Abstract
Nuisance, toxic cyanobacterial blooms are a persistent and globally expanding problem. Prevention of blooms requires that external and internal sources of nutrients are managed to levels where development of cyanobacterial blooms is restricted. Control of blooms, in which their presence is reduced to a level where they no longer pose a risk through additional measures such as biomanipulation or artificial mixing, demands that three elements come together: (1) understanding of the key ecological traits of the dominant cyanobacteria taxa, (2) system analysis of the lake, in particular its morphometry, water and nutrient balance, (3) adequate design and execution of the management methods of choice. All three elements are important for choosing effective management interventions and predicting their outcome. Mitigation of blooms reduces the risks and harmful effects of blooms if they cannot be prevented or sufficiently controlled, methods such as harvesting of surface scums or application of cyanocides may be used in those cases where water quality improvement is urgent. Ultimately, managing cyanobacterial blooms is most effective in the context of developing a Water Safety Plan. This is a risk assessment and management approach developed by the World Health Organization and provides a platform for bringing together the stakeholders who have a say about activities in the catchment causing eutrophication. Together, they can develop and implement control measures in the chain from catchment to drinking-water offtake which effectively mitigate eutrophication and thus protect humans and the lake ecosystem services they rely on from effects of toxic cyanobacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyanobacterial blooms are the ultimate consequence of a seemingly simple cause: when a lake is loaded with an excess of nutrients the water turns into a nuisance green soup. Yet underneath this deceptive simplicity in cause and effect, there is a world of complexity at the landscape, hydrological and ecological level. Never does this complexity become more apparent than when efforts are made to restore eutrophied lakes. The success rate in restoring disturbed aquatic ecosystems to a healthy state is highly variable, and this may in part be a consequence of insufficient understanding, planning and preparation. We distinguish prevention—blooms Footnote 1 do not appear in the system—from control—excessive cyanobacterial growth in the ecosystem is suppressed despite sufficient availability of nutrients—and mitigation—blooms exist but are reduced in size so that harmful effects, although still present are diminished. Successful prevention, control and/or mitigation requires that three elements to come together: (1) an in depth understanding of the ecology of the bloom forming cyanobacteria, in particular their key, niche defining traits (Litchman and Klausmeier 2007), e.g., buoyancy, N2-fixaton and colony formation; (2) an in depth analysis and quantification of the lake’s relevant physical, chemical and biological properties, e.g., water and nutrient balance, lake depth, basin morphometry or residence time; (3) a full understanding and comprehensive preparation of their execution of the management option(s) of choice (e.g., manipulation of fish stocks or artificial mixing). Hence, traits of the dominant cyanobacteria, lake characteristics and the chosen treatment must necessarily come together (see also Mantzouki et al. this issue 2016). Management of blooms should not be a process of trial and error but the result of a carefully planned process. This paper is the Synopsis of a Special Issue of Aquatic Ecology (eds. Visser, Ibelings, Fastner & Bormans, see Editorial by Ibelings et al. this issue 2016) and a product of a European COST Action, CYANOCOST (www.cyanocost.com).
Delays in recovery from eutrophication
Lake restoration efforts often face long delays. Attempts to control blooms started in the 1970s and 1980s when the negative consequences of eutrophication could no longer be ignored, since the blooms clearly interfered with lake ecosystem functioning (severe loss of aquatic biodiversity) and services like the provisioning of drinking water or recreation. To their frustration, lake managers often found that blooms remained even when nutrient loading to the lake was successfully brought under control, partly due to internal nutrient loading and partly due to insufficient reduction in external loading. Both the critical load for phosphorus and the lake retention time have been identified as decisive factors in determining the extent and rate to which cyanobacterial blooms are brought under control after implementation of restoration measures. Falling below the critical load through either diversion or stripping of phosphorus in the main inflow in combination with a relatively short retention times has led to the disappearance of cyanobacteria in Lake Washington (USA) and Schlachtensee (Germany) within a few years (Fastner et al. this issue 2016). As in the majority of cases comparable favorable hydrological (and financial) conditions are less likely to be present, substantial reduction in cyanobacteria can take decades, e.g., in Onondaga Lake (USA), it took 40 years for the lake reaching mesotrophic conditions and disappearance of cyanobacterial blooms (Fastner et al. this issue 2016).
