Abstract
Predation is one of the most fundamental and unifying concepts in ecology, and we are beginning to obtain a more complete understanding of how predators drive community structure and ecosystem function through their impacts on prey. We know considerably less about how predators affect each other through intraguild interactions, which is surprising considering predators often occur simultaneously and may compete for resources while avoiding being killed themselves. In the present study, we examined aspects of inter- and intra-specific resource use among three species of large-bodied predatory sharks (blacktip, bull, lemon) co-occurring within a subtropical, protected bay in the southeastern USA. Specifically, we inferred relative trophic position, isotopic niche overlap, and patterns of resource use of sharks using stable isotope analysis of carbon-13 and nitrogen-15 from blood and fin cartilage samples. We also combined these approaches with estimates of abundance and occurrence from empirical shark surveys to consider whether these species may exhibit resource partitioning in space and time. We found that all three species overlapped in space, and there was some isotopic niche overlap between the species. We also found evidence of temporal isotopic niche stability, suggesting that co-occurring shark species may compete for available prey resources, but individuals of those species may have similar patterns of resource use over time. We discuss our findings as they relate to the ecologies of the species in question and how sound conservation and management of ecosystems can allow for predator diversity, sympatry, and stable use of resources at the top of the food chain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foraging activities of predators can initiate trophic cascades via both predation and risk effects on their prey (Werner and Peacor 2003; Creel and Christianson 2008; Matassa and Trussell 2011). Furthermore, predator diversity can increase the prevalence and strength of trophic cascades (Estes et al. 1998; Silliman and Bertness 2002; Byrnes et al. 2006), a phenomenon which is reinforced by patches of predictable resources or nutrients (Frederiksen et al. 2006). Predators are typically considered to be dietary generalists (i.e., feeding on multiple prey types), and many have adopted strategies to exploit persistent and profitable resource regions (Costa 1993). However, at the community level, resource partitioning can also occur among sympatric predators (e.g., White and Potter 2004; Papastamatiou et al. 2006); yet, understanding the conditions under which varying degrees of resource portioning will occur among predators remains relatively poorly understood.
Sharks often occupy apex or top predatory trophic roles in aquatic ecosystems (Cortés 1999). In recent years, sharks have become the focus of conservation-based research primarily due to widespread population declines from overfishing (e.g., Baum et al. 2003; Gallagher et al. 2012; Dulvy et al. 2014). Research has shown that predation risk from large sharks may cause changes in the distribution, foraging, and habitat use of their prey species (Madin et al. 2010; Barnett and Semmens 2012; Hammerschlag et al. 2012). However, relatively less is known about how sharks affect the resource use of sympatric shark species (Kinney et al. 2011), depite the fact that many species often co-occur.
Stable isotope analysis uses isotopic ratios of carbon (C13/C12) and nitrogen (N15/N14), which are enriched within consumers in a stepwise and predictable way and can be used to infer patterns of resource use among consumers (Peterson and Fry 1987). Carbon ratios are used to infer the carbon source at the base of a food web (e.g., coastal vs. offshore, Peterson and Fry 1987), while nitrogen ratios are often correlated with trophic position (Post 2002). The isotopic niche of a species includes, but is distinct from, an ecological or dietary niche (reviewed in Layman et al. 2012). Since different consumer tissues incorporate stable isotopes from their environment at different rates (e.g., bone/cartilage: months to years; plasma/muscle: days to months), comparing isotopic signatures between two tissues can reveal the temporal stability of an individual’s patterns of resource usage (e.g., Beaudoin et al. 1999; Knudsen et al. 2011). Stable isotope analysis is increasingly being used to study aspects of elasmobranch resource use (reviewed in Hussey et al. 2012; Shiffman et al. 2012).
In this study, we took an eco-physiological approach to examine aspects of inter- and intra-specific resource use of top predatory fishes within a productive subtropical bay protected from commercial fishing (Florida Bay in Everglades National Park, USA). Specifically, we evaluated four primary questions: (1) Do shark species in this productive system occur similarly over space and time? (2) Do these shark species exhibit overlap in isotopic niche inferred by stable isotope analysis, and if so, what is the degree of this overlap? (3) Do the focal species exhibit temporal stability in isotopic niche space? Taken together, (4) Do these species show evidence of possible partitioning of their resource use (e.g., Kinney et al. 2011)? To answer these questions, we investigated inferred trophic position, isotopic niche overlap, and patterns of resource use for three species of sharks in our system, using stable isotope analysis of carbon-13 and nitrogen-15 from blood and fin samples. Further, we coupled this analysis with an examination of the difference in abundance of these three species within the study area. We discuss our findings as they relate to intraguild predator interactions, the importance of predator effects on marine food webs, and the conservation implications of these intraguild interactions.
