Abstract
Although diatoms are important bioindicators of ecological quality, their ecological traits are still not well understood. A major issue is that of substrate preferences, which may result in differences in production, and assemblage structure and composition, and which should therefore be taken into account for ecological quality assessment studies. Thus, in this work, the periphyton grown on sand and ceramic tiles in indoor controlled channels were compared to understand whether substrate differences lead to differences in: periphyton production (chlorophyll-a), chlorophyll-b and c concentrations, diatom assemblages (diversity-Shannon-Wiener, cell density, taxonomic composition, trait proportions), and ecological quality assessments (IPS-‘Indice de Polluosensibilité Spécifique’). A combined inoculum of periphyton from four Portuguese streams was introduced to the running channels (six sand and six tile) and left to colonize for 35 days. Epilithic (tiles) and epipsammic (sand) assemblages were sampled at days 14 and 35. We verified that there were no differences in chlorophyll-a concentration over time and between substrates. On both sampling occasions, the epipsammic assemblages had higher concentration of chlorophyll-c and diatom density but without significant differences over time in each substrate. The taxonomic composition was different between substrates and over time. However, these differences were not reflected in ecological quality assessment. The diversity was also similar between substrates in both sampling occasions, but it was higher at day 14. Mobile and stalked species were more abundant over the entire study and differed significantly between substrates, with the epipsammic assemblages presenting higher abundances of both traits. We concluded that the colonizing substrate influences diatom assemblages but not the ecological quality assessment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Streams are continuously affected by erosion and deposition processes, which along with lithology, slope, current, degree of disturbance, and distance from headwaters result in different sediment sizes (Cattaneo et al. 1997; Rolland et al. 1997). Therefore, there are streams where rocks dominate (large stable substrates), streams that within the same site have large stable and small unstable substrates (e.g. sand), and even streams with fine unstable substrates only (in particular lowland sites). Substrate type, texture, roughness, and stability/instability (granulometry) are relevant habitat criteria for the interactions between benthic algae and their substrates (Cattaneo et al. 1997; Hunt and Parry 1998; Janauer and Dokulil 2006; Bergey and Cooper 2015). Substrate size can affect the abundance and composition of the attached algae by providing different degrees of stability to colonizing organisms (Cattaneo et al. 1997). Indeed, the substrate size can be an important environmental disturbance as it may increase the probability of damage or destruction of the biomass of the colonizing organisms (Grime 1973). Several studies have addressed the distinct associations of periphyton on different substrates (e.g. rock surface, upper layer of sediment, or aquatic plants) through their biovolume, diversity, algal assemblages, and chlorophyll concentration (e.g. Cattaneo et al. 1997; Rolland et al. 1997; Sabater et al. 1998; Potapova and Charles 2005).
Among algae, diatoms have been selected in most European countries as representative of periphyton (Almeida and Feio 2012; Kelly et al. 2012; Feio et al. 2014) due to their good performance as ecological quality indicators (Lowe and Pan 1996; Kelly et al. 1998; Stevenson and Pan 1999). From all types of substrates found in rivers, most studies focus on epilithon (Winter and Duthie 2000), as hard surfaces are the preferred substrates used in ecological quality assessment (Kelly et al. 1998). However, other substrates like submerged macrophytes and sediments are also commonly found and may be dominant in some stream and river sections (Kelly et al. 1998; Elias et al. 2015). Some studies indicate that benthic diatom species present different biological characteristics that enable them to adapt to specific micro-habitats (Krejci and Lowe 1986; Soininen and Eloranta 2004). If these natural inter-substrate differences are reflected in ecological quality assessment metrics, this can potentially mask responses of algal assemblages to stresses associated with human activities and may interfere with ecological quality assessments based on the knowledge of these responses (Winter and Duthie 2000; Potapova and Charles 2005; Bere and Tundisi 2011; Mendes et al. 2012). However, some studies have tested the effect of different substrates in ecological quality assessments without finding significant differences (Kitner and Poulíčková 2003; Potapova and Charles 2005; Mendes et al. 2012). These studies were conducted under natural conditions where the assemblages are shaped simultaneously by many environmental factors which may lead to confounding effects (Stevenson and Pan 1999). In addition, the substrate effect is difficult to detect in large-scale, coarse resolution studies, when the role of other factors such as inter-stream differences in hydrology, physical habitat, and chemistry becomes more important than the role of substrate (Potapova and Charles 2005). Even when different diatom assemblages of the same river are compared and differences are verified (Cetin 2008), it is difficult to ensure that the assemblages have been exposed to the same environmental variations at the same time or even at the same developmental phase. Additionally, it is impossible to avoid contamination between substrates with diatoms migrating between habitats in wadeable streams.
Therefore, the present work was conducted ex situ under controlled experimental conditions to investigate: (1) whether algae assemblages establishing on new hard (ceramic tiles) and soft (sand) substrates become significantly different concerning chlorophyll-a (chl-a), b (chl-b), and c (chl-c) concentrations, diversity (Shannon-Wiener, H′), density (cells cm−2), taxonomic composition, and trait proportions; (2) how they evolve over time (up to five weeks of colonization) on the different substrates; and (3) whether differences in diatom assemblages on the different substrates result in differences in ecological quality assessment. The ecological quality of the channels was assessed by using a common autoecological diatom method which is also the Portuguese official index, the ‘Indice de Polluosensibilité Spécifique’ (IPS) for monitoring programs, in the context of the Water Framework Directive (INAG 2009).