In particular, eutrophication of shallow lakes is a classic example of the theory of alternative stable states, and turbid versus clear shallow lakes even played an important role in development of the concept (Scheffer et al. 2001). The Border lakes in the Netherlands, presented by Noordhuis et al. (this issue 2016), provide a perfect case study. These lakes were formed in the 1950s, and the lakes were originally clear with abundant growth of submerged macrophytes. But after years of eutrophication, the lakes fairly abruptly switched to a turbid state, characterized by dense blooms of Planktothrix agardhii. A range of restoration measures were taken, including removal of P at wastewater treatment plants, flushing with Ca-rich water and a reduction in the bream density. In the eventual return of the clear state, the re-colonization by dreissenid mussels (first zebra later quagga mussels, respectively Dreissena polymorpha and D.rostriformis bugensis) played an important role. Biomanipulation typically works best in combination with nutrient reduction supporting the lake in reaching a new equilibrium (Kasprzak et al. 2007).
The important lesson learned is that the long delay in a return to the clear water state is a consequence of both states—clear as well as turbid—being stable and resisting change through internal feedback mechanisms. For this reason, lake management had to reduce phosphorous levels to concentrations well below the concentration where the clear water state was lost during eutrophication. The turbid state lasted for many years despite huge efforts and costs to restore lake water quality and ecosystem functioning (Ibelings et al. 2007). This phenomenon of hysteresis teaches us that we should act before the loss of the desired, functional ecosystem state, since in the clear state resilience of the ecosystem works with us to maintain a functional state. Once lost, nature in the alternative stable state will work against restoration efforts, delay a return to the clear state and greatly inflate costs. In recent years, we have learned much more about early warning signals, in particular about a process known as critical slowing down measured as an increase in auto-correlation (Dakos et al. 2008), which indicates that a system is approaching a tipping point before it truly reaches the bifurcation. Those signals should be taken seriously and should lead to immediate action, to avoid a collapse into the disturbed, dysfunctional state.
Global problem, local differences
The role played by zebra mussels in restoring a clear water state and healthy ecosystem in the Borderlakes represents an interesting example of the role played by local circumstances and local perception of eutrophication problems (Van Dolah et al. this issue 2016). In the Netherlands, zebra mussels arrived in the nineteenth century and have been part of the ecosystem of Dutch lakes for many decades. They are seen as valuable components of the ecosystem, not only since they are efficient filter feeders, which provide strong top-down control of cyanobacterial blooms (supported by the fact that they seem hardly sensitive to cyanobacterial toxins like microcystin (Dionisio-Pires et al. 2004), but also because they are staple food for huge numbers of diving ducks that overwinter in the IJsselmeer area in the Netherlands. In contrast, in particular in North America, zebra and quagga mussels are perceived as a huge threat to lake water quality and native biodiversity. The mussels have played a role for instance in the return of the Microcystis blooms to some of the Great Lakes. The different roles played by Dreissena in promoting or reducing cyanobacterial blooms, in particular those of Microcystis, are not fully understood, although Knoll et al. (2008) suspect that the trophic state of lakes may play a decisive role, Dreissena only promoting blooms in oligo- to mesotrophic but not in eutrophic systems.
Although cyanobacterial blooms are a worldwide phenomenon, clearly they are not—or are not perceived to be—the same on all continents, and local knowledge is indispensable when control measures are planned. We should also admit that all authors and the vast majority of case studies and references given in this Special Issue come from temperate regions, whereas it is fully known that plankton dynamics in lakes at different latitudes, including the (sub)tropics unfold differently (De Senerpont Domis et al. 2013). The efficacy of biomanipulation for instance is much reduced in tropical systems as a consequence of distinct differences in the trophic structure of temperate versus tropical lakes (Jeppesen et al. 2007). Given that this Special Issue is the result of a European COST Action and given that the authors of the various contributions mainly come from within this Action, clearly the papers have their focus on temperate lakes.
Linking lakes, cyanobacteria and control measures
In this Special Issue, different methods are presented to prevent, control and mitigate blooms of cyanobacteria. The challenge is to choose the method—or combination of methods—which is most likely to yield success. What are the factors that determine success rate? Why for instance do Visser et al. (this issue 2016) demonstrate that artificial mixing of lakes or reservoirs can be a very effective means to manage blooms of buoyant cyanobacteria, whereas Lürling et al. (this issue 2016a, b) who review end of pipe methods whose efficacy is “questionable” at best, list mixing as one of the tools that should be avoided, given a lack of demonstrated success? The key difference is that Visser et al. (2016) properly link key traits of the cyanobacteria—here buoyancy—to key characteristics of the lake—here sufficient lake depth—and the main properties of the method—here adequately designed artificial mixing, resulting in turbulent mixing rates that exceed the flotation velocity of Microcystis spp. in the lake, whereas Lürling et al. (this issue 2016b) rightfully point out that mixing will not work in shallow lakes, or if the equipment is not powerful enough. Thus, the debate about artificial mixing demonstrates the importance of the three elements mentioned above—i.e., that measures are successful only if designed on the basis of understanding key aspects of the phyiscs, chemistry and biology of the respective system. Too often, artificial mixing has been installed as presumed “quick fix” of the problem—without properly analysing the system.