Methods
Study system and sample collection
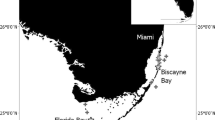
Sampling was conducted in the northwest edge waters of Everglades National Park, Florida, USA, (~25.0°N, 81.0°W; Fig. 1). This region within Florida Bay is a productive marine ecotone containing by a diverse assemblage of teleost fishes and sharks year-round (e.g., Torres et al. 2006). Shark fishing and research surveys were conducted in the study area monthly across the wet and dry seasons from December 2009 to February 2011. Our study focused on the three relatively large (i.e., >140 cm TL) and common shark species: blacktip sharks (Carcharhinus limbatus), bull sharks (Carcharhinus leucas), and lemon sharks (Negaprion brevirostris), sampled within an area of ~10 km2. We focused on these species for three reasons. First, all three co-occur in the study area (Wiley and Simpfendorfer 2007). Secondly, these species are known to feed primarily on fishes and occasionally elasmobranchs, and thus could potentially compete for the same prey in Florida Bay (Hoffmayer and Parsons 2003; Newman et al. 2010). Lastly, the area of greatest bull shark core habitat occurred right on our sampling area (Hammerschlag et al. 2012).
All sharks were captured using circle-hook drumlines, a standardized and minimally invasive fishing technique following Gallagher et al. (2014). The fishing gear consisted of a submerged weight tied to a line running to the surface by means of an attached inflatable buoy. A 23-m monofilament ganglion line (~400 kg test) was attached to the submerged weight by a swivel, which terminated at a baited 16/0 5°—offset circle hook. This method permitted sharks to swim in a 23-m radius circle around the base when captured. The proximal end of the monofilament line was connected to the weight. Two sets of five baited (barracuda. Sphyrna barracuda) drumlines were deployed roughly ~500 m apart from 1100 to 1430 h and were allowed to soak for an hour. After an hour from the first deployment, each drumline was sequentially checked for shark presence. If a shark was present, it was immediately brought to the boat for processing. A saltwater pump was placed in the sharks’ mouth to permit oxygenation of the gills during the workup process. All sharks were then sexed and measured for total length (TL, cm). Two tissue samples were taken: whole blood and a fin clip from the trailing edge of the dorsal fin (specifically to later analyze the basal cartilage/fin rays, hereafter termed ‘fin’). Whole blood was obtained through caudal venipuncture using 18-gauge needles and 10-mL syringes (Gallagher et al. 2014). Approximately, 7 mL of whole blood was taken from each shark and aliquoted into serum collection vacutainers for future stable isotope analysis. Fin clips were taken by trimming approximately 7 mm off the trailing edge of the first dorsal fin. Collected samples were put on an ice-slurry until we returned to dry land, after which they were all frozen at −20 °C until further processing.
Shark abundance and analyses
We used two metrics to evaluate relative abundance patterns of the focal species. First, we calculated catch per unit effort (CPUE) for each species expressed as the number of sharks captured per set of five drumlines, averaged by the total number of sets (i.e., number of sharks per set/number of total sets; with a ‘set’ assumed to be independent) following the approach of Hammerschlag et al. (2012). Second, we calculated the frequency of occurrence for each species expressed as the proportion of drumline sets positive for a given species (i.e., number of sets containing that species/number of total sets) following an approach modified from Hammerschlag and Serafy (2010). Since CPUE data were not normally distributed, we tested for differences among and between species using Kruskal–Wallis tests. For frequency of occurrence data, we applied Chi-squared analysis to test for statistical differences among and between species. To investigate whether species were potentially avoiding one another or spatially partitioning habitat, we used Spearman correlation to look for correlations between CPUE and occurrence data among species (i.e., whether abundance of one species was positively or negatively correlated with another).
Stable isotope ratios and analyses
Stable isotope analyses were conducted in a continuous flow isotope ratio mass spectrometer (CFIRMS) at the Laboratory of Stable Isotope Ecology in Tropical Ecosystems, at the University of Miami (Florida, USA). Approximately, 1 mL of whole blood and 0.5 mm of fin sample were dried for 48 h and then homogenized (we excluded the skin of fin samples). An aliquot of about 5.0 mg from each sample was then weighed in tin cups and combusted in a Eurovector Elemental Analyzer (Milan, Italy) at 1050 °C. The resulting gases were separated and analyzed in an Isoprime isotope ratio mass spectrometer (GV, Manchester, England) for carbon stable isotope ratios. CFIRMS involved the automated sequential measurement of samples (unknowns) together with reference material. Stable isotope ratios are expressed in δ-notation as parts per thousand (‰) deviations from the international standards PDB (carbon) according to the equation: δ13C or δ15N = [(R sample/R standard) − 1] * 1000 and R is 13C/12C or 15N/14N (the ratio of ‘heavy carbon’ to ‘light carbon’) and nitrogen, δ15N (the ratio of ‘heavy nitrogen’ 15N to ‘light nitrogen’ 14N). All isotope data were log-transformed prior to analysis to meet the assumptions of normality and homogeneity. All statistical analyses were performed on blood-isotope data, except for the evaluation between-tissue variation (described below). To investigate whether there were any effects of size on shark isotopic values, we evaluated for each species the relationship between shark length and δ13C and δ15N using regression analysis. Since we did not assume that the relationship between variables would be linear, both linear and second-order polynomial equations were used, with the best fit line reported when data were significant (defined by the highest R 2 value).
