multivariate multiscale spatial analysis. R package version 0.0-9
Du X, García-Berthou E, Wang Q et al (2015) Analyzing the importance of top-down and bottom-up controls in food webs of Chinese lakes through structural equation modeling. Aquat Ecol 49:199–210
Elmoor-Loureiro LMA (1997) Manual de identificação de cladóceros límnicos do Brasil
El-Sheimy N, Valeo C, Habib A (2005) Digital terrain modeling: acquisition, manipulation and applications (Artech House Remote Sensing Library). Artech House, Norwood, MA
Fares ALB, Calvão LB, Torres NR et al (2020) Environmental factors affect macrophyte diversity on Amazonian aquatic ecosystems inserted in an anthropogenic landscape. Ecol Indic 113:106231
Farr TG, Kobrick M (2000) Shuttle Radar Topography Mission produces a wealth of data. Eos, Trans Am Geophys Union 81:583–585
Farr TG, Rosen PA, Caro E et al (2007) The shuttle radar topography mission. Rev Geophys 45:2. https://doi.org/10.1029/2005RG000183
Fournier B, Coffey EED, van der Knaap WO et al (2016) A legacy of human-induced ecosystem changes: spatial processes drive the taxonomic and functional diversities of testate amoebae in Sphagnum peatlands of the Galápagos. J Biogeogr 43:533–543
França JS, Gregório RS, de Paula JD et al (2009) Composition and dynamics of allochthonous organic matter inputs and benthic stock in a Brazilian stream. Mar Freshw Res 60:990–998
Fulone LJ, Vieira LCG, Velho LFM, Lima AF (2008) Influence of depth and rainfall on testate amoebae (Protozoa-Rhizopoda) composition from two streams in northwestern São Paulo state. Acta Limnol Bras 20:29–34
García-Chicote J, Armengol X, Rojo C (2018) Zooplankton abundance: a neglected key element in the evaluation of reservoir water quality. Limnologica 69:46–54. https://doi.org/10.1016/j.limno.2017.11.004
Gomes LF, Vieira LCG, de Souza CA et al (2020) Environmental controls on zooplankton during hydrological periods of flooding and flushing in an Amazonian floodplain lake. Limnetica 39:35–48
Gonçalves JF Jr, França JS, Medeiros AO et al (2006) Leaf breakdown in a tropical stream. Int Rev Hydrobiol 91:164–177
Guenther G (2001) Digital elevation model technologies and applications, the DEM users manual: chapter 8 airborne lidar bathymetry. Am Soc Photogramm Remote Sensing Bethesda, MA, USA
Hauer C, Leitner P, Unfer G et al (2018) The role of sediment and sediment dynamics in the aquatic environment. Riverine Ecosystem Management. Springer, Cham, pp 151–169
Henley WF, Patterson MA, Neves RJ, Lemly AD (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8:125–139
Heugens EHW, Hendriks AJ, Dekker T et al (2001) A review of the effects of multiple stressors on aquatic organisms and analysis of uncertainty factors for use in risk assessment. Crit Rev Toxicol 31:247–284
Jézéquel C, Tedesco PA, Bigorne R et al (2020) A database of freshwater fish species of the Amazon Basin. Sci Data 7:1–9
Joko CY (2011) Taxonomia de rotíferos monogonontas da planície de inundação do alto rio Paraná (MS/PR)
Koste W (1978) Rotatoria die rädertiere Mitteleuropas begründet von Max Voigt–Monogononta. 2. Auflage neubearbeitet von Walter Koste Gebrüder Borntraeger Berlin, Stuttgart
Kozlowski DF, Hall RK, Swanson SR, Heggem DT (2016) Linking management and riparian physical functions to water quality and aquatic habitat. J Water Resour Prot 8:797
Kuczyńska-Kippen N, Basińska A (2014) Habitat as the most important influencing factor for the rotifer community structure at landscape level. Int Rev Hydrobiol 99:58–64