Nutrient management, lakes in the landscape
Before discussing the various methods and their potential application, we want to convey our key message. Lakes are not isolated elements in the landscape. Whatever happens in a catchment, to a large extent determines the state of a lake. For this reason, lakes act as sentinels of wider environmental change (Adrian et al. 2009). Management of cyanobacterial blooms cannot and should not be seen separate from management of catchment processes. Nutrient management in the catchment ultimately is the basis of sustainable prevention or control of blooms. Mitigation of blooms may be necessary to deal with emergencies and can be considered for short term problems or risks that require a quick solution (imagine the risks of cyanobacterial surface scums for peak summertime recreation). In a recent obituary of Brian Moss (Jeppesen and Johnes 2016), Brian Moss is quoted as saying “All things are connected and a solution to the rising tide of problems in aquatic ecosystems will only be found if we start to treat and manage waterbodies and landscapes as connected systems”. We agree and therefore we cannot overemphasize the importance of these landscape-lake links for sustainable management of blooms. For that reason, the paper by Hamilton et al. (this issue 2016) which describes nutrient management in catchments is a key contribution to the Special Issue. To ensure a strong link between catchment and lakes, we advocate the Water Safety Plan (WSP) approach as developed by the World Health Organization (WHO): This approach aims at safeguarding humans from exposure to hazards—including, e.g., toxins produced by cyanobacteria—on the basis of a comprehensive understanding of events and activities that may cause their occurrence in water and the respective system’s properties that affect the health risks hazards (see Ibelings et al. 2014). WSP will be central to the upcoming revision of the widely used handbook “Toxic cyanobacteria in water” (Sivonen and Jones 1999; 2nd edition in preparation).
Water Safety Plans
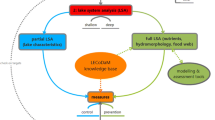
Cyanobacteria produce a wide range of bioactive compounds, including known toxins like microcystins, anatoxin-a(s), saxitoxin or cylindrospermopsin which may have acute or (sub)chronic effects on human health (see Special Issue on “Harmful algal blooms and public health”, Editorial by D’Anglada 2015). The most sustainable way to protect the public from exposure to toxic cyanobacteria is to limit cyanobacterial development in lakes, so that toxin concentrations do not exceed safe limits. The concentration of toxin is mainly, but not exclusively, set by the cyanobacterial biomass in the lake, which in turn is the outcome of resource controlled growth versus various loss processes. The COST- outcomes presented in this Special Issue support the use of the WSP approach for assessing and managing human health risks from toxic cyanobacteria. While in general, developing a WSP involves a comprehensive analysis of the hazards (i.e., including pathogens and other toxic substances) and assessing the health risks they pose, elements of WSP approach can also be applied only to cyanobacteria, without such a full system analysis, but retaining a system analysis targeted on cyanobacteria as advocated by Lürling et al. (2016a) which includes a water and nutrient balance as well as an in depth analysis of the biological functioning of the lake ecosystem. An outcome is the identification of the key control measures that can be taken to manage eutrophication and/or blooms. Control measures should be implemented at several levels to ensure maximum safety by the redundancy of having multiple barriers so that each level contributes incrementally to overall control (see Fig. 1). In the case of blooms, this first and foremost includes nutrient management (1) in the lake catchment (Hamilton et al. this issue 2016), (2) at the inflow to lakes (Fastner et al. this issue 2016) as well as (3) in-lake nutrient control, such as reducing P-release from the sediment by hypolimnetic aeration (Bormans et al. this issue 2016), capping of the sediment through binding P to metals or particles (Douglas et al. this issue 2016).
Prevention, control and mitigation measures for cyanobacterial blooms: nutrient management in the lake catchment, at the inflow to lakes and in-lake methods, including P-release from the sediment and in-lake methods can all be applied to prevent, control or mitigate cyanobacterial growth or biomass. This figure outlines the WHO Water Safety Plan approach so that control points are implemented at several levels (catchment, inflow, sediment in lake) to ensure maximum trust that the desired goals—a healthy lake ecosystem which supports important lake ecosystem services—are reached and can be maintained. Figure adapted from Fig. 4 Ibelings et al. (2014), courtesy of Dr Ingrid Chorus
The latter approach-binding P-is a geo-engineering concept. In geo-engineering, biochemical processes in lakes—usually P-release from the lake sediment—are engineered to achieve a desired ecosystem state (typically reduction in cyanobacterial blooms). For this, P-binding agents are added to the sediment to control legacy P-stores (Spears et al. 2014). Including a control measure to cap P in the sediment may be important in specific cases: For example, an outcome of system assessment for a given polymictic lake in the context of a WSP may be that increasing P-release is expected as a consequence of increasing anoxia near the sediment in the wake of enhanced microstratification caused by a changing climate, and that reducing external P-input alone will not be sufficient to reach the TP target needed to effectively control cyanobacterial blooms. An alternative scenario might be that the water exchange rate of a given lake is too low to export its excessive P-content within the targeted time scale. Such situations would render sediment capping or binding P necessary. We wish to express a warning, however, to apply geo-engineering in a solitary context, which is sometimes considered because of the relatively low costs and rapid results (Spears et al. 2014), without further measures to control external nutrient loading: continued P-input would quickly counteract any success in capping or binding the current P-content.