We also examined isotopic (a) niche width, (b) niche space, and (c) niche overlap between species pairs. To evaluate for potential differences in isotopic niche width between species, we calculated and compared a metric of dispersion on δ13C and δ15N bivariate data following the approach of Turner et al. (2010) and Hammerschlag-Peyer et al. (2011). Dispersion is a measure of the average trophic variability, and thus niche width, between shark species. To calculate dispersion, we computed the mean distance to centroid (δ13C and δ15N bivariate mean) for each shark species separately using nested linear models and a residual permutation procedure (Turner et al. 2010; Hammerschlag-Peyer et al. 2011). Next, the absolute of the difference in these mean values was calculated. An absolute value greater than zero indicates a difference in isotopic niche width between shark species (Turner et al. 2010). To evaluate potential differences in isotopic niche space, we calculated and compared difference in central tendency of δ13C and δ15N bivariate data between species. Central tendency is a measure of isotopic niche space, and differences between species are measured by computing the Euclidean distance between the centroids of the two species (Turner et al. 2010). The isotopic niche position between two shark species was considered to be different if the Euclidean distance between the two species was significantly greater than zero (R codes for the test of dispersion and central tendency are provided in Appendix of Turner et al. 2010). The test statistics for dispersion and central tendency are herein referred to as ‘mean distance to centroid’ and ‘Euclidean distance,’ respectively.
To measure isotopic niche, we calculated each species convex hull area (Vaudo and Heithaus 2012), which is the area of the smallest convex polygon that contains all individuals of a species in a δ13C–δ15N-biplot (Layman et al. 2007). We also calculated the small sample size corrected SIBER ellipse area (SEAc, Jackson et al. 2011), and we estimated isotopic niche overlap among species using species-specific SIBER ellipses generated via Bayesian modeling in the SIAR package in R (R Core Team 2013). Non-overlapping isotopic niche space suggests resource partitioning between species, but overlapping isotopic niche space does not necessarily suggest shared resource usage (reviewed in Layman et al. 2012). Additionally, larger values for convex hulls, SEAc, δ13C range, and δ15N range indicate a relatively larger isotopic niche.
Different tissues incorporate isotopes at different rates; shark whole blood has a faster turnover rate (up to 265 days; MacNeil et al. 2005) than shark fin (~400–600 days; Matich et al. 2011). To investigate the potential temporal isotopic niche stability of individuals, we regressed blood against fin values of δ13C and δ15N (for individuals with paired fin/blood samples, from each species separately and for all sharks combined). This allowed us to compare the isotopic niche from a hard tissue (i.e., cartilage/fin ray) to a more metabolically active tissue (whole blood), which have broadly different turnover rates, therefore to determine if it varied over time. We compared the paired blood and fin δ13C and δ15N values each using paired t tests. Analysis of occurrence data was computed using SAS Statistical Software (SAS Institute, NC, USA). All other analyses were performed in the program R (R Core Team 2013), and significance was declared at p < 0.05.
Results
A total of 1010 individual drumlines from 202 sets were analyzed for shark density, detecting a total of 176 sharks across the three species (blacktip, n = 86; bull, n = 22; lemon, n = 68).
The largest species in our study was the lemon shark (mean ± SD cm TL, 191.3 ± 24.5 cm, Table 1), followed by the bull shark (176.9 ± 14.8 cm) and the blacktip shark (145.1 ± 27.4 cm). The ranges of sizes encountered suggest that our sample of individuals captured was likely sub-adult or adult; no young-of-year or neonates of any species were captured (Table 1).
Significant differences in CPUE among sharks were detected (Kruskal–Wallis, p < 0.0001, Fig. 2). The CPUE of bull sharks was significantly lower (mean ± SD = 0.1 ± 0.37) than that of lemon (0.34 ± 0.67) and blacktip sharks (0.43 ± 0.67; p < 0.0001, Fig. 2a), whereas the CPUE between blacktip and lemon sharks was not significantly different (p = 0.06). Occurrence significantly differed among the three species (Chi-squared, p ≤ 0.0001, Fig. 2b), with the highest occurrence found for blacktip sharks (34% of sets), followed by lemon sharks (24%) and bull sharks (10%). Pairwise comparisons revealed significant differences between all species: lemon versus blacktip sharks (p < 0.05), blacktip versus bull sharks (p < 0.0001), and lemon versus bull sharks (p < 0.001). No correlations existed among the three species in CPUE or occurrence (Table 2). Thus, the abundance of one species did not appear to be correlated with the density of another species.
Bull sharks (n = 18) had the highest mean δ15N in whole blood (mean ± SD, 12.1 ± 1.2‰), followed by blacktip sharks (n = 40, 11.4 ± 0.6‰) and lemon sharks (n = 30, 10.2 ± 0.7‰; Table 1). The most enriched mean δ13C was found in lemon sharks (−12.6 ± 2.2‰), followed by blacktip sharks (−13.3 ± 1.2‰) and then bull sharks (−14.7 ± 2.8‰, Table 1). We also obtained means for both isotopes from fin samples for a subset of individuals and observed a smaller range in both δ15N and δ13C values among species compared to values from whole blood (Table 1). Blacktip sharks (n = 14) exhibited the highest mean fin δ15N (10.6 ± 0.7‰), followed by bull sharks (n = 9, 10.2 ± 0.7‰) and lemon (n = 21, 9.6 ± 1.0‰, Table 1). Lemon sharks displayed the most enriched mean fin δ13C (−11.2 ± 2.2‰), followed by blacktip (−12.5 ± 1.4‰) and bull sharks (−12.6 ± 2.2‰, Table 1).
