Abstract
The year-to-year variations of vertical distribution and biomass of anoxic phototrophic bacteria were studied during ice periods 2003–2005 and 2007–2008 in meromictic lakes Shira and Shunet (Southern Siberia, Russian Federation). The bacterial layers in chemocline of both lakes were sampled with a thin-layer hydraulic multi-syringe sampler. In winter, biomass of purple sulphur bacteria varied considerably depending on the amount of light penetrating into the chemocline through the ice and snow cover. In relatively weakly stratified, brackish Shira Lake, the depth of chemocline varied between winters, so that light intensity for purple sulphur bacteria inhabiting this zone differed. In Shira Lake, increased transparency of mixolimnion in winter, high chemocline position and absence of snow resulted in light intensity and biomass of purple sulphur bacteria exceeding the summer values in the chemocline of the lake. We could monitor snow cover at the lake surface using remote sensing and therefore estimate dynamics and amount of light under ice and its availability for phototrophic organisms. In Shunet Lake, the light intensities in the chemocline and biomasses of purple sulphur bacteria were always lower in winter than in summer, but the biomasses of green sulphur bacteria were similar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Under-ice limnological studies are few because the sampling and measurements are difficult under severe winter conditions. Usually, winter is considered to be a “dead” season with respect to primary production of phytoplankton because the presence of ice on lake surface tends to limit phytoplankton growth due to decreased light penetration, particularly if it is covered by snow (Sigee 2005). If the ice cover is thin, however, and snow is not present, light penetration may be sufficient to support algal growth (Kelley 1997). For example, in Lake Baikal the populations of diatoms under ice account for about 40% of annual primary productivity (Sigee 2005; see also Jewson et al. 2009, this volume).
Phototrophic anoxic bacteria may contribute substantially to total primary production in stratified lakes with anaerobic hypolimnion (Bieble and Pfennig 1979). These bacteria use sulphide and other reduced compounds of sulphur as electron-donors for anoxygenic photosynthesis. Dense accumulations of these bacteria can develop, if light reaches the deep sulphide-containing layers. Although blooms of anoxic photosynthetic bacteria are observed in the pelagial of many lakes and reservoirs, their ecological significance in these systems remains unclear (Overmann 1997).
Anoxic phototrophic bacteria are generally known to persist in chemoclines of temperate meromictic lakes under ice (Lawrence et al. 1978; Overmann et al. 1994; Tonolla et al. 2003). In winter, the light intensity and abundance of phototrophic bacteria in chemocline are generally lower than in summer. For example, in Lake Cadagno (Switzerland) in March 1999, the light intensity in the chemocline was 10% of that in summer due to ice and snow. The abundance of photosynthetic bacteria (PSB) in chemocline of Lake Cadagno was an order of magnitude lower in March 1999 than in August 1999 (Tonolla et al. 2003). In Lake Mahoney (Canada), during winter (February–March, 1992), average light intensity was between 20 and 50%, and the total average biomass of PSB was about 30% of that in summer (Overmann et al. 1994; Overmann 1997).
The present study concerns lakes Shira and Shunet in Siberia, Russia, which are both covered by ice during 5 months, and where the populations of phototrophic bacteria persist during ice period in the chemocline and the monimolimnion (Savvichev et al. 2005; Rogozin et al. 2005). As part of a comprehensive study of carbon and sulphur cycles in Shira and Shunet Lakes, we studied vertical distribution and biomass of anoxic phototrophic bacteria and profiles of physical and chemical characteristics during under-ice periods from 2003 to 2008. The under-ice samplings and measurements were carried out once per winter.
Materials and methods
Description of the lakes
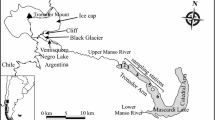
Shira Lake (90.11E, 54.30°N) is located in the northern part of the Republic of Khakassia, Siberia (Russian Federation). This elliptical (9.3 × 5.3 km) lake has an area of 35.9 km2 and maximum depth of 23.8 m. The lake water is brackish, with sulphate–chloride and sodium–magnesium composition (Kalacheva et al. 2002). The average salinity of water is ca. 15 g l−1 in mixolimnion and 19 g l−1 in monimolimnion (Rogozin and Degermendzhy 2008). The lake has no outflow. The main inflow is from Son River that provides about 42% of fresh water supply to the lake; other sources of water are precipitation and seepage water. The lake is in most years ice-covered from the end of November till the beginning of May.