Beside controlling nutrients, there is a choice of in-lake methods to help control or mitigate cyanobacteria, for example artificial mixing (Visser et al. this issue 2016), biomanipulation (Triest et al. this issue 2016), water level manipulation (Bakker and Hilt this issue 2016) or use of cyanocides like hydrogen peroxide (Matthijs et al. this issue 2016). Whereas well designed artificial mixing can be considered a control measure—see the work on Lake Nieuwe Meer by Visser et al. (1996) and Huisman et al. (2004) where mixing for many years now prevents Microcystis from developing a population in the lake—application of cyanocides in our definition could be either control or mitigation, depending on its efficacy, since existing blooms are reduced in size or sometimes fully taken out by use of the chemicals, allowing for instance lakes to remain open for recreation. Planning control measures—in the context of developing a WSP (see https://toxische-cyanobakterien.de/en/water-safety-plan/) or independently of this—implies choosing the locally most efficient controls, and this may require implementing multiple control measures, e.g., for control of external nutrient loading and measures taken within the waterbody in order to increase the likelihood that blooms are indeed effectively controlled. For instance in a setting where the critical load to a lake or reservoir appears controllable, this should obviously have priority. In most cases, indeed measures to control nutrient availability must be part of lake restoration efforts, but combining them with other measures may speed up lake recovery.
In circumstances, however, where the options to control nutrients in the catchment are not sufficiently available or where the urgency to have positive results is large, measures such as artificial mixing or cyanocides may be considered as stand alone, and have proven—at times—to be successful. Lake Nieuwe Meer in the Netherlands may serve as an example (Visser et al. 1996). Many families have their home on the lake, living on house-boats. Dense Microcystis scums for many years surrounded these boats, and a solution was urgently needed. Nutrient management was not a viable option. Lake Nieuwe Meer is part of the river Rhine watershed, and with the high nutrient levels of the 1980s and 1990s nutrient control would have taken many years to have an effect. Importantly, nutrient management was not under control of the local Amsterdam authorities, which would have required action at the catchment level, as nowadays promoted by the EU Water Framework Directive guidelines. Under these circumstances, the only viable control measures were in lake methods like artificial mixing, and given an appropriate technical design this clearly has worked well for many years.
Verification and validation procedures
After implementation of the appropriate combination of methods and control measures, the efficiency of the measures taken should be monitored, which in WSP involves both verification and validation. Validation entails checks that the measures put in place are indeed able to do the job—i.e., prevent, control or mitigate blooms—to the extent that relevant endpoints (e.g., cyanobacterial cell numbers or microcystin concentrations), are within the required range. Verification implies checking that the system indeed achieves targets in terms of the health hazard, i.e., for microcystin in recreational water that microcystin concentrations do not exceed 20 µg L−1 or cell numbers remain below 100,000 cells mL−1; Chorus and Bartram 1999). With respect to monitoring, the efficacy of bloom management and to ensure that lakes are secure for users it is important to realize that cyanobacterial blooms are highly dynamic events, with growth and loss processes operating at the time scale of days and (scums) appearing and disappearing at even shorter time scales. Traditional approaches to lake monitoring are not able to capture these dynamics. A growing number of lake ecologists—joined in GLEON (www.gleon.org)—therefore is developing and using autonomous, high frequency buoys and platforms (Weathers et al. 2012). Cyanobacteria can be monitored in real time using fluorescence or flow cytometry (Pomati et al. 2011), and this is a field showing rapid development. Furthermore in managing cyanobacteria and the risks they pose, modeling is an indispensable tool (Hipsey et al. 2015). Ibelings et al. (2003) demonstrated that in principle cyanobacterial scum formation can be predicted ahead of time using the medium term weather (wind) forecast, giving time for early warning and implementation of safety measures like warning signs for swimmers or even lake closures.