Based on our pairwise comparisons using the mean distance to centroid (mdc) measure, isotopic niche width between all species pairs was significantly different (blacktip–bull: mdc = 1.63, p < 0.005; blacktip–lemon: mdc = 0.8, p < 0.01; bull–lemon: mdc = 0.82, p < 0.05; Table 3). Similarly, our pairwise comparisons using the Euclidean distance measure (md) revealed that isotopic niche space differed significantly between all shark species’ pairs (blacktip–bull: md = 1.18, p < 0.05; blacktip–lemon: md = 1.64, p < 0.005; bull–lemon: md = 2.81, p < 0.005; Table 3).
Our isotopic niche overlap measures showed that blacktip sharks’ SIBER ellipse overlapped almost completely with that of bull sharks (93.6%), though only 20.11% of the bull shark ellipse overlapped with that of blacktips. Lemon sharks’ SIBER ellipse overlapped very little with blacktip sharks (4.1%) and bull sharks (2.02%). Bull sharks had the highest total occupied isotopic niche area (Figs. 3, 4), more than twice that of blacktip sharks, as well as the highest SEAc values (Tables 4, 5; Fig. 4). Lemon and bull sharks had similar δ15N ranges, which were each more than twice as large as that of blacktip sharks. Blacktip sharks had the highest δ13C range of any of the three species investigated (Table 4).
We fitted first- and second-order polynomial equations to carbon and nitrogen isotopes from whole blood as a function of length for each species. There were no significant relationships between length and blood δ13C for blacktip sharks (F 2,39 = 1.76, p = 0.09, R 2 = 0.04) and lemon sharks (F 2,29 = 1.57, p = 0.23, R 2 = 0.10), nor among blood δ15N for both species (F 2,39 = 1.22, p = 0.31, R 2 = 0.06; F 2,29 = 1.70, p = 0.18, R 2 = 0.12,). There was no significant trend between bull shark length and blood δ13C (F 2,17 = 1.54, p = 0.25, R 2 = 0.17), but we did detect a significant quadratic relationship between bull shark length and blood δ15N (F 2,17 = 9.10, p < 0.01, R 2 = 0.55).
For the individuals for which there were paired blood and fin samples, we evaluated blood–fin relationships for both isotopes. We detected tight, significant relationships between blood and fin δ13C values for blacktip sharks (F 1,13 = 60.17, p < 0.0001, R 2 = 0.834, Fig. 5a) and bull sharks (F 1,8 = 13.65, p < 0.01, R 2 = 0.66, Fig. 5b), as well as for both species’ δ15N (F 1,13 = 18.25, p < 0.01, R 2 = 0.60; F 1,8 = 27.94, p < 0.01, R 2 = 0.80, respectively, Fig. 6a, b). Lemon sharks also showed similar patterns for both δ13C (F 1,20 = 85.85, p < 0.0001, R 2 = 0.82, Fig. 5c) and δ15N (F 1,20 = 46.24, p < 0.0001, R 2 = 0.71, Fig. 6c). When pooling all individuals from all species, we detected strong and significant relationships for δ13C (F 1,43 = 272.30, p < 0.001, R 2 = 0.87, Fig. 5d) and δ15N (F 1,43 = 247.40, p < 0.001, R 2 = 0.85, Fig. 6d). Within individuals, fin cartilage δ13C values were significantly more enriched than those from the blood (p < 0.05, Fig. 7), whereas there were no significant differences between δ15N values between the two tissues (Fig. 7).
Linear regressions showing the relationship between δ13C values between fin (relatively slower turnover rate) and whole blood (relatively faster turnover rate) as a means for estimating temporal diet stability among individuals for a blacktip sharks, b bull sharks, c lemon sharks, and d all sharks pooled together. To promote ease of access and comparability with other studies, the data plotted represent untransformed values
Linear regressions showing the relationship between δ15N values between fin (relatively slower turnover rate) and blood (relatively faster turnover rate) as a means for estimating temporal diet stability among individuals for a blacktip sharks, b bull sharks, c lemon sharks, and d all sharks pooled together. To promote ease of access and comparability with other studies, the data plotted represent untransformed values
Discussion
For the three shark species evaluated here, we found spatial overlap and isotopic niche overlap, and we detected evidence of temporal stability in isotopic niche. The presence of partially overlapping isotopic niches may, but does not necessarily, suggest competition for resources between these shark species—at the very least it fails to provide strong evidence of resource partitioning between those species (with the possible exception of lemon sharks). Additionally, we detected little differences in isotopic signatures between two tissues with different isotopic turnover rates, suggesting isotopic niche stability over time. Together, these results suggest that shark species which are found in the same location may compete for resources, but individuals within those species may have consistent patterns of resource usage over time.
Many strategies for alleviating competition and predation risk in co-occurring predatory species have been identified (Lima and Dill 1990), ranging from the shifting of habitat or timing of reproduction (Ghalambor and Martin 2000; Heithaus et al. 2006), to utilizing fat reserves or hoarding food and hiding (Lucas and Walter 1991), and altering foraging habits or resource preferences (Hawlena and Pérez-Mellado 2009). We tested for and found temporal isotopic niche stability in tissues, in a population of overlapping shark species. Given low competition due to available resources (Simpfendorfer and Milward 1993; the lack of non-overlapping isotopic niches in the present study), we interpret this finding as sharks being selective and thus exploiting the same preferred resources over the time scale in which we investigated.