Shunet Lake (90.13E; 54.25°N) is located 8 km to the south-east of Shira Lake. This lake is also elliptical (1.2 × 0.4 km) but with much smaller in area (0.47 km2) and shallower depth (6.2 m). The ionic composition of the lake water is very similar to that of Shira Lake (Parnachev et al. 2002). The salinity of the lake between mixolimnion (17–20 g l−1) and monimolimnion (up to 66 g l−1) differs markedly. The lake has no outflow but an inflow from a small stream. The ice cover persists from early November to April or the beginning of May.
Both lakes have sulphide-rich monimolimnion and exhibit massive development of PSB dominated by species closely related to Lamprocystis purpurea (Chromatiaceae), which form “purple” layers in chemocline (Pimenov et al. 2003; Rogozin et al. 2005). The PSB in “purple” layer in Lake Shunet reached a very high density, >108 cells ml−1 (Rogozin et al. 2005).
Sampling and measurements
For sampling during winter, a hole was drilled in ice at the deepest site in the centre of each lake. The lakes were sampled at the same sites in both winter and summer. Data were collected once during the winter season except in 2008. During ice period of 2008, two surveys were carried out to check whether the biomass of anoxic phototrophic bacteria increased during period from February to March in response to increase in day length.
The chemocline in the lakes was detected on each sampling date using data of temperature, conductivity, dissolved oxygen, turbidity and redox-potential measured with Hydrolab Data-Sonde 4a submersible profiler (Hydrolab, Austin, Texas, USA). The profiler was fixed at the lowest part of a thin-layer hydraulic multi-syringe sampler (Rogozin and Degermendzhy 2008) that was used after locating the chemocline. Twenty 120-ml samples were taken simultaneously over a total depth of 1 m (Rogozin and Degermendzhy 2008). Otherwise, the lakes were sampled using 0.5 l Ruttner sampler. Shira Lake was sampled at depths of 3, 6, 9, 12, 15, 17, 19, 21, 23 m and Shunet Lake at 2, 3, 4, 5.5, 6 m.
Dissolved oxygen and sulphide in chemocline samples were measured by Aquamerck kits (Merck, Germany). If sulphide concentration exceeded 5 mg l−1, the subsamples were fixed with zinc acetate and determined according to Volkov and Zhabina (1990).
Irradiance (PAR) was measured using LI-193SA spherical quantum sensor and LI-COR 1400 datalogger (LI-COR Ltd. Lincoln, NE, USA), and using these data, vertical attenuation coefficients for the mixolimnion, chemocline and monimolimnion were calculated. For light measurements, the hole drilled in ice was covered with an opaque lid to prevent exposure of under-ice water to direct sunlight. The light intensity at the upper border of the chemocline was calculated for a normalized surface light intensity of 1,500 μE m−2 s−1 (Tonolla et al. 2003).
The increase in daily solar radiation during early spring was estimated from the time courses of solar radiation calculated for sampling dates of February 16 and March 22, 2008 by solrad.xls program (Washington State Department of Ecology, Olympia, WA). In this program, the solar position was calculated according to NOAA’s JavaScript solar position calculator, and clear-sky solar radiation was calculated according to Bras model (http://www.srrb.noaa.gov/highlights/sunrise/azel.htm). The coordinates of the lakes, surface ground elevation and dates of interest were input data for calculations. Then, the time curves of solar radiation were integrated to calculate values of daily solar radiation.
Pigment determinations
Samples were filtered through membrane filters (0.22-μm pore size; 32-mm filter diameter: Bio-Chrom, Russia) previously coated with a thin layer of BaCO3. The BaCO3 layer containing bacterial cells was suspended in 5 ml of 90% acetone at 4°C. After 24-h extraction (Montesinos et al. 1983), the samples were centrifuged at 7,000 rpm for 10 min, and the clear supernatant was decanted and used for pigment determinations. Absorption scans from 350 to 900 nm were used to identify the principal pigments. Bacteriochlorophylls a and d in acetone solution were derived from the absorption at 772 nm (Bchl a) and 654 nm (Bchl d) using the specific absorption coefficients of 92.3 l g−1 cm−1 (Jeffrey and Humphrey 1975) and 98 l g−1 cm−1 (Stanier and Smith 1960), respectively. Chlorophyll a was calculated from the absorption at 663 nm using the specific absorption coefficient of 87.67 l g−1 cm−1 (Jeffrey and Humphrey 1975). In the chemocline of Shunet Lake, the absorption maxima were detected between 654 and 663 nm indicating similar concentrations of both Bchl d and Chl a.