Methods
Experimental system description
The experimental system comprised twelve modular mesocosm systems (MMS). Each MMS was composed of one poly(methyl methacrylate) (PMMA, 8 mm thick) mesocosm channel (150 cm long, 10 cm wide, and 12 cm high) with a maximum functional volume of approximately 18 L, connected to a PMMA (8 mm thick) water reservoir (60 cm long, 10 cm wide, and 45 cm high), operating with a maximum functional water volume of approximately 27 L (Fig. 1).
The MMS was operated as a recirculated system. Water in the reservoir was pumped through a 25-mm PVC (polyvinyl chloride) inlet pipe system, which allowed flow direction regulation in the mesocosm channel, by a submerged water pump (EHEIM compact + 3000, Germany) that can operate with a regulated water flow from 200 to 2000 L h−1. The water outlet pipe system, from the mesocosm channel to the reservoir, was built with 50 mm PVC pipe, with an adjustable damper placed close to the end of the mesocosm channel, which allowed the regulation of water level.
Each mesocosm channel was illuminated from above with T5 HO 80 W tubular fluorescent lamps, Lumilux—8000 K (Osram, Germany). The distance from the illumination system and the water surface was adjustable in order to control the photosynthetic active radiation (PAR).
Experimental set-up
At the beginning of the experiment, 25 L of tap water was fed to the water deposit of each channel, after passing through 5-µm wound polypropylene and active carbon (AC) filters to remove suspended particulate matter and free chlorine, respectively. The water depth in mesocosm tanks was kept at 5 cm. Water velocity was maintained at approximately 0.05 m s−1. The illumination systems were positioned at about 1 m above the channels to provide light to the attached algae (≈200 µmol m−2 s−1) with a 12-h:12-h light–dark cycle. Water temperature was measured by K-type thermocouples (Testo 176T4 data logger) and maintained within 17 and 20 °C during the experiment. This was possible with the help of an air conditioning system and a well-isolated experimental room that was able to minimize air temperature variations.
The bottom of all mesocosm channels (n = 12) was covered with 62 unglazed ceramic tiles-T (40 × 50 mm in size). In 6 of the channels (n = 6), the tiles were completely covered with about 1 cm depth of sand bed-S (98% SiO2, 2-mm particles). A two-week preliminary study revealed that the tiles did not influence the chemical composition of the circulating water. However, in order to exclude any previously undetected differences due to the tile chemical composition, and as the sand is almost inert (SiO2), the tiles were kept under the sand bed in the sand channels. Ceramic tiles were used in replacement of natural substrate due to the necessity of having a substrate with a known area and comparable conditions across channels. The use of artificial substrates (e.g. clay tiles, acrylic, and glass) for the study of diatom communities is common (Lowe and Gale 1980; Biggs 1990; Lane et al. 2003; Ndiritu et al. 2006; Dalu et al. 2014a, b).
Biofilm samples were collected from four streams in the Portuguese littoral region and mixed in 1-L bottle to be used as inoculum in the channels. These biofilms included epilithic and epipsammic assemblages. Each channel (n = 12) was seeded with 60 mL inoculum at the beginning of the experiment. Thereafter, 10 L (40%) of water was removed from each channel on a weekly basis and immediately replaced by 10 L of new filtered tap water. The total duration of the experiment was 35 days (d35). The streams were selected to obtain a diverse inoculum community representative of the different streams found in the central western Portuguese region (Atlantic-temperate climate). This includes a variety of streams with streambeds ranging from rocks to sand, and with a variety of human pressures (from least to highly disturbed) (Feio et al. 2010; Elias et al. 2015).
Water, periphyton sampling, and treatment
The inoculum was left to colonize the substrates in the channels for an initial period of seven days, after which water replacement and sampling was initiated. Water samples from the channels and from the tap were collected weekly (d0, d7, d14, d21, d28 and d35) for determination of alkalinity (mg HCO3 − L−1), nitrate (mg NO3 − L−1), silica (mg Si L−1), chloride (mg Cl− L−1), phosphate (mg PO4 3− L−1), and sulphate (mg SO4 2− L−1). The analysis of nitrate, chloride, phosphate, and sulphates was carried out on a Dionex 2000i/SP Ion Chromatograph with conductivity detector. Anions were separated on a AS4A SC (25 cm × 4 mm I.D) with an AG4A-SC ground column 4 mm I.D and were detected by suppressed conductivity detector using an anion micro-membrane AMMS-I with regenerant of 25 mN sulphuric acid. The injection volume was 10 μL, and the flow rate was 2.0 mL min−1. Soluble silica was determined by UV–Vis spectrophotometry using the reduced molibdosilicic acid (EPA 370.1 Method), and alkalinity was determined by titration with HSO4 (0.1 N). Several (from one to every two days) in situ measurements of pH, conductivity (µS cm−1), and percentage of total dissolved oxygen were taken using a Multiparameter Probe 3430 WTW throughout the experiment. Periphyton samples were randomly retrieved from each channel on two sampling occasions: day 14 (d14) and day 35 (d35). For a given sampling occasion, periphyton samples were taken from the same position in each channel; however, the sampling position varied with the sampling occasion and was always done downstream to upstream to minimize disturbance effects. For each sampling occasion, four subsamples (four tiles) were collected along the channel in order to embrace all the possible assemblages variability that can exist within the channel. Afterwards, these subsamples were all merged in just one sample per channel in each sampling occasion.
