Abstract
The wastewater generated from the industrial activities are considered as one of the prime sources of water contamination. The yearly production of synthetic dyes are ~ 700 tons worldwide. Synthetic dyes have detrimental effects on the human as well as animal health. Therefore, there is an urgent need to treat the water containing synthetic dyes. Dye treatment methods can be divided in to three categories namely; chemical, physical, and biological. The chemical procedure includes; photocatalytic degradation, ozonation, fenton reagent, and aerobic and anaerobic degradation are the examples of the biological procedures. However, the physical procedures consists of filtration/coagulation, adsorption, ion exchange etc. Further, these techniques may have its own drawbacks including generation of hazardous sludge and expensive to operate along with high maintenance cost. The most appealing techinque for abatement of dye from the contaminated water is adsorption owing to its ecofriendly, flexibility, affordability, sustainability, and abundant availability of raw materials to produce adsorbents. It has been noticed that over 80% of dye adsorption processes on adsorbent surfaces were endothermic in nature which means the adsorption processes were self-sustaining in terms of energy consumption. In present review paper, the discussion has been focused on the removal of anionic dyes from water using low-cost biochar adsorbents which is not reported in any previous review papers. Further, it will significantly help to the budding researchers to develop continuous water treatment system (in column mode), if wish to work of anionic dyes remediation from water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to industrialization and urbanisation, water resources have been continuously polluted and impacted the health of flora and fauna. Environmental degradation occurs when massive amounts of chemicals are liberated into the water bodies by various industries including the textile, rubber, tannery, paper, cosmetics, printing, dyes, petrochemical, pharmaceutical, plastics, and food processing industries [1]. Some of the industries, whose effluents deposit colours or colourants into the aquatic environment, includes, paints, leather, paper, textiles, resins, pharmaceuticals, cosmetics, plastics, food, and industrial dyes [2]. Dye-induced effluents affect not only the aesthetic nature but also the photosynthetic action by blocking the penetration of sunlight in to the water bodies and hence reducing the biological oxygen demand (BOD) value and increasing the chemical oxygen demand (COD) value [3]. Currently, there are more than 10,000 different types of dyes are in application around the globe, and 700 tonnes of dyes are produced annually [4]. Various processes including bleaching, dyeing, and printing processes involved in the textile industry requires around 3600 different dyes and 8000 distinct chemicals [5]. Substantial amount of water and colours are required in the dyeing process, and 8% to 20% of the dyes are being discharged into the textile industry's effluents [6]. The bulk of textile dyes used in the industry are azo groups as they have biggest structural class (over 60%) than any other group [7,8,9]. Inefficient textile dying procedures, according to Chung [10], result in the release of 15–50% of azo dyes into produced wastewater that are not bonded to fibres and fabrics. Some textile companies cleanse their effluent before releasing it into the environment to dissolve the free azo dyes, whereas some release industrial wastewater directly into water bodies endangering wildlife and creating major ecotoxicological concerns. Utilisation of industrial wastewater decreases the soil quality and also affects the crop germination rate [11,12,13]. Along with increase in the number of textile industries, generation of enormous amount of wastewater containing dyes had increased immensely. Therefore, removal of dyes from wastewater effluents has become vital using effective as well as low-cost treatment techniques to preserve the health of ecosystem. However, during the selection of the best treatment approach, presence of various inorganic and organic contaminants (competing pollutants), their toxicity, and the pertinent environmental discharge levels are the most important factors which should be considered carefully [14, 15]. Further, these dyes can be classified into two groups ionic and non-ionic on the basis of their ionic nature (as shown in Fig. 1). The ionic groups have further been categorised into anionic and cationic dyes. Anions are major active groups in the anionic dyes, whereas as cations are the major active group in cationic dyes. There are few non-ionic dyes, like vat and disperse dyes. These dyes are converted into soluble anionic forms before employing on a substrate. The synthetic dyes could be categorised into direct, acid, and reactive dyes on the basis of their application process.