Bull sharks in this study exhibited the highest mean δ15N value although this could be related to some transient inshore feeding or other factors not evaluated here. Whereas much of the blacktip total occupied niche area fell within that of lemon or bull sharks, bull sharks had a wider isotopic niche area that largely did not overlap with that of either other species (Table 3; Figs. 3, 4). Lemon sharks’ total occupied niche area overlapped with blacktip and bull sharks to a relatively intermediate degree. Our isotopic results are consistent with these findings based on stomach content analyses, suggesting that the three species investigated here are occupying approximately the same relative trophic level in the Everglades system. Based on previously published stomach content analysis, lemon and blacktip sharks are considered to be generalist teleost predators occupying a similar trophic level, with 92.9 and 88.9% of their standardized diet, respectively, composed of teleost prey (Cortés 1999). Also, based on previously published stomach contents, bull sharks are considered to occupy a similar, but slightly higher, trophic level (4.3, Cortés 1999) than both lemon and blacktip sharks (4.2, Cortés 1999), despite having a more variable diet, primarily composed of teleosts (52.3%) and chondrichthyans (sharks, rays, skates, chimaeras; 35.4%, Cortés 1999). Taken together, these findings suggest that bull sharks may exhibit wider and more diverse use of resources than either other species, including feeding on higher trophic level prey, results consistent with diet studies by Cortés (1999). Moreover, our comparison of isotopic signatures of whole blood and fin suggests that these patterns of resource use are consistent over a 8- to 18-month period (blood and fin tissue turnover times, MacNeil et al. 2005). Recent work evaluating energetics in large sharks via plasma lipids from the area found that blacktip and bull sharks had high concentrations of metabolic fuels such as free-fatty acids, with bull sharks showing the highest cholesterol of all sharks studied (Gallagher et al. 2017). The authors hypothesized this could be a result of feeding on high protein diets. Determining the actual resources used via additional non-lethal methods (i.e., stomach content analyses, energetic metabolism sensu Gallagher et al. 2017), combined with fine scale tracking studies could provide greater insights into this.
Information on the behavior and movements of the species in our study and other locations may help explain some of our findings. Telemetry studies of adult bull sharks off Florida have revealed high residency to coastlines with spatial exploitation of regional hot-spots (likely in pursuit of food, Hammerschlag et al. 2012), whereby individuals will then initiate coastal migrations (Graham et al. 2016). Acoustic tracking data from Australia and Southern Africa indicate that bull sharks also appear to range up and down regional coastlines and individuals will venture to adjoining continental shelves in accordance with summer months (Werry et al. 2012; Daly et al. 2014); however, this species is generally more restricted in their migratory movements than other large predatory sharks (i.e., tiger sharks). Juvenile bull sharks have high affinities and residency in coastal estuaries and river systems in the southeastern USA, which serve as nursery habitats for this species as well as for blacktip and lemon sharks (which show loosely similar movement patterns; Yeiser et al. 2008; Ortega et al. 2009; Heupel et al. 2003).
By comparing the range of carbon isotope signatures from our sharks to other species from Florida Bay (Chasar et al. 2005), it is possible lemon sharks could be primarily consuming small fishes inhabiting the seagrasses such as snapper (δ13C range from ~−11.0 to −13.0‰), whereas a slightly similar pattern of inshore feeding may be occurring in blacktips, albeit on slightly higher trophic level fishes such as jacks (δ13C range −12.0 to −13.0‰; Chasar et al. 2005). These inferences are entirely speculative since we did not study prey species (and we cannot comment on the legacy of the isotopes collected >10 years ago); however, they help put our findings into context. Our isotope signatures may be more a function of the production resources (e.g., mangrove vs. seagrass). Bull sharks exhibited the greatest range in isotopic niche breadth, with less enrichment in carbon suggesting possible greater use of offshore resources, possibly including more mobile prey such as small coastal sharks and larger fishes known to be consumed by this species (Cortés 1999). Large sharks do predate naturally on other smaller shark species (e.g., Springer 1961; Snelson et al. 1984), and the area of Florida Bay studied here has been previously found to contain the highest proportion of sharks year-round in the entire Florida Bay and Everglades ecosystem (Torres et al. 2006). Indeed, within this region, Torres et al. (2006) found a positive correlation between the abundance of teleost prey and the abundance of sharks, suggesting that the food web is dynamic over large spatial scales. During this study, we encountered instances of shark depredation on line-caught blacktip and bull sharks. Thus, it is reasonable to assume this is an area of high predation risk for the shark community.
In this study, we did not find significant effects of total length on δ13C or δ15N for any of our three study species with the exception of bull shark δ15N blood. One reason for this finding may be that the individuals we sampled in our study were generally all relatively larger (and thus older) than the size range over which many ontogenetic shifts in resource use may occur. For example, Shiffman et al. (2014) found an ontogenetic shift in isotopic niches between young-of-year and juvenile sandbar sharks (Carcharhinus plumbeus), but not a second shift between juvenile and adult individuals. Similarly, Fisk et al. (2002) found a significant effect of total length on δ15N values in Greenland sharks (Somniosus microcephalus), but their study encompassed a larger size range of individual sharks than the present study. Clearly, more data are needed to better understand the complexities of resource use among sympatric large sharks, and future work using stable isotopes could be integrated with acoustic telemetry, diet data, and spatial modeling to correlate foraging with movement (e.g., Papastamatiou et al. 2010; Daly et al. 2014; Papastamatiou et al. 2015).