For the epilithic biofilm (tiles), the upper surface of 4 tiles (80.0 cm2 per channel) was scraped with a toothbrush and washed with distilled water into a flask. In epipsammon biofilm (sand) sampling, an area of 57.8 cm2 of the upper surface of the sand bed was collected into a flask using a syringe. Two distinct samples of each channel were collected at each sampling occasion, one for diatom analysis and another for chlorophyll determination (chl-a, chl-b, and chl-c). The area contained in the volume treated was always assured so that the results obtained could be expressed per unit area of substrate.
For the determination of chlorophylls, the total area sampled (80.0 cm2 and 57.8 cm2 for epilithic and epipsammon, respectively) of each channel was used. Following Branco et al. (2010), the samples were repeatedly centrifuged (2000 rpm) for five minutes until all the water was removed and only a pellet remained. Pigments were extracted with 2 mL of acetone (90%v) from the pellet; the extract was protected from light and maintained in the cold. To break the cells, samples on ice were sonicated in 4 cycles of 15 s. The extract was stirred for 30 min in a refrigerated environment and then centrifuged at 4000 rpm for 10 min at 4 °C. Chlorophylls-a, b, and c were quantified spectrophotometrically, and its concentration (in µg cm−2) calculated following the procedure of Jeffrey and Humphrey (1975). Chlorophyll-a is used as an indicator of primary production as its found in all algae and in Cyanobacteria, chl-c is used as indicator of the presence of diatoms, as its one of its major pigment, and chl-b will give us an indication of the biomass from other taxonomic algal groups such as Chlorophyta and Euglenophyta.
For diatom assemblage analysis, a subsample of the total area sampled was oxidized with concentrated nitric acid and potassium dichromate for about 24 h at room temperature. The remaining sample was preserved with formaldehyde (5–10% final concentration). Thereafter, a known volume of the oxidized sample was deposited on a coverslip and allowed to dry at room temperature. Permanent slides were mounted using Naphrax®. Using a light microscope (100 × objective and 1.32 numerical aperture), all the unbroken diatom valves for each sample were counted and identified to species or infra-specific rank mainly using Krammer and Lange-Bertalot’s floras (1986; 1988; 1991a, b) and Krammer (2000; 2001; 2009). From those, diatom cell density was determined and extrapolated to the unit area (cm2) of each sample; species diversity (H′) and assemblage analysis were also derived. The diatom index IPS (Coste in CEMAGREF 1982) was also calculated with the OMINIDIA software (Lecointe et al. 1993).
Permanent slides, with the inoculum of biofilm introduced in the channels, were also mounted, and up to 400 diatom valves counted according to the aforementioned description.
Prior to oxidation, microscopic identification of all the samples (from the channels and inoculum) was also carried out to verify whether other groups of photosynthetic organisms besides diatoms were present and dominating in the samples.
Selection and calculation of biological traits
The biological characteristics that enable species to adapt more easily to specific substrates (here tile vs sand) are the capacity to resist dislodgement caused to substrates by the stream current and the capacity of moving vertically into or out of the sand depending on water velocity. Thus, variations in the diatom biological trait life form were also investigated in this study by analysing changes in proportions of categories that have high potential to distinguish epipsammic and epilithic diatom assemblages: mobile, planktonic, adnate, and pad (Berthon et al. 2011; Rimet and Bouchez 2011, 2012).
Each species was assigned to all the trait categories that it could display, according to Rimet and Bouchez (2012). For each sample, the total number of valves of all the species presenting a given trait category was counted. To obtain the total number of valves of each trait category in each channel, the average of all the samples from the same treatment was calculated.
Data analysis
A completely randomized design was used in the experiment. Statistical differences in chlorophyll concentrations, H′, diatom cell density, IPS, and biological diatom traits resulting from treatment effects were tested with the univariate analysis of variance (equivalent to one-way models) PERMANOVA global tests with unrestricted permutations (Euclidean dissimilarity measure). Additionally, PERMANOVA pairwise tests with unrestricted permutations were performed to assess possible differences between sampling occasions (d14 and d35) and substrates (S and T). These two sampling occasions were selected as we consider that at the 7th day differences between substrates were still not evident, and at least one month has been recommended for sampling the equivalent of the ‘mature’ community occurring on natural substrates in the stream (Oemke and Burton 1986; Kelly et al. 1998).
To determine whether the treatments lead to differences in the diatom assemblages, a multidimensional scaling analyses, MDS (Bray–Curtis dissimilarity measure; data square-root-transformed), was performed. The statistical differences resulting from the treatment effects were tested with a permutational multivariate analysis of variance (MANOVA equivalent) (PERMANOVA) global tests (unrestricted permutations). PERMANOVA pairwise tests (unrestricted permutations) were performed to assess at which sampling occasions the treatments (two substrates) were different and whether the differences across treatments were consistent over time. SIMPER analysis (data square-root-transformed, Bray–Curtis similarity) was used to determine the most representative taxa (those contributing the most to the average similarity within groups) of the different treatments and sampling occasions. In addition, SIMPER analysis (presence/absence data, Bray–Curtis similarity) was used to determine the species that were present in a given substrate and absent from the other at the same sampling occasion and that contributed more to the group average dissimilarity.
All the data analyses were performed with PRIMER 6 & PERMANOVA software (PRIMER-E Ltd, Plymouth, UK).
Results
Physical and chemical parameters
One of the tile channels was eliminated from the analysis due to difficulties in maintaining the water temperature constant. Therefore, the results presented are from six channels with sand and five with tiles.