With specific fibre active sites like hydroxyl group (–OH) or amino group (–NH2), reactive dyes can establish a covalent link [19]. The abundance of hydroxyl groups surrounding the structures of lignocellulosic biomasses makes them accessible for interaction with reactive dyes. Dichlorotriazine, trichloropyrimidine, aminochloro-s-triazine, sulphatoethylsulphone, dichloroquinoxaline, aminofluoro-s-triazine, and difluorochloropyrimide groups are frequently found in commercial reactive dyes [20]. Electrolytes (such sodium chloride or Glauber salt) are frequently employed to neutralise the negatively charged surface of cellulosic fibres and reactive dyes in order to reduce the repulsion charge between them [21].
Whereas, direct dyes are having strong affinity towards cellulose-based fibre systems which can be directly applied for the dying process. The phthalocyanine, anthraquinoid, azo, and metal complexes are the main chromophores in the structure of direct dyes [19]. Although, these dyes are not very much reactive, but presence of these dyes has increased substantially in the water.
A group of acidic dyes are applied to noncellulosic fibres like protein and polyamides under acidic conditions as they include acidic groups in their structural makeup. Sulphonated azo, triphenylmethane, anthraquinone, xanthenes, and nitrodiphenylamine are some of the chromophoric complexes that are found in acid dyes [22]. Through an electrostatic force, cationized fibres can get connected with an acid dye anion [23]. Aquatic flora is negatively impacted by textile dyes that have been released into aquatic environments by various industries. One of the severe issues related to dye contaminated water body is the blockage of sunlight into the water. It stops the light from introducing in to the photic zone of the aqueous environment [24]. This has important ecological impacts, such as altering the aquatic habitat and lowering photosynthesis process of aquatic plants and hence impacting the aquatic plant life [25]. The dye contaminated water has the potential to affect water quality and may induce skin disease, allergies, dermatitis, malignancies, and mutations in humans [26]. Release of high quantities of textile dyes in the atmosphere reduces the level of oxygen, block the sunlight, and impair the biological function of aquatic flora and wildlife [27].
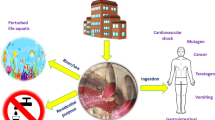
Wastewater containing dyes and dye pigments has a bright colour, fluctuating pH values, high levels of suspended particles, biological oxygen demand, chemical oxygen demand, and also elevated total organic carbon. According to Berradi et al. [28], suspended contaminants due to presence of dyes can inhibit exchange of gases in the fishes from through their gills, which could potentially slow or can stop their growth. On the other hand, consumption of dye contaminated fish and other aquatic animals for sources of protein can cause cramping, fever, and hypertension in human beings [29]. Extremely toxic and perhaps cancer-causing textile dyes have been associated with a number of illnesses in both humans and animals [30, 31]. These problems might result from the replacement of enzyme cofactors that would render the enzymes inactive themselves [32]. Bioaccumulation of dyes (as organic contaminants) can be seen in the freshwater flora, including fish and algae and hence ultimately may affect whole food chain or other parts of the environment as represented in Fig. 2.
The ingested or inhaled textile dyes particularly connected with dust particles would irritate the skin, eyes, and lungs [33]. According to Hanger [34], those who work with reactive dyes run the risk of developing dermatitis, allergic rhinitis, allergic conjunctivitis, occupational asthma, and other allergic reactions. Additionally, 60–70% of azo dyes are hazardous, carcinogenic, and have resistant to standard therapeutic approaches due to their sensitivity to physicochemical breakdown and poor biodegradability [35].