We realize that, although stable isotope analysis can be a valuable and flexible method for studying aspects of food web interactions and habitat usage of elasmobranchs under certain scenarios, there are many limitations as they relate to our study (Hussey et al. 2012; Layman et al. 2012; Shiffman et al. 2012). Firstly, we did not specifically test for individual specialization; although we believe our comparison of two metabolically different tissues provided a valid comparison of patterns of resource use over time (albeit shorter time periods if we had used blood plasma). Also, if the isotopic signature of prey or producers at the base of the food web varies spatially or temporally (e.g., if there are slight unpredictable interannual fluctuations in the isotopic signature at the base of the food web in Florida Bay; Chasar et al. 2005), changes in the isotopic signature of a consumer could have also resulted due to temporal variation in producer isotopic signature rather than distinct patterns of resource usage (e.g., Vizzini and Mazzola 2003). We also realize that while non-overlapping isotopic niches would likely suggest strong resource partitioning between species, overlapping isotopic niches (as detected here) do not necessarily suggest shared resource usage or competition (reviewed in Layman et al. 2012). Thus, due to the spatial scale of our study (despite the restricted home range of the species evaluated here), we cannot extrapolate these findings to the greater ecosystem, as these findings may occur at the local level (i.e., Torres et al. 2006). Many isotopic models require the use of an accurate diet-tissue discrimination factor, which have not been calculated for many elasmobranchs to date (Hussey et al. 2010). A number of factors beyond diet or resource use could lead to variability in isotopic signatures, such as urea and lipids, which were not removed from our samples here but could still be present in blood and cartilage tissue, and could still skew the δ13C values (Hussey et al. 2011). Different storage and sample preparation techniques can also affect the isotopic signature of some elasmobranch tissues (Kim and Koch 2012). Stable isotope analyses also only present inferred averaged resource use, and complementary data, such as movement or stomach content analysis, can provide additional insights that can also be used to validate or obtain more insights from results of stable isotope analysis. We do not know the degree of movement that our coastal sharks exhibited during the study, although recent work on bull shark movements in this area suggested that these animals generally remain within the sampling area throughout the year (Graham et al. 2016).
Conclusions
Understanding how large consumers utilize important inshore ecosystems can provide key insights into how to best manage and conserve these areas. Our study provides new insights into how individuals of large, co-occurring marine predators are able to coexist in localized, prey-rich systems. Specifically, we found that three species of sympatric, large sharks, likely did not partition the resources available to them but instead they may have been selective in their prey over time. Our work adds to the growing body of work on resource use and partitioning among sharks using isotopic data (e.g., Abrantes and Barnett 2011; Matich et al. 2011; Speed et al. 2012; Kiszka et al. 2015). This type of information collected for multiple species has added value since the ecological roles of sharks as top predators are currently debated, largely due to insufficient data (Frisch et al. 2016; Roff et al. 2016). When prey and good habitat are not limiting, top predators (even those of different trophic levels) can be diverse, abundant, and can co-occur and lead to stable resource use, a finding that has implications for continued sound management and protection of ecologically important environments.
References
Abrantes KG, Barnett A (2011) Intrapopulation variations in diet and habitat use in a marine apex predator, the broadnose sevengill shark Notorynchus cepedianus. Mar Ecol Prog Ser 442:133–148
Barnett A, Semmens JM (2012) Sequential movement into coastal habitats and high spatial overlap of predator and prey suggest high predation pressure in protected areas. Oikos 121:882–890
Baum JK, Myers RA, Kehler DG, Worm B, Harley SJ, Doherty PA (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299:389–392
Beaudoin CP, Tonn WM, Prepas EE, Wassenaar LI (1999) Individual specialization and trophic adaptability of northern pike (Esox lucius): an isotope and dietary analysis. Oecologia 120:386–396
Byrnes J, Stachowicz JJ, Hultgren KM, Hughes RA, Olyarnik SV, Thornber CS (2006) Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol Lett 9:61–71
Chasar LC, Chanton JP, Koenig CC, Coleman FC (2005) Evaluating the effect of environmental disturbance on the trophic structure of Florida Bay, USA: multiple stable isotope analyses of contemporary and historical specimens. Limnol Oceanogr 50:1059
Cortés E (1999) Standardized diet compositions and trophic levels of sharks. ICES J Mar Sci 56:707–717
Costa DP (1993) The relationship between reproductive and foraging energetics and the evolution of the Pinnipedia. Symp Zool Soc Lond 66:293–314