The water used throughout the experiment showed similar physical and chemical characteristics in both treatments (see Table 1). The nutrient and conductivity values were relatively high for tap water, e.g. the mean concentration of nitrate found in the channels was 19.5 mg NO3 − L−1 (Table 1).
Chlorophylls
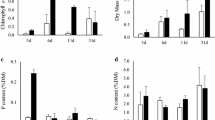
The total periphyton biomass, measured as the ubiquitous pigment chlorophyll-a (chl-a), did not vary over time (d14 vs d35) nor between substrates (T vs S) (pseudo-F = 3.10, p(perm) > 0.05) (Fig. 2a). The mean highest chl-a concentration (0.134 µg cm−2) was obtained on sand at day 35, while the mean lowest concentration (0.075 µg cm−2) was observed on tile at day 14. Chl-c concentration showed statistical differences between substrates. In both sampling occasions, the epipsammic assemblages presented a significantly higher concentration of chl-c (Td14 vs Sd14 t = 2.80, p(MC) = 0.02; Td35 vs Sd35 t = 2.80, p(MC) = 0.03) than the epilithic assemblages. The mean highest chl-c value (0.025 µg cm−2) was obtained on sand at d35, while the mean lowest concentration (0.007 µg cm−2) was obtained on tile at both d14 and d35. This variable did not vary significantly along time for a given substrate (Td14 vs Td35 t = 0.18, p(MC) = 0.92; Sd14 vs Sd35 t = 1.93, p(MC) = 0.08) (Fig. 2b).
a Chlorophyll-a (Chl-a), b chlorophyll-c (Chl-c) and c chlorophyll-b (Chl-b) concentrations (mean ± standard deviation) of the algal assemblages developing on tiles (T) and sand (S) substrates at days 14 and 35 in the artificial channels. Treatment means labelled with the same letter (a, b, c) do not significantly differ (p > 0.05; PERMANOVA pairwise test)
The chl-b concentration values were inferior to the values of the other chlorophylls. The mean highest chl-b concentration (0.009 µg cm−2) was obtained on the sand at d14. Chl-b only varied along time on the epipsammic assemblages (Td14 vs Td35 t = 2.36, p(MC) = 0.05; Sd14 vs Sd35 t = 8.84, p(MC) < 0.01) (Fig. 2c). Considering sampling occasions, the epilithic assemblages never presented significantly higher chl-b concentrations (Td14 vs Sd14 t = 1.90, p(MC) = 0.12; Td35 vs Sd35 t = 2.77, p(MC) = 0.08) (Fig. 2c).
Periphyton assemblages
The microscopic analyses of the unoxidized samples revealed a clear dominance of diatoms on the periphyton assemblages. The other two groups from which we identified more individuals were Chlorophyta (e.g. Scenedesmus, Ankistrodesmus, Coelastrum and Monoraphidium) and Cyanobacteria (e.g. Chroococcus).
The analysis of the inoculum sample used to seed the channels revealed that the most abundant species were Staurosira venter (Ehrenberg) Cleve & J. D. Möeller, Achnanthidium minutissimum (Kützing) Czarnecki, and Asterionella formosa Hassall even though they never exceeded 10% abundance.
In total, 174 diatom species were identified during the counting of all the samples collected from the channels. The corresponding total number of valves counted ranged from 568 to 2586. The epipsammic diatom assemblages presented higher cell density in both sampling occasions when compared to the epilithic assemblages (Td14 vs Sd14 t = 2.59, p(perm) = 0.03; Td35 vs Sd35 t = 2.39, p(perm) = 0.04); however, this variable was not different over time within each substrate (Td14 vs Td35 t = 1.44, p(perm) = 0.23; Sd14 vs Sd35 t = 1.76, p(perm) = 0.11) (Fig. 3).
In terms of diatom taxonomic composition, we verified a segregation regarding both the sampling occasion (Td14 vs Td35 t = 1.74, p(perm) < 0.01; Sd14 vs Sd35 t = 1.68, p(perm) < 0.01) and substrates (Td14 vs Sd14 t = 1.68, p(perm) < 0.01; Td35 vs Sd35 t = 1.88, p(perm) < 0.01) (Fig. 4). For both substrates, the MDS and SIMPER analysis revealed that the assemblages of different channels at day 35 were less similar to each other than at day 14 (within-group average similarity: T14 = 68.8%, S14 = 71.6%, T35 = 66.6%, S35 = 69.5%) (Fig. 4).
Species analysis (Table 2) showed that the species that contributed more to the within-group average similarity in both sampling occasions and substrates were the same but with different contributing percentages to in-group similarity: Achnanthidium minutissimum (13.6–23.9%), Fragilaria cf. parva (Grunow) A. Tuji & D. M. Williams (6.0–11.6%), and Navicula notha Wallace (5.1–7.1%) (Table 2). The higher contribution of these species to the average similarity was verified at day 35 on both substrates. All these three species were also found in the inoculum: A. minutissimum presented an abundance of 9%, the species F. cf. parva and N. notha presented abundances of 2 and 6%, respectively.