In addition, in 2007 and 2008, Chl a was measured with fluorescent chlorophyll probe 6025 installed in YSI 6600 sonde (Yellow Springs, Ohio, USA). The suspension of green alga Chlorella vulgaris was used to calibrate the probe. The selectivity of the probe was checked using suspensions of pure cultures of PSB and green sulphur bacteria (GSB) containing no Chl a. Because a high fluorescent signal was also recorded in the two cultures, the selectivity of the probe is questionable in mixed suspensions of anoxic phototrophic bacteria and Chl a-containing phytoplankton. Thus, the probe data were not used for chemocline and monimolimnion of both lakes. Instead, in mixolimnia, Chl a measured by the probe corresponded well to Chl a determinations.
Pigments were not determined in 2004 and 2005 but computed from the PSB and GSB counts. For computing this, a calibration factor of 2.65 × 10−14 g Bchl a cell−1 was derived from parallel determinations of Bchl a and PSB numbers in samples of 2007 and 2008. A calibration factor of 13.4 × 10−14 g Bchl d cell−1 (Lunina et al. 2007a) was used to convert the GSB numbers into Bchl d.
Microbial counting
For enumeration of bacteria, subsamples were preserved in glutaraldehyde (final concentration 1%). Purple sulphur bacteria filtered through 0.2-μm pore size black Nuclepore filters and stained using DAPI (Porter and Feig 1980) were counted by Zeiss Axioskop 40 FL (Carl Zeiss, Germany) epifluorescence microscope using a filter set 02 (G365, FT 395, LP420, Carl Zeiss, Germany). We distinguished PSB from other bacteria by their distinct shape, size and type of aggregation (Pfennig and Truper 1989). The shape, size and autofluorescence of the cells dominating in chemocline were similar to those for pure cultures (accession number AJ633676 EMBL/GenBank) of PSB isolated from Shira Lake and related to Lamprocystis purpureus (Rogozin, unpublished). In addition, cyanobacterial and algal cells were identified by their orange and red autofluorescence using filter set 15 (BP 546/12, FT 580, LP 590, Carl Zeiss, Germany). PSB were counted at 1,000× magnification in 40 fields, each comprising 0.01 mm2. GSB were counted unstained on 0.2-μm pore size black Nuclepore filters in 40 fields, using a light microscope MBI-11 (LOMO, Russia) in the reflected visible light at 1,100× magnification. In light field, the GSB appear blue or yellow-green (Kopylov et al. 2002a), whereas PSB appear white due to reflection of gas vacuoles. Heterotrophic bacteria were not visible in reflected light.
Remote estimation of snow cover
The 8-day multi-spectral composite images obtained from MODIS space spectroradiometer (product MOD09A1 downloaded from http://edcimswww.cr.usgs.gov/pub/imswelcome were used to estimate snow conditions at the surface of Shira Lake. The percentage of reflectance (ratio of reflected radiation to incident radiation × 100) at the wavelength of 858 nm was used as an index of snow cover. This wavelength provides the best contrast between snowy and snow-free ice surfaces in reflected spectra. This is because maximal reflectance of snow is observed in visible light, but maximal absorbance of water and clear ice is observed in near-infrared light (Hall et al. 2002). The whole surface of Shira Lake included 162 pixels, 500 × 500 m each. The reflectance was calculated for each pixel, and mean value was used to estimate snow cover of the whole surface. In most cases, standard deviation expressed in units of reflectance was <15%. Atmospheric effects were removed using the standard MODIS algorithm of atmospheric correction. This included corrections for the effect of atmospheric gases, aerosols and thin cirrus clouds. An estimate of the snow conditions of Shunet Lake using remote sensing methods was not possible because of its small surface area.
Results
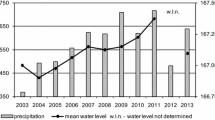
In both lakes, the ice thickness differed insignificantly from year to year, whereas the snow cover varied between no snow and full snow cover (Tables 1, 2). The reflectance of the Shira Lake surface measured by space spectroradiometer corresponded well to the nature of snow cover: the reflectance was low when there was no snow on ice in March 2007 and March 2008 but high when snow entirely covered the ice surface in February 2004, March 2005 and February 2008 (Fig. 1). In February 2003 when snow covered about 50% of the lake area, the reflectance was also high (Fig. 1).