Creel S, Christianson D (2008) Relationships between direct predation and risk effects. Trends Ecol Evol 23:194–201
Daly R, Smale MJ, Cowley PD, Froneman PW (2014) Residency patterns and migration dynamics of adult bull sharks (Carcharhinus leucas) on the east coast of southern Africa. PloS one 9:e109357
Dulvy NK et al (2014) Extinction risk and conservation of the world’s sharks and rays. eLife 3:ee590
Estes JA, Tinker MT, Williams TM, Doak DF (1998) Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282:473–476
Fisk AT, Tittlemier SA, Pranschke JL, Norstrom RJ (2002) Using anthropogenic contaminants and stable isotopes to assess the feeding ecology of Greenland sharks. Ecology 83:2162–2172
Frederiksen M, Edwards M, Richardson AJ, Halliday NC, Wanless S (2006) From plankton to top predators: bottom–up control of a marine food web across four trophic levels. J Anim Ecol 75:1259–1268
Frisch AJ et al (2016) Reassessing the trophic role of reef sharks as apex predators on coral reefs. Coral Reefs 35:459–472
Gallagher AJ, Kyne PM, Hammerschlag N (2012) Ecological risk assessment and its application to elasmobranch conservation and management. J Fish Biol 80:1727–1748
Gallagher AJ, Serafy JE, Cooke SJ, Hammerschlag N (2014) Physiological stress response, reflex impairment, and survival of five sympatric shark species following experimental capture and release. Mar Ecol Prog Ser 496:207–218
Gallagher AJ, Skubel RA, Pethybridge HR, Hammerschlag N (2017) Energy metabolism in mobile, wild-sampled sharks inferred by plasma lipids. Conserv Physiol 5(1):cox002
Ghalambor CK, Martin TE (2000) Parental investment strategies in two species of nuthatch vary with stage-specific predation risk and reproductive effort. Anim Behav 60:263–267
Graham F, Rynne P, Estevanez M, Luo J, Ault JS, Hammerschlag N (2016) Use of marine protected areas and exclusive economic zones in the subtropical western North Atlantic Ocean by large highly mobile sharks. Div Distrib 22:534–546
Hammerschlag N, Serafy JE (2010) Nocturnal fish utilization of a subtropical mangrove–seagrass ecotone. Mar Ecol Prog Ser 31:364–374
Hammerschlag N, Luo J, Irschick DJ, Ault JS (2012) A comparison of spatial and movement patterns between sympatric predators: bull sharks (Carcharhinus leucas) and Atlantic tarpon (Megalops atlanticus). PLoS ONE 7:e45958
Hammerschlag-Peyer CM, Yeager LA, Araújo MS, Layman CA (2011) A hypothesis-testing framework for studies investigating ontogenetic niche shifts using stable isotope ratios. PLoS ONE 6:e27104
Hawlena D, Pérez-Mellado V (2009) Change your diet or die: predator-induced shifts in insectivorous lizard feeding ecology. Oecologia 161:411–419
Heithaus MR, Hamilton IM, Wirsing AJ, Dill LM (2006) Validation of a randomization procedure to assess animal habitat preferences: microhabitat use of tiger sharks in a seagrass ecosystem. J Anim Ecol 75:666–676
Heupel MR, Simpfendorfer CA, Hueter RE (2003) Running before the storm: blacktip sharks respond to falling barometric pressure associated with Tropical Storm Gabrielle. J Fish Biol 63:1357–1363
Hoffmayer ER, Parsons GR (2003) Food habits of three shark species from the Mississippi Sound in the northern Gulf of Mexico. Southeast Nat 2:271–280
Hussey NE, MacNeil MA, Fisk AT (2010) The requirement for accurate diet-tissue discrimination factors for interpreting stable isotopes in sharks. Hydrobiologia 654:1–5
Hussey NE, Dudley SF, McCarthy ID, Cliff G, Fisk AT (2011) Stable isotope profiles of large marine predators: viable indicators of trophic position, diet, and movement in sharks? Can J Fish Aquat Sci 68:2029–2045
Hussey NE et al (2012) Stable isotopes and elasmobranchs: tissue types, methods, applications and assumptions. J Fish Biol 80:1449–1484
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J Ani Ecol 80:595–602
Kim SL, Koch PL (2012) Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ Biol Fish 95:53–63
Kinney MJ, Hussey NE, Fisk AT, Tobin AJ, Simpfendorfer CA (2011) Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Mar Ecol Prog Ser 439:263–276
Kiszka JJ, Aubail A, Hussey NE, Heithaus MR, Caurant F, Bustamante P (2015) Plasticity of trophic interactions among sharks from the oceanic south-western Indian Ocean revealed by stable isotopeand mercury analyses. Deep Sea Res Part I Oceanogr Res Pap 96:49–58
Knudsen R, Siwertsson A, Adams CE, Garduño-Paz M, Newton J, Amundsen PA (2011) Temporal stability of niche use exposes sympatric Arctic charr to alternative selection pressures. Evol Ecol 25:589–604
Layman CA, Arrington DA, Montaña CG, Post DM (2007) Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88:42–48
Layman CA et al (2012) Applying stable isotopes to examine food web structure: an overview of analytical tools. Biol Rev 87:542–562
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Lucas JR, Walter LR (1991) When should chickadees hoard food? Theory and experimental results. Anim Behav 41:579–601
MacNeil MA, Skomal GB, Fisk AT (2005) Stable isotopes from multiple tissues reveal diet switching in sharks. Mar Ecol Prog Ser 302:199–206
Madin EM et al (2010) Field evidence for pervasive indirect effects of fishing on prey foraging behavior. Ecology 91:3563–3571