The differences between the substrates at the same sampling occasion were due to less abundant taxa and to presence or absence of certain taxa. Comparing both substrates at day 14, the epipsammic assemblages presented less species of the genus Navicula contributing to the group similarity (Table 2) and a smaller number of species that were only present in the sand assemblages (7 species vs 18 in the tile) (Table 3). Species such as Adlafia minuscula var. muralis (Grunow) Lange-Bertalot, Surirella linearis W. Smith, and Nitzschia sociabilis Hustedt were only found in the epipsammic assemblages, while species such as Hippodonta capitata (Ehrenberg) Lange-Bertalot, Metzeltin & Witkowski, Reimeria sinuata (Gregory) Kociolek & Stoermer, and Navicula lanceolata Ehrenberg were only present in the epilithic assemblages (Table 3). However, the diversity was similar between substrates (Td14 vs Sd14 t = 1.47, p(perm) = 0.16) with mean values of 2.73 and 2.51, for epilithic and epipsammic assemblages, respectively (Fig. 5). At day 35, the species belonging to the genus Nitzschia contributed more to the sand group average similarity (Table 2). Once more, the epipsammic assemblages presented a smaller number of species that were only present in the sand assemblages and that contributed to the group average dissimilarity (3 species vs 8 in the tile) (Table 3). The species Diadesmis confervacea Kützing, Gomphonema cf. affine Kützing, and Nitzschia acicularis (Kützing) W. Smith were only found in the epipsammic assemblages, while species such as Nitzschia fonticola (Grunow) Grunow, Tryblionella hungarica (Grunow) Frenguelli, and Planothidium lanceolatum (Brébisson ex Kützing) Lange-Bertalot were only present in the epilithic assemblages (Table 3). Despite these differences, the diversity was similar between substrates (Td35 vs Sd35 t = 0.98, p(perm) = 0.35) with mean values of 1.88 and 1.71 for epilithic and epipsammic assemblages, respectively (Fig. 5).
The comparison of the same substrate over time revealed that in both cases, the number of species contributing to the within-group average similarity decreased (up to 80% cumulative contribution; Table 2) with Achnanthidium minutissimum, Navicula notha, and Fragilaria cf. parva becoming most relevant at d35. From day 14 to day 35, there was a significant decrease in the diversity, independently of the substrate (Td14 vs Td35 t = 5.19, p(perm) = 0.01; Sd14 vs Sd35 t = 4.94, p(perm) < 0.01) (Fig. 5).
Despite the different multidimensional patterns of the epilithic and epipsammic communities at the same sampling occasion, the IPS values among the two substrates had a good agreement (Td14 vs Sd14 t = 1.32, p(MC) = 0.23; Td35 vs Sd35 t = 0.32, p(MC) = 0.76) (Fig. 6). There was a significant increase in the IPS values over time in both substrates (Td14 vs Td35 t = 4.46, p(MC) < 0.01; Sd14 vs Sd35 t = 5.07, p(MC) < 0.01) (Fig. 6).
Biological traits
In both substrates, mobile species were more frequent than planktonic (Fig. 7a, b). Between substrates, the epipsammic assemblages presented higher number of mobile and planktonic valves in both sampling occasions (days 14 and 35) (Fig. 7a, b). However, the number of mobile and planktonic valves did not change over time in both substrates (Figs. 7a, b). Concerning the form of attachment (pad, stalked, or adnate), a higher abundance of species with the ability to attach to the substrate by stalk was found (Fig. 7c–e).
Number of valves per cm2 (mean ± standard deviation) found in the diatom assemblages developing on tiles (T) and sand (S) substrates at days 14 and 35 in the artificial channels with the trait life form categories: a mobile, b planktonic, c pad, d stalk, and e adnate. Treatment means labelled with the same letter (a, b, c) do not significantly differ (p > 0.05; PERMANOVA pairwise test)
In both sampling occasions (d14 and d35), the sand assemblages presented higher number of stalked species than the tile assemblages (Fig. 7d). Within sand assemblages, there was a significant increase in the number of stalked diatoms from d14 to d35 (Fig. 7d). Despite the apparent increase in the number of stalked valves in the tile over time, statistical differences were not found (Fig. 7d). The majority of species that contributed to the stalked category belonged to the genus Achnanthidium. The number of diatoms with adnate habit and pads was similar between substrates and over time (Fig. 7c, e).
Discussion
The results of this mesocosm experiment show that the substrate affects diatom assemblage’s composition. This is in agreement with other studies that indicated that the composition of diatom assemblages on different substrates was different (Round 1991; Cattaneo et al. 1997; Potapova and Charles 2005). Yet, contrary to our findings, other studies have not found differences between substrates (Rothfritz et al. 1997; Bere and Tundisi 2011; Winter and Duthie 2000). The differences found between the assemblage compositions of the two substrates might be due to the species which dominated and were common to both substrates which is probably related to other factors. For example, both Achnanthidium minutissimum and Navicula notha have high oxygen requirements (polyoxybionte; van Dam et al. 1994) which was a condition satisfied by our experimental design.
We also found a significantly higher number of mobile cells in sand compared with tile substrates, which is in accordance with other studies (Cattaneo et al. 1997; Potapova and Charles 2005). Stalked species were also always significantly more abundant in sand compared with tile, contrary to what we were expecting, as in the sand, the majority of the species present will be those which have the necessary traits to tolerate the abrasion of moving grains (Townsend and Gell 2005) or be able to move (Soininen and Eloranta 2004) in order to avoid entrapment by the sand grains. However, the species that contributed most to the stalk categories were from the genus Achnanthidium, in particular A. minutissimum which stalk is simple, i.e. linked to one cell, instead of a stalk that is linked to several cells (Rimet and Bouchez 2012). This species has been found to dominate in highly hydrological disturbed habitats, suggesting that it may have resistance to the dislodgement induced by current shear forces (Soininen and Eloranta 2004).