The water column of both lakes on all sampling dates was distinctly separated into an aerobic mixolimnion and an anaerobic monimolimnion (Figs. 2, 3). In winter, water temperature and conductivity were distributed evenly throughout the whole oxygenated mixolimnions. The winter and summer profiles of sulphide were similar in the two lakes (Figs. 2, 3). In Shunet Lake, the vertical position of the chemocline, the transition zone between oxygenated and sulphide-rich waters, was almost constant (Table 2), but in Shira Lake it varied between 11 and 16 m (Table 1).
Characteristics of Shunet Lake (a) in winter and (b) in summer. The highest concentration of bacteriochlorophyll a observed in June 2007 is shown at the proper scale on inserted small panel. Summer 2004 light profile is indicated by arrow on the panel of light profile of March 2008 for comparison. Bacteriochlorophylles in February 2003 are taken from Lunina et al. (2007a)
In both lakes, the light penetration during ice periods depended on snow cover. In both 2007 and 2008, the maximal light intensity under ice was recorded when snow was absent. The lowest light intensity was in February 2003 and 2008 when snow covered the ice surface (Figs. 2a, 3a; Tables 1, 2). Under the snow-free ice, the light intensity for a known depth was about 10-times higher in Shira Lake and 3–6 times higher in Shunet Lake than under the snow-covered ice (Tables 1, 2). Since the light transmittance of snow is much less than that of ice, the thin snow cover of 2 cm resulted in drastic decrease in under-ice light intensity compared with snow-free ice cover conditions (Tables 1, 2). In contrast, the variation of snow thickness from 2 to 20 cm resulted in relatively insignificant variations of under-ice light intensity (Tables 1, 2).
In winter, the vertical light attenuation coefficient (K d) of mixolimnion of Shira Lake was lower than in summer (Table 1). Thus, the water column in winter was more transparent than in summer; in Shunet Lake, however, the K d values in the mixolimnion were similar in winter and summer (Table 2); the maximal light intensity reaching the chemocline in winter was only ca. 20% of that in summer (Table 2). Therefore, in Shunet Lake, the light intensity in chemocline decreased due to ice and snow cover, i.e., due to meteorological factors. On the other hand, in Shira Lake, the maximal light intensity in winter in the chemocline was even higher than in summer (Table 1). This was due to increase in water column transparency combined with snow-free conditions. Therefore, in Shira Lake, the decrease in plankton biomass in winter mixolimnion resulted in improved light conditions for anoxic phototrophic bacteria in chemocline. This was not the case for Shunet Lake because the transparency of mixolimnion, i.e., plankton biomasses, in summer and winter were similar in this lake.
In winter, K d in both lakes was constantly low throughout the mixolimnion but sharply increased in chemocline implying the presence of bacterial plume. In summer, the PAR intensity below the chemocline in Shunet Lake was under detection limit due to extremely dense “purple” layer (data not shown).
In Shira Lake, the Chl a concentration in mixolimnion was lower in winter than in summer (Table 1; Fig. 2). This is in contrast with Shunet Lake where Chl a concentration in mixolimnion in winter was even higher than in summer (Table 2; Fig. 3). In mixolimnion of both lakes, the Chl a was distributed uniformly in winter. In Shira Lake, the summer profile of Chl a concentration had a peak in oxic mixolimnion near the thermocline (Fig. 2b). The sharp Chl a peak detected in March 2007 in chemocline coincided with peak of “purple” layer of PSB (Fig. 2a). In Shunet, the Chl a concentrations always abruptly increased by order of magnitude at the depths near “purple” layer (Fig. 3). In Shira Lake, Chl a under the chemocline was always detectable in absorbtion spectra of acetone extracts. The profiles of Chl a in anoxic monimolimnion of Shira (Fig. 2) were similar in summer and winter.