Matassa CM, Trussell GC (2011) Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92:2258–2266
Matich P, Heithaus MR, Layman CA (2011) Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J Anim Ecol 80:294–305
Newman SP, Handy RD, Gruber SH (2009) Diet and prey preference of juvenile lemon sharks Negaprion brevirostris. Mar Ecol Prog Ser 398:221–234. doi:10.3354/meps08334
Ortega LA, Heupel MR, Van Beynen P, Motta PJ (2009) Movement patterns and water quality preferences of juvenile bull sharks (Carcharhinus leucas) in a Florida estuary. Environ Biol Fish 84:361–373
Papastamatiou YP, Wetherbee BM, Lowe CG, Crow GL (2006) Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitive exclusion. Mar Ecol Prog Ser 320:239–251
Papastamatiou YP, Friedlander AM, Caselle JE, Lowe CG (2010) Long-term movement patterns and trophic ecology of blacktip reef sharks (Carcharhinus melanopterus) at Palmyra Atoll. J Exp Mar Biol Ecol 386:94–102
Papastamatiou YP, Meyer CG, Kosaki RK, Wallsgrove NJ, Popp BN (2015) Movements and foraging of predators associated with mesophotic coral reefs and their potential for linking ecological habitats. Mar Ecol Prog Ser 521:155–170
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18:293–320
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Roff G et al (2016) The ecological role of sharks on coral reefs. Trends Ecol Evol 31:395–407
Shiffman DS, Gallagher AJ, Boyle MD, Hammerschlag-Peyer CM, Hammerschlag N (2012) Stable isotope analysis as a tool for elasmobranch conservation research: a primer for non-specialists. Mar Fresh Res 63:635–643
Shiffman DS, Frazier B, Kucklick J, Abel D, Brandes J, Sancho G (2014) Feeding ecology of the sandbar shark (Carcharhinus plumbeus) in South Carolina estuaries revealed through δ13C and δ15N stable isotope analysis. Mar Coast Fish 6:156–169
Silliman BR, Bertness MD (2002) A trophic cascade regulates salt marsh primary production. Proc Nat Acad Sci 99:10500–10505
Simpfendorfer CA, Milward NE (1993) Utilisation of a tropical bay as a nursery area by sharks of the families Carcharhinidae and Sphyrnidae. Environ Biol Fish 37:337–345
Snelson FF, Mulligan TJ, Williams SE (1984) Food habits, occurrence, and population structure of the bull shark, Carcharhinus leucas, in Florida coastal lagoons. Bull Mar Sci 34:71–80
Speed CW, Meekan MG, Field IC, McMahon CR, Abrantes K, Bradshaw CJA (2012) Trophic ecology of reef sharks determined using stable isotopes and telemetry. Coral Reefs 31:357–367
Springer S (1961) Dynamics of the feeding mechanism of large galeoid sharks. Am Zool 1:183–185
Torres LG, Heithaus MR, Delius B (2006) Influence of teleost abundance on the distribution and abundance of sharks in Florida Bay, USA. Hydrobiologia 569:449–455
Turner TF, Collyer ML, Krabbenhoft TJ (2010) A general hypothesis-testing framework for stable isotope ratios in ecological studies. Ecology 91:2227–2233
Vaudo JJ, Heithaus MR (2012) Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system. Mar Fresh Res 63:1077–1084
Vizzini S, Mazzola A (2003) Seasonal variations in the stable carbon and nitrogen isotope ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Mar Biol 142:1009–1018
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100
Werry JM, Lee SY, Lemckert CJ, Otway NM (2012) Natural or artificial? Habitat-use by the bull shark, Carcharhinus leucas. PLoS ONE 7:e49796
White WT, Potter IC (2004) Habitat partitioning among four elasmobranch species in nearshore, shallow waters of a subtropical embayment in Western Australia. Mar Biol 145:1023–1032
Wiley TR, Simpfendorfer CA (2007) The ecology of elasmobranchs occurring in the Everglades National Park, Florida: implications for conservation and management. Bull Mar Sci 80:171–189
Yeiser BG, Heupel MR, Simpfendorfer CA (2008) Occurrence, home range and movement patterns of juvenile bull (Carcharhinus leucas) and lemon (Negaprion brevirostris) sharks within a Florida estuary. Mar Fresh Res 59:489–501
Acknowledgements
We are grateful for the field support provided by D. Lazarre, V. Ansaldi, C. Shepard, as well as the rest of the interns and staff from the Shark Research and Conservation Program at the University of Miami. We also thank L. Sternburg for his assistance during laboratory assays. We thank C. Rohner and P. Prebble for their constructive comments on this submission. This work was supported by the Batchelor Foundation Inc., the Disney Conservation Fund, and the University of Miami Citizen’s Board. All of the work here conformed to the legal requirements in the USA and was permitted and conducted under the Everglades National Park Permit (EVER-2011-SCI-0012), and the University of Miami Institutional Animal Care and Use Committee (Protocol # 09–187).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest, and all authors gave consent.
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
About this article
Cite this article
Gallagher, A.J., Shiffman, D.S., Byrnes, E.E. et al. Patterns of resource use and isotopic niche overlap among three species of sharks occurring within a protected subtropical estuary. Aquat Ecol 51, 435–448 (2017). https://doi.org/10.1007/s10452-017-9627-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9627-2