Contrary to what we were expecting (see also Potapova and Charles 2005), at the same sampling occasion, the sand assemblages were never more diverse than the tile assemblages. It is expected that epilithic diatom assemblages are more stable than epipsammic ones because much less disturbance due to moving substrate particles occurs on firm stony substrates. Therefore, in natural environments the higher diversities found in natural epipsammic assemblages may be also due to the fact that in many occasions, the sampled assemblage is not an undisturbed mature one. According to Tuji (2000) when a community is in the last phase of colonization of a substrate and is affected by a disturbance, the resulting community architecture becomes similar to the first phase. Although the epipsammon represents a specialized diatom assemblage that seems well adapted to a variable environment, disturbance probably plays an important role in structuring the assemblage, keeping it in a ‘pioneer’ state (Miller et al. 1987). So, when we allowed the assemblages to develop during the 35 days without additional disturbances, it resulted in similar development states for both substrates and consequently similar diversities. This is in agreement with some studies dealing with differences in diatom assemblages among different substrates, where the role of factors such as hydrology (Soininen and Eloranta 2004) and pollution (Bere and Tundisi 2011) was found to overcome that of substrates. In some situations, the diversity differences may also be the result of significant differences at the population level often associated with small algal populations that exert little influence on density and diversity, and these differences may be an artefact of a chance encounter of a rare population during algal enumeration (Lowe et al. 1996).
Regarding the colonization process, and according to chl-c and diatom density by the fourteenth colonization day, diatom assemblages were already stable, independently of the substrate as there were no significant differences between sampling occasions. In agreement, a study by Oemke and Burton (1986) dealing with diatom colonization dynamics (diatom cell densities) growing on glass slides showed that the rate of increase in diatom density slowed after 10 or 14 days with the colonization curves reaching an apparent plateau by days 21 or 28. Yet, contrary to diatom density, the last 21 days of colonization of our experiment contributed to changes in the diatom assemblage’s composition in both sand and tile channels. These results suggest a decline in less abundant species and the dominance of a small number of species. Oemke and Burton (1986) also found a gradual decline in diversity after an early peak as a result of an increased dominance of few species. In addition, changes in traits also occurred over time, with a significant increase in the category ‘stalked’ on tiles from d14 to d35.
Considering the ecological quality assessment, the IPS values obtained at the same sampling occasion did not reflect the differences in epipsammic and epilithic diatom assemblages that were obtained in terms of multivariate patterns. As in other studies, this suggests that hard and soft (sand) substrates can be exchangeable in assessment methods that are based on autoecological methods (Soininen and Könönen 2004; Potapova and Charles 2005; Mendes et al. 2012), considering that communities are in the same state of development. Apparently, and considering the IPS results, the sand substrate assemblages were not more influenced by the sediment-bound chemicals than the epilithic ones (Kelly et al. 1998).
The significant increase in the IPS values over time in both substrates can be attributed to the dominance of sensitive species at day 35, which is the case of Achnanthidium minutissimum, in the IPS index. This index is based on weighted average between the relative abundance and the sensitivity (tolerance) and indicator value of a group selected species. Therefore, the high abundance of a sensitive species, as A. minutissimum, may cause such increase. This species has been considered indifferent to nutrient concentrations (van Dam et al. 1994); however, a laboratory experiment carried out by Manoylov (2009) suggests that A. minutissimum is a good competitor for nutrients when they are in low supply compared with other taxa. Therefore, this adaptation may have allowed A. minutissimum to outgrow the other species by the end of the 35 days of colonization.
In conclusion, we verified that both substrates reached an almost maximum production (diatom cell density and chl-c concentration) after two weeks of colonization although we did not find any clear patterns among diatom assemblage diversity. The type of colonizing substrate influences diatom assemblages (production, density and composition, traits) but not ecological quality assessment. Therefore, we can argue that in streams where the preferential substrate (usually stones or rocks) is not available and sand is the only substrate available, this can be used as alternative if the aim is to assess ecological quality using an autoecological index.