Bchl a was always absent from mixolimnion of both lakes, but it was present in traces at the upper boundary of the chemoclines. Local peaks of Bchl a were almost regularly detected in the chemoclines of both lakes, but concentration of Bchl a as well as PSB abundance in Shunet Lake were from one to two orders higher than in Shira (Figs. 2, 3). In Shunet, Bchl a was a 5–10 cm thick, a “purple” layer present at the oxic–anoxic interface except in February 2003. In this “purple” layer, the concentrations of PSB and Bchl a were lower in winter than in summer for all study years. The peak of PSB in March 2007 was more spread out in depth than at other times (Fig. 3a), and the total concentration of Bchl a in chemocline was maximal (Table 2). In Shira, the peak of PSB was detectable only in March 2007 as “purple” layer in sampler syringes; concurrently, the concentration of Bchl a exceeded all reported values for Shira Lake (Fig. 2a).
Bchl d was not detected at all in Shira Lake, implying the absence of green sulphur bacteria (GSB), which was also confirmed by microscopic examinations. This is in contrast with Shunet Lake where Bchl d was always detected in the both chemocline and monimolimnion (Fig. 3). The total amount of Bchl d in monimolimnion varied less with depth than that of Bchl a (Table 2), and the depth profiles of Bchl d were invariably similar (Fig. 3). In summer, local peaks ranging from 0.6 × 107 to 1.6 × 107 cell ml−1 of GSB were detected in chemocline of Shunet Lake either in the “purple” layer or at 5–10 cm deeper (data not shown). In winter, no peaks of GSB were detected in the chemocline.
In the chemocline of Shira Lake, temperature conditions in winter and summer were similar in most years (Fig. 2; Table 1). The PSB populations developed at temperatures ranging from 0 to 1.7°C for most seasons, except during warm period in June 2007 (Fig. 2). In contrast, in Shunet Lake, the summer temperatures in chemocline were 5–15°C higher than in winter (Fig. 3).
Discussion
Despite the inconveniences of working in field under severe winter conditions, a sampling strategy comparable with that in summer period is certainly crucial for investigation into stratified lakes, especially those exhibiting meromixis. During summer, for sampling of deeper layers of stratified lakes, even slight movements of the boat may disturb the micro-stratification of microorganisms and physical and chemical factors. In winter, in contrast, an almost complete sampling precision and replication can be achieved because of solid ice surface.
Generally, the oxic–anoxic interface positioning in a stratified lake can be affected by both mixing processes and microbial activity such as oxygen and sulphide production. In Shira Lake, the winter oxic–anoxic interface was positioned at the depth of maximal salinity gradient for all years, except 2004 (Fig. 2a). In 2004, the vertical position of oxic–anoxic interface was 1.5 m below the position of maximal salinity gradient (data not shown). Since the position of maximal salinity gradient can be scarcely affected by microbial activity, one can assume that mainly mixing processes are responsible for chemocline and oxic–anoxic interface positioning in Shira Lake in winter. Because the under-ice light conditions of anoxic phototrophic bacteria depend on position of chemocline, the knowledge of mixing processes is necessary for the prediction of phototrophic bacterial production in Shira Lake. Higher salinities in mixolimnion during winter in 2003 and 2008 than in 2007 (Fig. 2a) indicate more intensive mixing with the deeper water layers with higher salinity. Therefore, annual variability in turbulent mixing during the open-water period is responsible for the variability in positioning of chemocline in winter. In Shunet Lake, the salinity gradient between mixolimnion and monimolimnion was notably stronger than in Shira Lake (Fig. 3), and this explains why stratification in Shunet is more stable and the depth of chemocline almost constant.
A relatively more transparent mixolimnion in winter than in summer in Shira Lake cannot be per se due to unfavourable prevailing conditions for phototrophic organisms. The combination of “high” chemocline with absence of snow on ice surface appears to have resulted in relatively more light reaching the chemocline in March 2007. From the time course of the lake surface reflectance (Fig. 1), one can assume that the absence of snow from ice surface probably resulted in higher time-integrated amount of light energy available for photosynthesis during under-ice period of 2007 compared with other winters. Hence, the snowless winter conditions appear to favour PSB in Shira Lake and can explain the anomalously high biomass of PSB observed in March 2007 (Fig. 2a). Since the reflectance of the lake surface corresponded well to snow conditions for fixed dates of measurements (Fig. 1), we noted that remote satellite data can be used to monitor the horizontal distribution and thickness of snow cover of Shira Lake. Therefore, the light conditions under ice can be deduced by monitoring of snow cover dynamics in studied lakes.