Prevention and control measures
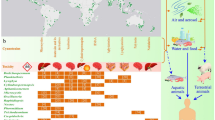
Having firmly established that nutrient management is essential to manage cyanobacterial blooms, we now further discuss other techniques that are on offer for the control or mitigation of blooms. For this, we refer to the paper by Stroom and Kardinaal (this issue 2016), who provide guidance to managers in choosing appropriate control measures, as well as to the paper by Lürling et al. (this issue 2016b) who describe several (commercially offered) methods that should be treated with caution. In the paper by Stroom and Kardinaal, many more measures are described than we will discuss in this synopsis. Figure 2 brings together key functional traits of cyanobacteria [following the system designed by Reynolds et al. (2000) adapted for use in this Special Issue by Mantzouki et al. (2016)] with key lake properties such as trophic state, lake mixing type and residence time with several management options. Prevention measures (upper part of Fig. 2) are effective for all groups of cyanobacteria, independent of their traits or taxon. Prevention—see the sections above—always means a reduction in external and—often—internal nutrients, which makes it unnecessary in oligotrophic systems (i.e., no symbols in that column). In meso- and eutrophic lakes and reservoirs, both external and internal measures can or should be taken to prevent growth of cyanobacteria. Which nutrient control measures to select depends inter alia on the depth of the system, e.g., control of P-loading from the sediment through dredging is less feasible in deep systems, while this can be helpful in shallow lakes, whereas hypolimnetic aeration is only, though not always, effective in stably stratified, deep systems and also chemical control of nutrients in lake sediments (flocculation and capping) will not be very effective in shallow, well-mixed systems (Bormans et al. this issue 2016; Douglas et al. this issue 2016).
Schematic overview of methods for the prevention, control and mitigation of cyanobacterial blooms as presented in the Special Issue of Aquatic Ecology. The table brings together key functional traits of cyanobacteria (following the system designed by Reynolds et al. (2000) adapted for use in this Special Issue on management of cyanobacteria by Mantzouki et al. (2016)] with key lake properties like tropic state, mixing type and residence time with the several management options (see explanation of the symbols underneath the table). Symbols used in the figure: M, Monomictic; D, Dimictic; P, Polymictic; S, Short; L, Long;  , P and N-control in catchment;
, P and N-control in catchment;  , P and N internal control—chemically;
, P and N internal control—chemically;  , P and N internal control—hypolimnetic aeration;
, P and N internal control—hypolimnetic aeration;  , P and N internal control—dredging; HP, Hydrogen peroxide;
, P and N internal control—dredging; HP, Hydrogen peroxide;  , Flushing;
, Flushing;  , Water level management;
, Water level management;  , Biomanipulation with mussels;
, Biomanipulation with mussels;  , Biomanipulation with fish;
, Biomanipulation with fish;  , Artificial mixing;
, Artificial mixing;  , Weakening of stratification;
, Weakening of stratification;  , Abstraction depth;
, Abstraction depth;  , Bubble screen;
, Bubble screen;  , Withdraw (scums) from surface;
, Withdraw (scums) from surface;  , Information for bathers;
, Information for bathers;  , Closing of bathing sites
, Closing of bathing sites
Control measures such as flushing, biomanipulation or artificial lake mixing cannot be used in all systems but are one again dependent on lake depth. Artificial mixing will only be effective to combat cyanobacteria in relatively deep systems with in general an average depth of more than 15 m (Visser et al. this issue 2016). On the other hand, water level management and biomanipulation will tend to be only effective in shallow systems. Thus just on basis of the depth or mixing type of a lake, a selection of suitable measures can begin to be made. Furthermore, choosing control measures should take traits of the dominant cyanobacteria in the system into account. Based on a cyanobacterium’s key traits, a control measure may have a high or low likelihood to work. We will discuss a few examples. Artificial mixing is mainly effective for cyanobacteria that are buoyant and grow as tufts or colonies, such as Dolichospermum, Aphanizomenon, Gloeotrichia, Woronichinia and Microcystis. Buoyancy provides these genera with a clear competitive advantage over non-buoyant algae in lakes with a stable watercolumn. This is most obvious for cyanobacteria growing in aggregates since the flotation rate is primarily dependent on the size of the unit, i.e., the larger the colony, the faster it floats. Colonies thus can escape turbulent mixing in a lake’s epilimnion—provided mixing is not too vigorous—and remain positioned in the well illuminated surface layer. In an artificially mixed system, this advantage will be lost and bring about a reduced abundance of cyanobacteria. For filaments with a small diameter, the flotation rate is very low and they will mostly remain entrained in the turbulent flow in the water column. For the filamentous genera Planktothrix and Cylindrospermopsis, artificial mixing may still work but only if it is applied intermittently (Antenucci et al. 2005).