Lampert W, Sommer U (2007) Limnoecology: the ecology of lakes and streams. Oxford University Press, Oxford
Lathuillière MJ, Coe MT, Johnson MS (2016) A review of green-and blue-water resources and their trade-offs for future agricultural production in the Amazon Basin: what could irrigated agriculture mean for Amazonia? Hydrol Earth Syst Sci 20:2179–2194
Leão H, Siqueira T, Torres NR, de Assis Montag LF (2020) Ecological uniqueness of fish communities from streams in modified landscapes of Eastern Amazonia. Ecol Indic 111:106039
Legendre P, Legendre L (2012) Numerical ecology, 3rd edn. Elsevier, Amsterdam
Marcuzzo FFN, Andrade LR, Melo DC de R (2011) Métodos de interpolação matemática no mapeamento de chuvas do estado do Mato Grosso
Medeiros ÍLS, Santos FA dos, El-Deir ACA, Melo Júnior M de (2019) Does riparian vegetation influence the composition and structure of the zooplankton community in temporary ponds? Iheringia Série Zool 109. https://doi.org/10.1590/1678-4766e2019037
Mitchell EAD, Payne RJ, Lamentowicz M (2008) Potential implications of differential preservation of testate amoeba shells for paleoenvironmental reconstruction in peatlands. J Paleolimnol 40:603–618
Mullan K, Sills E, Pattanayak SK, Caviglia-Harris J (2018) Converting forests to farms: the economic benefits of clearing forests in agricultural settlements in the Amazon. Environ Resour Econ 71:427–455. https://doi.org/10.1007/s10640-017-0164-1
Neves GP (2011) Copépodes planctônicos (Crustacea, Calanoida e Cyclopoida) em reservatórios e trechos lóticos da bacia do Rio da Prata (Brasil, Paraguai, Argentina e Uruguai): taxonomia, distribuição geográfica e alguns atributos ecológicos
Nimer E, Brandão AMPM (1989) Balanço hídrico e clima da região dos cerrados. Secretaria de Planejamento e Coordenação da Presidência da República, Fundação Instituto Brasileiro de Geografia e Estatística, Diretoria de Geociências, Departamento de Recursos Naturais e Estudos Ambientais
Nobre RLG, Caliman A, Cabral CR et al (2020) Precipitation, landscape properties and land use interactively affect water quality of tropical freshwaters. Sci Total Environ 716:137044
Nóbrega RLB, Guzha AC, Lamparter G et al (2018) Impacts of land-use and land-cover change on stream hydrochemistry in the Cerrado and Amazon biomes. Sci Total Environ 635:259–274
Ogden CG (1991) The biology and ultrastructure of an agglutinate testate amoeba Difflugia geosphaira sp. nov. (Protozoa, Rhizopoda). Arch für Protistenkd 140:141–150
Ogden GG, Hedley RH (1980) An atlas of freshwater testate amoebae. Soil Sci 130:176
Oksanen J, Blanchet FG, Kindt R, et al (2013) Package ‘vegan.’ Community Ecol Packag version 2
Pfafstetter O (1989) Classificação de bacias hidrográficas: metodologia de codificação. Rio Janeiro, RJ Dep Nac Obras Saneam 1989:19
Prudente BS, Pompeu PS, Juen L, Montag LFA (2017) Effects of reduced-impact logging on physical habitat and fish assemblages in streams of Eastern Amazonia. Freshw Biol 62:303–316
Reyer CPO, Rammig A, Brouwers N, Langerwisch F (2015) Forest resilience, tipping points and global change processes. J Ecol 103:1–4
Richter BD, Baumgartner JV, Powell J, Braun DP (1996) A method for assessing hydrologic alteration within ecosystems. Conserv Biol 10:1163–1174
Ríos-Touma B, Ramírez A (2019) Multiple stressors in the neotropical region: environmental impacts in biodiversity hotspots. In: Multiple stressors in river ecosystems. Elsevier, pp 205–220
Rockström J, Steffen W, Noone K et al (2009) A safe operating space for humanity. Nature 461:472