Since the concentrations of PSB in the chemoclines of both lakes were lower in February than in March, PSB activity in early spring (March) was probably attributable to an increase in light intensity. The under-ice samplings in 2008 (February and March) allowed us to test the hypothesis of start in spring of PSB growth under-ice. The time-integrated, daily solar radiation calculated using Solrad.xls for 16 February was 6 × 106 J m−2, whereas for March 22 it was 13.8 × 106 J m−2, i.e., 2.3 times higher. Between these samplings dates, the snow cover disappeared from lakes Shira and Shunet, resulting in 7 and 6 times higher light intensity in the chemocline of these lakes, respectively (Tables 1, 2). Therefore, in March 2008, the increase in PSB densities compared with February can be explained by increase in light intensity rather than that in day length. Our study generally indicates that densities of purple sulphur bacteria in meromictic lakes in winter may vary depending on the amount of light penetrating the lake and reaching the chemocline through the snow cover and ice.
In Shira Lake, several studies (Kopylov et al. 2002a; Gaevsky et al. 2002; Pimenov et al. 2003; Lunina et al. 2007b, this study) have substantiate that thermocline is formed in summer at 6–8 m depth. Since the chemocline is always positioned 4–8 m deeper below thermocline, thermal stratification prevents chemocline waters from warming-up during summer. That is why the PSB environment in Shira Lake is not essentially colder in winter than in summer. Both summer and winter populations of PSB in Shira are probably adapted to low temperature. In contrast, in Shunet Lake, the winter populations of anoxic phototrophic bacteria, both PSB and GSB, appear to develop in winter under lower temperature than summer populations.
The winter light conditions for GSB are not necessarily unfavourable. For example, in 2003, the decrease in light due to snow cover was compensated by higher positioning of chemocline and increase in chemocline transparency due to almost complete disappearance of “purple” layer. As a result, depth of PAR was located below the chemocline where GSB developed; notably, available light intensity for GSB below the chemocline was higher than in summer. Assumedly, seasonal variations of light conditions for GSB are regulated by variations of PSB densities in the layers above. In winter, the decrease in PSB density may provide increase in light intensity available for GSB. Such effects of light shading by PSB on GSB have also reported by Montesinos et al. (1983) for Spanish lakes.
The presence of Chl a in anoxic monimolimnion of Shira Lake has been observed in a previous study (Gaevsky et al. 2002). This may be explained by passive sedimentation of phytoplankton. Although the quantification of Chl a in anoxic zone of Shunet was not possible, both the increase in Chl a above the chemocline (Fig. 3) and absorbance spectra of acetone extracts of filtered water samples suggest that concentrations of Chl a under the chemocline cannot be neglected. Since no assimilation of inorganic carbon in light period was noted in Shira at below 16 m in summer and winter (Kopylov et al. 2002b; Pimenov et al. 2003; Savvichev et al. 2005), photosynthetic microorganisms probably were not active in deep anoxic waters of the lake. The higher dissolved oxygen concentrations observed in both lakes under ice in March than in February (Figs. 2, 3) imply photosynthetic activity of oxygenic phytoplankton in mixolimnion thus probably provide an evidence of spring start of phytoplankton growth (Figs. 2, 3).
Previous workers (Pimenov et al. 2003; Savvichev et al. 2005; Lunina et al. 2007a, b) observed that rates of photosynthesis (14C method) in these two lakes were lower in winter than in summer. Moreover, the contribution of anoxic photosynthesis to total primary production was rather low in all seasons (Pimenov et al. 2003; Savvichev et al. 2005). In both lakes, the oxic photosynthesis contributed from 93 to 100% of total photosynthetic inorganic carbon assimilation for both summer and winter (Pimenov et al. 2003; Savvichev et al. 2005). In each lake, the differences in primary production between summer and winter were more due to differences in oxic photosynthesis. However, the winter measurements were made only for 2003 (Savvichev et al. 2005) when light conditions were very poor in both lakes. Indeed, in the winters of 2003 and 2008, the light intensity in chemocline of Shira Lake was <0.4 μE m−2 s−1 (Table 1). This value appears to the lowest light intensity to support growth of pure PSB cultures (van Gemerden et al. 1989). Therefore, anoxic photosynthesis is negligible in the winters in Shira Lake and relatively low in Shunet Lake. Obviously, in the winter of 2007, the rates of oxic and anoxic photosynthesis in both lakes were higher because of considerably higher under-ice light intensities. Although direct measurements have not been made for other winters, in present study we show that variation of under-ice light values suggest that under-ice photosynthesis production may vary significantly depending on environmental conditions.