Flushing will be a good measure to combat almost all cyanobacteria as long as the flushing results in a residence time which is shorter than the growth rate of the cyanobacteria. Supporting evidence comes from observations on rivers where blooms of cyanobacteria are typically absent, except when discharge rates drop strongly during periods of drought, e.g., in the river Darling in Australia (Bowling and Baker 1996) and river Rhine in the Netherlands (Ibelings et al. 1998). Using a model study of the population dynamics of Microcystis, calibrated with data from a 2 years lake monitoring program, the effect of enhanced flushing as management strategy could be tested (Verspagen et al. 2006). This showed that Microcystis blooms will be suppressed when the flushing rate is sufficiently increased to result in residence times of ca. <20 days. Similarly, blooms of Dolichospermum have been suppressed by increasing flow in regulated weir pools in several Australian rivers (Bormans and Condie 1998; Mitrovic et al. 2011). For metalimnetic populations of P. rubescens, flushing will likely have little effect, since flushing will typically affect only the epilimnion of stratified lakes. Other measures listed in Fig. 2 can be considered too. For the control of blooms of Planktothrix agardhii, typical of nutrient rich shallow lakes, water level management and biomanipulation may have positive effects, because these measures can promote development of submerged macrophytes and help to tip the balance to a stable clear water state. Hydrogen peroxide has been tested to be very effective for Planktothrix agardhii, Dolichospermum, Aphanizomenon and Microcystis both in the laboratory and in whole-lake treatments (Matthijs et al. this issue 2016), but has not been tested yet for Gloeotrichia, Woronichinia, Cylindrospermopsis raciborskii and Planktothrix rubescens (therefore, no HP treatment is advised as yet for these species in Fig. 2).
Mitigation measures
Mitigation techniques (lower part of Fig. 2) can typically be performed to reduce the negative effects or mitigate the risks of all cyanobacterial groups independent of their traits, and also for lakes of varying trophic state or mixing type and both short and long retention times, except that methods like variation of abstraction depth for drinking water and hypolimnetic withdrawal will not be effective in shallow lakes. Harvesting of algal scums or surface curtains/bubble screens to avoid floating scums from entering sheltered areas like harbors have proven effective—in some cases—in both shallow and deep lakes but have limited likelihood of success in large lakes and require repeated action given that the conditions for cyanobacterial development remain favorable in the lake. As noted in Fig. 2, most mitigation techniques can be used in oligotrophic waters, but there are generally few nuisance by cyanobacteria, and therefore, information for recreation and closing lakes for contact sports will usually not be needed (although even in oligotrophic lakes a low cyanobacterial biomass which gets concentrated on the shore may occasionally cause problems).
Financial and political aspects of bloom management
Besides the lake characteristics and traits of the cyanobacterial taxa, also economical aspects will be an important selection criterion for water managers. How much money a water board or municipality is willing to pay for a treatment in a lake will depend on the ecosystem services supported by the lake, for instance recreational use. Very high costs in the range of millions to billions can arise for upgrading of waste water treatment plants and measures in the catchment as shown for Onondaga Lake and Lake Constance (Fastner et al. this issue 2016). However, nutrient reduction is the most effective and sustainable control of cyanobacterial blooms and thus protection of human and ecosystem health. In contrast, control and mitigation measures are substantially cheaper, though there are differences for the respective methods. Artificial mixing devices for example are expensive to install and are also rather expensive in their maintenance and running—energy—costs. Other treatments, like hydrogen peroxide addition, are relatively cheap and can be effective in the short run but need to be repeated at least every year and sometimes twice a year. It is good to realize that doing nothing also comes with considerable costs through loss of ecosystem services (Sharma et al. 2014). Next to these financial considerations also political choices are an important criterion. In our experience, most water boards strive after a sustainable solution, like the prevention measures shown in Fig. 2. However, if these are outside the budget of the water board or municipality or if the time needed to result in water quality improvements is too long, authorities may prefer to choose from the listed control measures. Mitigation measures will typically be used as a last resort only, but political pressure for swift results may sometimes interfere with the wisdom of the management measures chosen.
Conclusion
Our key message is that in managing cyanobacterial blooms nutrient must come first. Controlling nutrients is the most sustainable way to safeguard lake ecosystems and public health from the negative effects of cyanobacterial blooms. Having said this, there are many circumstances where additional measures for the control or mitigation of blooms is advisable or mandatory. In devising the management strategy, a good lake system analysis must go hand in hand with a deep understanding of the key ecological traits of the dominant cyanobacteria and a thorough design and implementation of the management measures. Managing blooms in lakes is more than just application of scientific knowledge, and social, financial and political aspects all play a role in lake management. We express our hope that the science in this Special Issue will be helpful in making choices for a (cost) effective and sustainable approach, following guidelines from the WHO Water Safety Plans.
Notes
We define blooms as an elevated cyanobacterial biomass that is above the biomass in the reference state of a given lake—admittedly not always easy to define—and which interferes with the ecosystem functioning and—services of this lake. Cyanobacteria are part of the normal plankton community of a lake, so it is not their presence per se, but the level of their biomass that defines—nuisance—blooms.