Roriz PAC, Yanai AM, Fearnside PM (2017) Deforestation and Carbon Loss in Southwest Amazonia: Impact of Brazil’s Revised Forest Code. Environ Manage 60:367–382
Schuler MS, Chase JM, Knight TM (2017) Habitat size modulates the influence of heterogeneity on species richness patterns in a model zooplankton community. Ecology 98:1651–1659
Schwind LTF, Arrieira RL, Bonecker CC, Lansac-Tôha FA (2016) Chlorophyll-a and suspended inorganic material affecting the shell traits of testate amoebae community. Acta Protozool 2016:145–154
Statzner B, Gore JA, Resh VH (1988) Hydraulic stream ecology: observed patterns and potential applications. J North Am Benthol Soc 7:307–360
Steffen W, Richardson K, Rockström J et al (2015) Planetary boundaries: guiding human development on a changing planet. Science 347(6223):1259855. https://doi.org/10.1126/science.1259855
Štěpánek M, Jiří J (1958) Difflugia gramen Penard, Difflugia gramen var. achlora Penard and Difflugia gramen f. globulosa fn. Hydrobiologia 10:138–156
Stoch F, Artheau M, Brancelj A et al (2009) Biodiversity indicators in European ground waters: towards a predictive model of stygobiotic species richness. Freshw Biol 54:745–755
Strecker AL, Brittain JT (2017) Increased habitat connectivity homogenizes freshwater communities: historical and landscape perspectives. J Appl Ecol 54:1343–1352
Sweeney BW, Bott TL, Jackson JK et al (2004) Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc Natl Acad Sci 101:14132–14137
Team RC (2018) R: A language and environment for statistical computing
Thomas SM, Neill C, Deegan LA et al (2004) Influences of land use and stream size on particulate and dissolved materials in a small Amazonian stream network. Biogeochemistry 68:135–151. https://doi.org/10.1023/B:BIOG.0000025734.66083.b7
Trumbore S, Brando P, Hartmann H (2015) Forest health and global change. Science (80-) 349:814–818
Velho LFM, Lansac-Tôha FA, Bini LM (2003) Influence of environmental heterogeneity on the structure of testate amoebae (Protozoa, Rhizopoda) assemblages in the plankton of the upper Paraná river floodplain, Brazil. Int Rev Hydrobiol A J Cover all Asp Limnol Mar Biol 88:154–166
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. Environ Manag 21:203–217
Xiong W, Ni P, Chen Y et al (2019) Biological consequences of environmental pollution in running water ecosystems: a case study in zooplankton. Environ Pollut 252:1483–1490
Zhai M, Hřívová D, Peterka T (2015) The harpacticoid assemblages (Copepoda: Harpacticoida) in the Western Carpathian spring fens in relation to environmental variables and habitat age. Limnol Manag Inl Waters 53:84–94
Zhang M, Liu N, Harper R et al (2017) A global review on hydrological responses to forest change across multiple spatial scales: Importance of scale, climate, forest type and hydrological regime. J Hydrol 546:44–59
Zimbres B, Machado RB, Peres CA (2018a) Anthropogenic drivers of headwater and riparian forest loss and degradation in a highly fragmented southern Amazonian landscape. Land use policy 72:354–363
Zimbres B, Peres CA, Penido G, Machado RB (2018b) Thresholds of riparian forest use by terrestrial mammals in a fragmented Amazonian deforestation frontier. Biodivers Conserv 27:2815–2836
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gomes, A.C.A.M., Gomes, L.F., Roitman, I. et al. Forest cover influences zooplanktonic communities in Amazonian streams. Aquat Ecol 54, 1067–1078 (2020). https://doi.org/10.1007/s10452-020-09794-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09794-6