2 Treatment techniques for dye removal from water
Dyes are coloured substances that are used to colour textiles, wools, and fibre in variety of industries along with leather, inks, printer inks, cosmetics, plastics, pharmaceutics, and foods industry [36]. Dyes must be removed after its application in order to prevent pollution of the marine environment and ecosystem and hence causing human health risks. The removal and recovery of toxic dye contaminants from industrial effluent has been accomplished using a variety of dye removal technologies, including oxidation, chemical precipitation, coagulation, reduction, ion exchange, reverse osmosis, solvent extraction, flocculation, adsorption, evaporation, separation, filtration, electrolysis, and membrane [37]. Each of the aforementioned procedures has some advantages and disadvantages (Table 1) that must be carefully considered before applying or researching through removal processes of dye.
2.1 Chemical precipitation
Chemicals like carbonates, sulfides, and hydroxides are used to develop the precipitate of the impurities (including dyes) present in the water and ultimatelty can be settled down and separated accordingly. According to Ahmad et al. [38], hydroxide precipitation is the most popular chemical precipitation technique for the removal of dyes. Dye removal process using precipitation along with adsorption and ion-exchange have ability to remove > 70% of dye [39]. Production of sludge during dye removal, leads to increase in the operational expenditure due to extra cost requirement for the disposal of sludge and high expenditure requirement for maintenance of the dye removal process.
2.2 Ion-exchange
One of the most popular approaches for the treatment of water, which relies on the use of ion-exchange resins (both cationic and anionic), that reacts extensively with functional groups to remove different types of dyes from aqueous solutions. According to Ahmad et al. [38], the ion-exchange resins can be either anion exchangers or cation exchangers. Strong bindings between dyes and resins are created during the ion-exchange procedure through the interchange of positive and negative ions. Theoretically, cationic and anionic dyes form complexes in the form of huge flocs can be separated by filtration using ion-exchange resins. Quarternized cellulose and quarternized sugarcane bagasse are ion-exchange resins that have the ablity to bind hydrolyzed reactive dyes among other resins [40]. On the magnetic ion-exchange resin, ion exchange with magnetic properties predominates in the process of Congo red adsorption [41, 42]. Although reusing the solvent after utilization is possible and can successfully remove soluble colours in ion-exchange processes but the technology and organic solvent required are costly [43].
2.3 Membrane filtration
Membrane filtration is one of the most sophisticated physical techniques for purifying coloured wastewater. In this technique membrane is used that have tiny pores, as a result solutes that are larger than the pores aggregating behind them, leading to generation of dye-free solution. Despite the ease and efficacy of this process, membranes are required to be repalced periodically [44]. Nanofiltration is another innovative membrane technique used to purify wastewater containing dyes. These membranes are having typically diameters ranged between 0.5 and 0.2 nm [45]. Therefore, using the principle of electrostatic repulsion and size, dye molecules can be removed efficiently from wastewater [46]. To remove organic dyes from textile effluent, ultrafiltration (UF) membranes with membrane widths varying from 0.1 to 0.001 µm can be utilised. Although ultrafiltration is more cost-effective than nanofiltration and requires less pressure but the rate of separation is inadequate as membrane pores are too small [47]. In order to eliminate wastewater that contains dye and to deliver water of a high quality, reverse osmosis (RO), a membrane filtration technique is frequently employed in the industries [48, 49].
2.4 Electrodialysis
In this procedure the ion-exchange membranes are placed between the anode and cathode electrodes which does not require any extra pressure for the effectively treatment of coloured water [50]. However, the main problem associated with this process is fouling and that will reduce the efficiency [51].
2.5 Advanced oxidation processes (AOP)
AOPs require the production of oxidizing species in enough quantity to interact with the medium's organic molecules, like hydroxyl and sulphate radicals [52, 53]. In this method hydroxyl radical's are produced and reacts quickly with the majority of organic contaminants [54, 55]. AOPs based on zero-valent aluminum was used to remediate textile effluent and which could remove 94.4% dye from water [56]. The main disadvantage of application AOPs in dye removal involves production of sludge on using Fenton reagent, formation of toxic byproducts on using photocatalysts, high costs owing to the requirement of expensive reagents (for example, H2O2), and energy consumption (generation of O3 or UV radiation) [57, 58].