This study concerns only few aspects of winter ecology of stratified lakes, mainly the under-ice light conditions. Certainly, winter study of other aspects, e.g., phytoplankton primary production and zooplankton studies can add to summer data in understanding the functioning of microbial food web and its role in understanding the total ecosystem dynamics.
Conclusions
-
1.
Winter biomass of purple sulphur bacteria in the studied meromictic lakes may vary depending on amount of light penetrating into the chemocline through the snow cover and ice on a lake surface.
-
2.
The amount of light reaching deeper layers depends on snow-cover characteristics. The nature of snow cover can be remotely sensed by reflectance of the lake surface. Therefore, the under-ice light conditions can be roughly derived by monitoring of snow cover dynamics in studied lakes.
-
3.
In relatively less strongly stratified Shira Lake, the vertical position of chemocline in winter can vary resulting in considerable changes in light conditions of chemocline. Hence, the under-ice light conditions of anoxic phototrophic bacteria depend on chemocline position.
-
4.
Due to increase in transparency of mixolimnion in winter, the combination of shallower chemocline with absence of snow results in higher light intensity in the chemocline of Shira Lake. Therefore, the biomass of purple sulphur bacteria in chemocline of Lake Shira may exceed the summer values under these conditions.
References
Bieble H, Pfennig N (1979) Anaerobic CO2 uptake by phototrophic bacteria. A review. Arch Hydrobiol Beih Ergeb Limnol 12:48–58
Degermendzhy AG, Gaevsky NA, Belonog NP, Ivanova EA, Rogozin DY, Koltashev AA, Gribalev ES (2003) Study of the physico-chemical and biological characteristics of two balineological lakes (Matarak, Shunet, Republic Khakassia). Vestnik Krasnoyarskogo Gosudarstvennogo Universiteta (Proceedings of Krasnoyarsk State University) 5:107–115 [In Russian]
Gaevsky NA, Zotina TA, Gorbaneva TB (2002) Vertical structure and photosynthetic activity of Lake Shira phytoplankton. Aquatic Ecol 36:165–178
Hall DK, Riggs GA, Salomonson VV, DeGirolamo NE, Bayr KJ, Jin JM (2002) MODIS Snow-cover products. Remote Sens Environ 83:181–194
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c in higher plants algae and natural phytoplankton. Biochem Physiol Pflanz 167:161–194
Jewson DH, Granin NG, Zhdanov AA, Gnatovsky RY (2009) Effect of snow depth on under-ice irradiance and growth of Aulacoseira baicalensis in Lake Baikal. This volume
Kalacheva GS, Gubanov VG, Gribovskaya IV, Gladchenko IA, Zinenko GK, Savitsky SV (2002) Chemical analysis of Shira Lake water (1997–2000). Aquat Ecol 36:123–141
Kelley D (1997) Convection in ice-covered lakes: effects on algal suspension. J Plankt Res 19:1859–1880
Kopylov AI, Kosolapov DB, Romanenko AV, Degermendzhy AG (2002a) Structure of planktonic microbial food web in a brackish stratified Siberian lake. Aquatic Ecol 36:179–204
Kopylov AI, Kosolapov DB, Degermendzhy NN, Zotina TA, Romanenko AV (2002b) Phytoplankton, bacterial production and protozoan bacterivory in stratified, brackish-water Lake Shira (Khakasia, Siberia). Aquatic Ecol 36:205–217
Lawrence JR, Haynes RC, Hammer UT (1978) Contribution of photosynthetic green sulphur bacteria to total primary production in a meromictic saline lake. Verh Int Verein Limnol 20:201–207
Lunina ON, Bryantseva IA, Akimov VN, Rusanov II, Rogozin DY, Barinova ES, Lysenko AM, Pimenov NV (2007a) Seasonal changes in the structure of the anoxygenic photosynthetic bacterial community in Lake Shunet, Khakassia. Microbiology 76:368–379 [Translated from Mikrobiologiya]
Lunina ON, Bryantseva IA, Akimov VN, Rusanov II, Barinova ES, Lysenko AM, Rogozin DY, Pimenov NV (2007b) Anoxic phototrophic bacteria community of Shira Lake (Khakassia). Microbiology 76:469–479 [Translated from Mikrobiologiya]