References
Adrian R, OReilly C, Zagarese H et al (2009) Lakes as sentinels of climate change. Limnol Oceanogr 84:2283–2297
Antenucci JP, Ghadouani A, Burford MA, Romero JR (2005) The long-term effect of artificial destratification on phytoplankton species composition in a subtropical reservoir. Freshwater Biol 50:1081–1093
Bakker ES, Hilt S (2016) Impact of water level fluctuations on cyanobacterial blooms: options for management. Aquat Ecol. doi:10.1007/s10452-015-9556-x
Bormans M, Condie SA (1998) Modelling the distribution of Anabaena and Melosira in a stratified river weir pool. Hydrobiologia 364:3–13
Bormans M, Maršálek B, Jančula D (2016) Controlling internal phosphorus loading in lakes by physical methods to reduce cyanobacterial blooms—a review. Aquat Ecol. doi:10.1007/s10452-015-9564-x
Bowling LC, Baker PD (1996) Major cyanobacterial bloom in the Barwon–Darling river, Australia, in 1991, and underlying limnological conditions. Mar Freshwater Res 47:643–657
D’Anglada LV (2015) Editorial on the special issue “Harmful Algal Blooms (HABs) and Public Health: Progress and Current Challenges”. Toxins 7:4437–4441
Dakos V, Scheffer M, van Nes EH, Brovkin V, Petoukhov V, Held H (2008) Slowing down as an early warning signal for abrupt climate change. Proc Natl Acad Sci USA 38:14308–14312
De Senerpont Domis LND, Elser JJ, Gsell AS, Huszar VLM, Ibelings BW, Jeppesen E, Kosten S, Mooij WM, Roland F, Sommer U, van Donk E, Winder M, Lurling M (2013) Plankton dynamics under different climate conditions in tropical freshwater systems (a reply to the comment by Sarmento, Amado and Descy 2013). Freshwater Biol 58:2211–2213
Dionisio-Pires LMD, Karlsson KM, Meriluoto JAO, Kardinaal E, Visser PM, Siewertsen K, Van Donk E, Ibelings BW (2004) Assimilation and depuration of microcystin-LR by the zebra mussel, Dreissena polymorpha. Aquat Toxicol 69:385–396
Douglas GB, Hamilton DP, Robb MS et al (2016) Guiding principles for the development and application of solid phase phosphorus-adsorbents for freshwater ecosystems. Aquat Ecol. doi:10.1007/s10452-016-9575-2
Fastner J, Abella S, Litt A et al (2016) Combating cyanobacterial proliferation by avoiding or treating inflows with high P load—experiences from eight case studies. Aquat Ecol. doi:10.1007/s10452-015-9558-8
Hamilton DP, Salmaso N, Pearl HW (2016) Mitigating harmful cyanobacterial blooms: strategies for control of nitrogen and phosphorus loads. Aquat Ecol. doi:10.1007/s10452-016-9594-z
Hipsey MR, Hamilton DP, Hanson PC et al (2015) Predicting the resilience and recovery of aquatic systems: a framework for model evolution within environmental observatories. Water Resour Res 51:7023–7043
Huisman J, Sharples J, Stroom JM, Visser PM, Kardinaal WEA, Verspagen JMH (2004) Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85:2960–2970
Ibelings BW, Fastner J, Bormans M, Visser PM (2016) Cyanobacterial blooms, ecology, prevention, control, mitigation: Editorial to a CYANOCOST Special Issue. Ecol Appl. doi:10.1007/s10452-016-9595-y
Ibelings BW, Admiraal W, Bijkerk R, Ietswaart T, Prins H (1998) Monitoring of algae in Dutch rivers: does it meet its goals? J Appl Phycol 2:171–181
Ibelings BW, Portielje R, Lammens EHRR, Noordhuis R, van den Berg MS, Joosse W, Meijer ML (2007) Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10:4–16
Ibelings BW, Backer LC, Kardinaal WEA, Chorus I (2014) Current approaches to cyanotoxin risk assessment and risk management around the globe. Harmful Algae 40:63–74
Jeppesen E, Johnes PJ (2016) Obituary: Brian Moss (1943–2016). Hydrobiologia 778:1
Jeppesen E, Meerhoff M, Jacobsen BA, Hansen RS, Sondergaard M, Jensen JP, Lauridsen TL, Mazzeo N, Branco CWC (2007) Restoration of shallow lakes by nutrient control and biomanipulation—the successful strategy varies with lake size and climate. Hydrobiologia 581:269–285
Kasprzak P, Benndorf J, Gonsiorczyk T, Koschel R, Krienitz L, Mehner T, Hülsmann S, Schultz H, Wagner A (2007) Reduction of nutrient loading and biomanipulation as tools in water quality management: long-term observations on Bautzen Reservoir and Feldberger Haussee (Germany). Lake Reserv Manage 23:410–427