2.6 Adsorption
Adsorption has been found to be the most effective, cost-effective, and well-liked dye removal method [59]. Adsorbents derived from waste organic materials (such as peels, leaves, and barks) and microbial biomasses (fungus, bacteria and green algae) have become more and more popular as biosorbents because of thier effectiveness, affordablility, and environmental friendly nature [36].
3 Sustainable biochar adsorbents
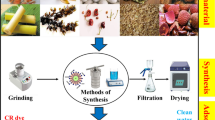
Peter Read initially utilised the term "biochar" in 2015 [60]. Biochar is consider as an environmental friendly, economical and biomass-based adsorbent. Biochar adsorbents have shown vast efficiency for the adsorption of dyes and it also requires less time along with economically viable materials for water purification [61, 62]. It is produced through the pyrolysis or anaerobic digestion of organic materials and is considered as thermally processed, stable, and carbon-rich molecule especially in oxygen free environment [63, 64]. Biomass can be classified into two categeries. First one is lignocellulosic which includes lignin and cellulose and second one are non-lignocellulosic that does not contain lignin and cellulose. The moisture content, cellulose, lignin, nutrients, minerals, inorganic elements, density, particle size, volatiles, percentage of carbon and ash, and hemicellulose content of the biomass affect the quality and efficacy of biochar [65]. Bioenergy crops, forestry byproducts, and agricultural byproducts are all categorised as lignocellulosic materials. Algae, dung, animal hair, and sewage sludge are some examples of non-lignocellulosic materials [66]. The non-lignocellulosic biomass has a higher composition of mineral and nutrition as compared to the lignocellulosic biomass [67]. According to Rashidi et al. [66], the primary component for manufacturing of biochar is thought to be agricultural waste. Agricultural waste contains 35 to 50% cellulose and 15 to 25% lignin while hemicelluloses content 15 to 40% all of these have sigificant influence on the features of the biochar. The residues of crop waste from agriculture fields, municipal wastes, food wastes, and animal manures are the biomass sources that could be applied for the production of biochar [68,69,70]. The carbon content of biochar produced from biomass via pyrolysis in an oxygen-limited atmosphere has been found very high [71, 72]. The high porosity, greater potential of cation exchange, higher stability, surface area porosity, and functional groups are the unique characteristics of the biochar and that make them main reason for multiple applications in various fields [73, 74]. The key factors affecting the biochar production are temperature, biomass type, residence time, heating rate, and pressure [75, 76]. In order to determine stability, the elemental composition, structure of biochar, and surface functional groups, numerous characterization techniques like Raman spectroscopy, BET, FTIR, XRD, NMR, SEM, TGA, etc. has been applied [77, 78]. Treatment of biochar with acid, alkali, or oxidising agents can enhance its physicochemical qualities as these treatment can change their surface area [79,80,81]. By thorough study of literatures on the properties, methods for analysing and quantifying the biochar will help in understanding the effectiveness of biochar in many industries. Moreover, the by-products generated during the production of biochar adsorbents like synthetic oil etc. can be used as an additional asset i.e. for energy requirements [82].
4 Scientific methods for biochar adsorbents production
For producing biochar adsorbents, biomass conversion process can be broadly classified as physicochemical, biochemical as well as thermochemical techniques. By the virtue of mechanical force, bioactive part of the biomass can be exctrated during physicochemical conversion. However, catalysis, heat and pressure are applied during thermal conversion of the biomass to produce biochar adsorbents [83]. Gasification, liquefaction, pyrolysis and torrefaction are the examples of thermochemical process of biomass conversion [84] as mentioned below in details.
4.1 Pyrolysis
Pyrolysis is the technique of burning the feedstock at high temperatures (250–900 °C) with little or no oxygen present. Moreover because of its widespread application on variety of biomass for production of biochar, pyrolysis considered as optimum approach among all biochar manufacturing procedures [85]. In pyrolysis, the biomass can undergo through multiple phases of development, comprising solid, liquid, and gas. The amount of biochar produced is significantly influenced by the various pyrolysis parameters including temperature, residence time, and biomass type [86]. According to the temperature and residence time, pyrolysis can be categorised into three categories: slow, fast, and microwave.