References
Almeida SFP, Feio MJ (2012) DIATMOD: diatom predictive model for quality assessment of Portuguese running waters. Hydrobiologia 695:185–197. doi:10.1007/s10750-012-1110-4
Bere T, Tundisi JG (2011) The effects of substrate type on diatom-based multivariate water quality assessment in a tropical river (Monjolinho), São Carlos, SP, Brazil. Water Air Soil Pollut 216:391–409. doi:10.1007/s11270-010-0540-8
Bergey EA, Cooper JT (2015) Shifting effects of rock roughness across a benthic food web. Hydrobiologia 760:69–79. doi:10.1007/s10750-015-2303-4
Berthon V, Bouchez A, Rimet F (2011) Using diatom life-forms and ecological guilds to assess organic pollution and trophic level in rivers: a case study of rivers in south-eastern France. Hydrobiologia 673:259–271. doi:10.1007/s10750-011-0786-1
Biggs BJF (1990) Use of relative specific growth rates of periphytic diatoms to assess enrichment of a stream. New Zeal J Mar Fresh 24:9–18. doi:10.1080/00288330.1990.9516398
Branco D, Lima A, Almeida SFP, Figueira E (2010) Sensitivity of biochemical markers to evaluate cadmium stress in the freshwater diatom Nitzschia palea (Kützing) W. Smith. Aquat Toxicol 99:109–117. doi:10.1016/j.aquatox.2010.04.010
Cattaneo A, Kerimian T, Roberge M, Marty J (1997) Periphyton distribution and abundance on substrata of different size along a gradient of stream trophy. Hydrobiologia 354:101–110
CEMAGREF (1982) Etude des Méthodes Biologiques d’Appréciation Quantitative de la Qualité des Eaux. Ministère de l’Agriculture, CEMAGREF, Division Qualité des Eaux, Pêche et Pisciculture, Lyon
Cetin AK (2008) Epilithic, epipelic, and epiphytic diatoms in the Göksu Stream: community relationships and habitat preferences. J Fresh Ecol 23:143–149. doi:10.1080/02705060.2008.9664565
Dalu T, Froneman PW, Chari LD, Richoux NB (2014a) Colonisation and community structure of benthic diatoms on artificial substrates following a major flood event: A case of the Kowie River (Eastern Cape, South Africa). Water SA 40:471–480. doi:10.4314/wsa.v40i3.10
Dalu T, Richoux NB, Froneman PW (2014b) Using multivariate analysis and stable isotopes to assess the effects of substrate type on phytobenthos communities. Inland Waters 4:397–412. doi:10.5268/IW-4.4.719
Elias CL, Calapez AR, Almeida SFP, Feio MJ (2015) Determining useful benchmarks for the bioassessment of highly disturbed areas based on diatoms. Limnologica 51:83–93. doi:10.1016/j.limno.2014.12.008
Feio MJ, Alves T, Boavida M, Medeiros A, Graça MAS (2010) Functional indicators of stream health: a river-basin approach. Freshw Biol 55:1050–1065. doi:10.1111/j.1365-2427.2009.02332.x
Feio MJ, Aguiar FC, Almeida SFP, Ferreira J, Ferreira MT, Elias C, Serra SRQ, Buffagni A, Cambra J, Chauvin C, Delmas F, Dörflinger G, Erba S, Flor N, Ferréol M, Germ M, Mancini L, Manolaki P, Marcheggiani S, Minciardi MR, Munné A, Papastergiadou E, Prat N, Puccinelli C, Rosebery J, Sabater S, Ciadamidaro S, Tornés E, Tziortzis I, Urbanič G, Vieira C (2014) Least disturbed condition for European Mediterranean rivers. Sci Total Environ 476–477:745–756. doi:10.1016/j.scitotenv.2013.05.056
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347. doi:10.1038/242344a0
Hunt AP, Parry JD (1998) The effect of substratum roughness and river flow rate on the development of a freshwater biofilm community. Biofouling 12:287–303. doi:10.1080/08927019809378361
INAG IP (2009) Critérios para a classificação do estado das massas de água superficiais—Rios e Albufeiras. In: Ministério do Ambiente, do Ordenamento do Território e do Desenvolvimento Regional. Instituto da Água, IP
Janauer G, Dokulil M (2006) Macrophytes and algae in running water. In: Ziglio G, Siligardi M, Flaim G (eds) Biological monitoring of rivers: applications and perspectives. Wiley, Chichester, pp 89–109. doi:10.1002/0470863781.ch6
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochem Physiol Pflanzen 167:191–194
Kelly MG, Cazaubon A, Coring E, Dell’uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B, Kwandrans J, Laugaste R, Lindstrøm E-A, Leitao M, Marvan P, Padisák J, Pipp E, Prygiel J, Rott E, Sabater S, van Dam H, Vizinet J (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224
Kelly MG, Gómez-Rodríguez C, Kahlert M, Almeida SFP, Bennett C, Bottin M, Delmas F, Descy J-P, Dörflinger G, Kennedy B, Marvan P, Opatrilova L, Pardo I, Pfister P, Rosebery J, Schneider S, Vilbaste S (2012) Establishing expectations for pan-European diatom based ecological status assessments. Ecol Indic 20:177–186. doi:10.1016/j.ecolind.2012.02.020
Kitner M, Poulíčková A (2003) Littoral diatoms as indicators for the eutrophication of shallow lakes. Hydrobiologia 506–509:519–524
Krammer K (2000) Diatoms of Europe: diatoms of the European inland waters and comparable habitats. Vol 1. The genus Pinnularia, vol 1. ARG Gantner-Verlag KG, Ruggell, Liechtenstein
Krammer K (2001) Diatoms of Europe: diatoms of the European inland waters and comparable habitats. Vol 2. Navicula sensu stricto. 10 Genera separated from Navicula sensu lato Frustulia. ARG Gantner-Verlag KG, Ruggell, Liechtenstein
Krammer K (2009) Diatoms of Europe: diatoms of the European inland waters and comparable habitats. Vol 5. Amphora sensu lato, vol 5. ARG Gantner-Verlag KG, Ruggell, Liechtenstein
Krammer K, Lange-Bertalot H (1986) Die Süßwasserflora von Mitteleuropa 2: Bacillariophyceae. 1 Teil: Naviculaceae. Gustav Fisher-Verlag, Stuttgart, Germany
Krammer K, Lange-Bertalot H (1988) Die Süßwasserflora von Mitteleuropa 2: Bacillariophyceae. 2 Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. Gustav Fisher-Verlag, Stuttgart, Germany