Knoll LB, Sarnelle O, Hamilton SK, Kissman CEH, Wilson AE, Rose JB, Morgan MR (2008) Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Can J Fish Aquat Sci 3:448–455
Litchman E, Klausmeier C (2007) A trait-based community ecology of phytoplankton. Annu Rev Ecol Evol Syst 39:615–639
Lürling M, Mackay E, Reitzel K, Spears BM (2016a) Editorial—a critical perspective on geo-engineering for eutrophication management in lakes. Water Res 97:1–10
Lürling M, Waajen G, de Senerpont Domis LN (2016b) Evaluation of several end-of-pipe measures proposed to control cyanobacteria. Aquat Ecol. doi:10.1007/s10452-015-9563-y
Mantzouki E, Visser PM, Bormans M, Ibelings BW (2016) Understanding the key ecological traits of cyanobacteria as a basis for their management and control in changing lakes. Aquat Ecol. doi:10.1007/s10452-015-9526-3
Matthijs HCP, Jančula D, Visser PM, Marsalek B (2016) Existing and emerging cyanocidal comppunds, new perspectives for cyanobacterial bloom mitigation. Aquat Ecol. doi:10.1007/s10452-016-9577-0
Mitrovic SM, Hardwick L, Dorani F (2011) Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J Plankt Res 2:229–241
Noordhuis R, van Zuidam BG, Peeters ETHM, Van Geest G (2016) Further improvements in water quality of the Dutch Borderlakes: two types of clear states at different nutrient levels. Aquat Ecol. doi:10.1007/s10452-015-9521-8
Pomati F, Jokela J, Veronesi M, Ibelings BW (2011) An automated platform for phytoplankton ecology and aquatic ecosystem monitoring. Environ Sci Technol 45:9658–9665
Reynolds C, Dokulil M, Padisak J (2000) Understanding the assembly of phytoplankton in relation to the trophic spectrum: where are we now? Hydrobiologia 424:147–152
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Sharma NK, Rai AK, Stal LJ et al (2014) Costs of harmful blooms of cyanobacteria. Wiley, New Jersey
Sivonen K, Jones G (1999) Cyanobacterial Toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water. A guide to their public health consequences, monitoring and management. E&FN Spon, London, pp 41–111
Spears BM, Maberly SC, Pan G et al (2014) Geo-engineering in lakes: a crisis of confidence? Environ Sci Technol 48:9977–9979
Stroom JM, Kardinaal WEA (2016) How to combat cyanobacterial blooms: strategy toward preventive lake restoration and reactive control measures. Aquat Ecol. doi:10.1007/s10452-016-9593-0
Triest L, Stiers I, Van Onsem S (2016) Biomanipulation as a nature based solution to reduce cyanobacterial blooms. Aquat Ecol. doi:10.1007/s10452-015-9548-x
Van Dolah ER, Paolisso M, Sellner K, Place A (2016) Employing a socio-ecological systems approach to engage HAB stakeholders. Aquat Ecol. doi:10.1007/s10452-015-9562-z
Verspagen JHM, Passarge J, Johnk KD, Visser PM, Peperzal L, Boers P, Laanbroek HJ, Huisman J (2006) Water management strategies against toxic Microcystis blooms in the Dutch Delta. Ecol Appl 16:313–327
Visser PM, Ibelings BW, Vanderveer B, Koedood J, Mur LR (1996) Artificial mixing prevents nuisance blooms of the cyanobacterium Microcystis in Lake Nieuwe Meer, the Netherlands. Freshwater Biol 2:435–450
Visser PM, Ibelings BW, Bormans M, Huisman J (2016) Artificial mixing to control cyanobacterial blooms: a review. Aquat Ecol. doi:10.1007/s10452-015-9537-0
Weathers K, Hanson PC, Arzberger P et al (2012) The Global Lake Ecology Observatory Network (GLEON): the evolution of grassroots science. Limnol Oceanogr Bull 22:71–73
Acknowledgments
This Special Issue of Aquatic Ecology was developed as a product of the CYANOCOST Action, and we are grateful for the opportunity CYANOCOST has given us to put this Issue together, as well as for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Guest editors: Petra M. Visser, Bas W. Ibelings, Jutta Fastner and Myriam Bormans/Cyanobacterial blooms. Ecology, prevention, mitigation and control.
Rights and permissions
About this article
Cite this article
Ibelings, B.W., Bormans, M., Fastner, J. et al. CYANOCOST special issue on cyanobacterial blooms: synopsis—a critical review of the management options for their prevention, control and mitigation. Aquat Ecol 50, 595–605 (2016). https://doi.org/10.1007/s10452-016-9596-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-016-9596-x