4.1.1 Slow pyrolysis
In this procedure, the biomass is pyrolyzed at temperatures between 300 and 500 °C for an extended period of time, ranging from a few seconds to days [87]. Since slow pyrolysis produces mostly solid products (biochar), with production of just a minor amount of gaseous (biogas) and liquid (bio-oil) products and hence it considered as the most efficient method for biochar production. In general, less biochar and less biogas are produced with increase in the temperature of pyrolysis. The higher quantity of biochar yield is produced during slow pyrolysis as it uses less heat and a longer residence time [88]. Study conducted by Tomczyk et al. had founded that 35.0% of biochar was formed by slow pyrolysis of dry biomass [89].
4.1.2 Fast pyrolysis
Biomass is transformed into bio-oil by a thermochemical process called fast pyrolysis, which is very helpful for creating energy [64]. The temperature range is between 500 and 700 °C. Greater heating rates (> 10–10,000 °C/min) and brief residence durations (seconds) define this kind of pyrolysis. As indicated in the previous section, fast pyrolysis as compared to slow pyrolysis, due to rise in temperature and fall in residence time results in relatively meagre production of biochar. Instead, rapid pyrolysis produces the majority of the bio-oil. The characteristics of the biochar that is created also depend on the type of pyrolysis technique used. In contrast to improved surface functional group and cation exchange capacity (CEC), higher pyrolysis temperatures lead to larger surface areas, higher pH, and more volatiles [89]. In accordance with Ghani et al. [90], biochar has a hydrophobic propensity and high heat conductivity at temperatures above 650 °C and becomes more hydrophilic at temperatures below 500 °C (slow pyrolysis).
4.1.3 Microwave pyrolysis
Intense microwave radiation is used in microwave pyrolysis in order to break down the biomass [91]. Microwave pyrolysis elevates the temperature of the feedstock's within in as compared to outside, in contrast to conventional pyrolysis [92]. This method is fast with consistent heating and because of this reason it is one of the cost-effective methods for biochar production [93, 94]. Preheating and dehumidification are not necesaarily important for application of this techinique. In contrast to other traditional methods, microwave pyrolysis only produces a very little amount of bio-oil. Using microwave-assisted pyrolysis, Wang et al. [95] produced biochar from camellia (Camellia oleifera) peel with outputs of 37.45% and 27.45% for production of biochar and bio-oil respectively.
4.2 Hydrothermal carbonization (HTC)
Water surrounds the biomass during the hydrothermal carbonization reaction, often known as "wet pyrolysis" [96]. This procedure uses low pressure and reaction temperatures between 180 and 230 °C in a closed system [97]. The pressure and temperature are kept under control in the HTC method so that the water is kept in liquid state throughout the process. High yield, low temperature (approximately 180 °C) requirement and energy efficient biochar production is the benefit of HTC [98]. It encompasses various chemical processes, including dehydration, condensation, decarboxylation, and hydrolysis [99]. The final HTC product is referred to as "hydrochar" to distinguish it from the biochar produced via pyrolysis. Additional functional groups, including oxygen, are incorporated into the hydrochar that are used in order to enhance the adsorption capacity of heavy metals from aqueous environments [100].
4.3 Gasification
Gasification of biomass is also considered as another potential method for production of biochar. The biochar using this method can be prepared at a temperature of 700ο in an oxidising atmosphere (single or mixed gases) [101]. In addition, syngas, a combination of H2, CO, CO2, and CH4, are produced during the partial combustion of the feedstock in an oxidising environment and that can be further used as fuel. The primary element influencing the generation of syngas is the reaction temperature [64]. On comparing with other techniques of biochar production, gasification yields the most gaseous output with least amount of biochar quantity. This process results in biochar that is highly stable, resistant to chemical oxidation, and smaller in particle size. Biochar contains minerals like N, K, P, and Ca that have the ability to improve soil fertility and can hence enhance the plant growth.