Krammer K, Lange-Bertalot H (1991a) Die Süßwasserflora von Mitteleuropa 2: Bacillariophyceae. 3 Teil: Centrales, Fragilariaceae, Eunotiaceae. Gustav Fisher-Verlag, Stuttgart, Germany
Krammer K, Lange-Bertalot H (1991b) Die Süßwasserflora von Mitteleuropa 2. Bacillariophyceae. 4 Teil: Achnanthaceae Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. Gustav Fisher-Verlag, Stuttgart, Germany
Krejci ME, Lowe RL (1986) Importance of sand grain mineralogy and topography in determining micro-spatial distribution of epipsammic diatoms. J North Am Benthol Soc 5:211–220
Lane CM, Taffs KH, Corfield JL (2003) A comparison of diatom community structure on natural and artificial substrata. Hydrobiologia 493:65–79. doi:10.1023/A:1025498732371
Lecointe C, Coste M, Prygiel J (1993) Omnidia: software for taxonomy, calculation of diatom indexes and inventories management. Hydrobiologia 269:509–513
Lowe RL, Gale WF (1980) Monitoring river periphyton with artificial benthic substrates. Hydrobiologia 69:235–244. doi:10.1007/BF00046798
Lowe RL, Pan Y (1996) Benthic algal communities as biological indicators. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic, San Diego, pp 705–739
Lowe RL, Guckert JB, Belanger SE, Davidson DH, Johnson DW (1996) An evaluation of periphyton community structure and function on tile and cobble substrata in experimental stream mesocosms. Hydrobiologia 328:135–146
Manoylov KM (2009) Intra- and interspecific competition for nutrients and light in diatom cultures. J Freshw Ecol 24:145–157. doi:10.1080/02705060.2009.9664275
Mendes T, Almeida SFP, Feio MJ (2012) Assessment of rivers using diatoms: effect of substrate and evaluation method. Fundam Appl Limnol 179:267–279
Miller AR, Lowe RL, Rotenberry JT (1987) Sucession of diatom communities on sand grains. J Ecol 75:693–709
Ndiritu GG, Gichuki NN, Triest L (2006) Distribution of epilithic diatoms in response to environmental conditions in an urban tropical stream, Central Kenya. Biodivers Conserv 15:3267–3293. doi:10.1007/s10531-005-0600-3
Oemke MP, Burton TM (1986) Diatom colonization dynamics in a lotic system. Hydrobiologia 139:153–166
Potapova M, Charles DF (2005) Choice of substrate in algae-based water-quality assessment. J North Am Benthol Soc 24:415–427
Rimet F, Bouchez A (2011) Use of diatom life-forms and ecological guilds to assess pesticide contamination in rivers: lotic mesocosm approaches. Ecol Indic 11:489–499. doi:10.1016/J.ECOLIND.2010.07.004
Rimet F, Bouchez A (2012) Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl Manag Aquat Ecosyst 406:01. doi:10.1051/KMAE/2012018
Rolland T, Fayolle S, Cazaubon A, Pagnetti S (1997) Methodical approach to distribution of epilithic and drifting algae communities in a French subalpine river: Inferences on water quality assessment. Aquat Sci 59:57–73
Rothfritz H, Jüttner I, Suren AM, Ormerod SJ (1997) Epiphytic and epilithic diatom communities along environmental gradients in the Nepalese Himalaya: implications for the assessment of biodiversity and water quality. Arch Hydrobiol 138:465–482
Round FE (1991) Diatoms in river water-monitoring studies. J Appl Phycol 3:129–145
Sabater S, Gregory SV, Sedell JR (1998) Community dynamics and metabolism of benthic algae colonizing wood and rock substrata in a forest stream. J Phycol 34:561–567
Soininen J, Eloranta P (2004) Seasonal persistence and stability of diatom communities in rivers: are there habitat specific differences? Eur J Phycol 39:153–160. doi:10.1080/0967026042000201858
Soininen J, Könönen K (2004) Comparative study of monitoring South-Finnish rivers and streams using macroinvertebrate and benthic diatom community structure. Aquat Ecol 38:63–75
Stevenson RJ, Pan Y (1999) Assessing environmental conditions in rivers and streams with diatoms. In: Stoermer EF, Smol JP (eds) The diatoms: applications for the environmental and earth sciences. Cambridge University Press, Cambridge, pp 11–40
Townsend SA, Gell PA (2005) The role of substrate type on benthic diatom assemblages in the Daly and Roper Rivers of the Australian wet/dry tropics. Hydrobiologia 548:101–115. doi:10.1007/s10750-005-0828-7
Tuji A (2000) Observation of developmental processes in loosely attached diatom (Bacillariophyceae) communities. Phycol Res 48:75–84
van Dam H, Mertens A, Sinkeldam J (1994) A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth J Aquat Ecol 28:117–133. doi:10.1007/BF02334251
Winter JG, Duthie HC (2000) Stream epilithic, epipelic and epiphytic diatoms: habitat fidelity and use in biomonitoring. Aquat Ecol 34:345–353
Acknowledgements
This study was possible due to the financial support of the FOUNDATION FOR SCIENCE AND TECHNOLOGY (Portugal) through the Ph.D. scholarship SFRH/BD/68973/2010 of the first author and through the strategic project UID/MAR/04292/2013 granted to MARE and UID/GEO/04035/2013 granted to GeoBioTec. We thank GeoBioTec Research Centre and Biology Department, University of Aveiro. We thank to the Engineer Acácio Pascoal from the company Gres Panaria, Portugal S.A.—LOVE TILES division for the offer of the ceramic tiles and to the company Water Technologies for all the technical and equipment support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
About this article
Cite this article
Elias, C.L., Rocha, R.J.M., Feio, M.J. et al. Influence of the colonizing substrate on diatom assemblages and implications for bioassessment: a mesocosm experiment. Aquat Ecol 51, 145–158 (2017). https://doi.org/10.1007/s10452-016-9605-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-016-9605-0