Montesinos E, Geurrero R, Abella C, Esteve I (1983) Ecology and physiology of the competition for light between Chlorobium limicola and Chlorobium phaeobacteroides in natural habitats. Appl Environ Microbiol 46:1007–1016
Overmann J (1997) Mahoney Lake: a case study of the ecological significance of phototrophic sulphur bacteria. In: Jones RI (ed) Advances in microbial ecology, vol 15. Saunders, Philadelphia, pp 251–288
Overmann J, Beatty JT, Hall KJ (1994) Photosynthetic activity and population dynamics of Amoebobacter purpureus in a meromictic saline lake. FEMS Microbiol Ecol 15:309–320
Parnachev VP, Degermendzhy AG (2002) Geographical, geological, hydrochemical distribution of saline lakes in Khakasia, Southern Siberia (2002). Aquatic Ecol 36:107–122
Pfennig N, Truper H (1989) Anoxygenic phototrophic bacteria. In: Staley JT, Bryant MP, Pfennig N, Holt JG (eds) Bergey’s manual of systematic bacteriology, vol 3. Williams and Wilkins, Baltimore, pp 1635–1653
Pimenov NV, Rusanov II, Karnachuk OV, Rogozin DY, Bryantseva IA, Lunina ON, Yusupov SK, Parnachev VP, Ivanov MV (2003) Microbial processes of the carbon and sulphur cycles in Lake Shira (Khakasia). Microbiology 72:221–229 [Translated from Mikrobiologiya]
Porter KG, Feig YS (1980) Use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Rogozin DY, Degermendzhy AG (2008) Hydraulically-operated thin-layer sampler for sampling heterogeneous water columns. J Sib Fed Univ 1:111–117
Rogozin DY, Pimenov NV, Kosolapov DB, Chan’kovskaya JV, Degermendzhy AG (2005) Thin layer vertical distributions of purple sulphur bacteria in chemocline zones of meromictic Lakes Shira and Shunet (Khakassia). Doklady Biological Sciences (Proceedings of the Russian Academy of Sciences) 400:54–56 [Translated from Doklady Akademii Nauk 400:426–429]
Savvichev AS, Rusanov II, Rogozin DY, Zakharova EE, Lunina ON, Yusupov SK, Pimenov NV, Degermendzhy AG, Ivanov MV (2005) Microbiological and Isotopic–Geochemical Investigations of Meromictic Lakes in Khakasia in Winter. Microbiology 74:477–485 [Translated from Mikrobiologiya]
Sigee D (2005) Freshwater microbiology. Wiley, Manchester, p 67
Stanier RY, Smith JHC (1960) The chlorophylls of green bacteria. Biochim Biophys Acta 41:478–484
Tonolla M, Peduzzi S, Hahn D, Peduzzi R (2003) Spatio-temporal distribution of phototrophic sulphur bacteria in the chemocline of meromictic Lake Cadagno (Switzerland). FEMS Microbiol Ecol 43:89–98
Van Gemerden H, Tughan CS, de Wit R, Gerbert RA (1989) Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiol Ecol 62:87–102
Volkov II, Zhabina NN (1990) A method for evaluating reduced sulphur compounds in sea water. Okeanologiya 30:778–782 [In Russian]
Acknowledgments
We thank Mr. Fedor Kozlov for assistance in winter sampling, Dr. Galina Kalacheva and Mrs. Inna Gladchenko for chemical analyses and all others who helped us in winter expeditions. We thank Prof. Kalevi Salonen (University of Jyväskylä, Finland) for helpful comments. This work was partly supported by the Russian Foundation for Basic Research, Grants 09-05-00915-a and 09-04-01114-a, by the Netherlands Organization for Scientific Research NWO), Grant 047.011.2004.030, by award no. PG07-002-1 of the Ministry of Education and Sciences of Russian Federation and US Civilian Research & Development Foundation for the Independent States of the Former Soviet Union (CRDF), by Siberian Branch of Russian Academy of Sciences, Integrative Project No. 95, by Russian Academy of Sciences, Programme No. 23.15.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rogozin, D.Y., Zykov, V.V., Chernetsky, M.Y. et al. Effect of winter conditions on distributions of anoxic phototrophic bacteria in two meromictic lakes in Siberia, Russia. Aquat Ecol 43, 661–672 (2009). https://doi.org/10.1007/s10452-009-9270-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-009-9270-7