5 Application of biochar for the removal of dyes from water
Roy et al. [102] produced biochar using Indian Bael shell (Aegle marmelos) for the adsorption of the patent blue dye (V). Bael shell sample was subjected to heat at 500 °C for a period of 3 h in a pyrolyzer. The highest binding capacity of 3.7 mg/g at pH 2.7 was observed using the prepared biochar of Indian Bael shell. The temperature of 303 K and contact time of 60 min was observed as the optimal condition for the elimination of pollutants. The pseudo-second order kinetic model and Langmuir isotherm model was the best fit for the adsorption of blue pigment on the Bael shell. Van der Waal forces, hydrogen bonds, and electrostatic interaction are applied in order to understand the mechanism of adherence of Patent Blue (PB) pigment on Bael shell biochar (BSB). At higher pH levels (> 8), the carboxylic group on the surface of BSB completely dissociates, forming an ionic bond with the negatively charged PB dye that was responsible for removal of PB dye from wastewater. Hanoon et al. [103] derived biochar from sawdust raw material in order to treat contaminated effluent containing toxic methyl orange dye. Methyl orange showed a maximum adsorption of 138.2 mg/g at temperature 289 K, initial concentration 100 mg/L, and pH 2. The adsorption process reached equilibrium within 360 min. The isothermal data was better explained by the Langmuir isotherm model. The biochar derived from the Date Palm Petiole (DPB) by pyrolysis process (temperature range from 350 to 700 °C) for the remediation of methyl orange (MO) contaminated water [104]. According to the investigation, DPB has shown adsorption capacity of 461 mg/g for MO. The experimentation was performed at different temperatures of 293 K, 303 K, 313 K, and 323 K. The specific pH for MO adsorption on DPB was 4 with an initial dye concentration of 100 mg/L and contact time 60 min. The Langmuir isotherm model was identified as the best isotherm model, whereas pseudo-second order was found to be the best kinetic model [104]. Kapoor et al. [105] used pineapple fruit peel biochar (PFPB) for the elimination of the dye was happened due to Patent Blue (PB) dye from wastewater [105]. PFPB demonstrated the maximum 95% removal of PB dye at pH 2 at initial dye concentration of 600 mg/L. Equilibrium studies showed the monolayer adsorption with adsorption capacity of 10.29 mg/g and resembled with Langmuir isotherm. Kinetic study supported the pseudo second-order model. Eleryan et al. [106] produced biochar using mandarin peel (MP) waste. To develop mandarin biochar-C-TETA (MBCT), biochar was treated with 80% H2SO4, boiled with water, and then heated with triethylenetetramine. The maximum adsorption capacity was found to be 312.5 mg/g at optimized pH of 2.0, adsorbent dosage of 0.25 g/L, and initial concentration of 100 mg/L. The analysis of kinetic and isothermal data revealed that the pseudo-second-order and Langmuir isothermal model was the best-fitted model for adsorption. The electrostatic attractive interactions between the positively charged sites on the sorbent surface and the negatively charged anionic dye molecules were the primary method of the MBCT sorption of the AO7 dye's anion absorption. In a study by El Nemr et al. [107], leftover pea peels (Pisum sativum) impregnated with ZnCl2 were heated at 600, 700, and 800 °C with the addition of CO2 to produce Pea Peels-Activated Carbon (PPAC) at respective temperature. Novel biochar was then evaluated for the removal of the AY11 dye from an aqueous solution. The pH 2 was found to be suitable for the removal of the AY11 dye. The maximum AY11 dye elimination percentage of 99.10% was achieved with an initial AY11 dye concentration of 100 mg/L and adsorbent dose of 1.0 g/L. The maximum adsorption capacity of the PPAC was achieved as 515.46 mg/g. The experimental data from PPAC were fitted by the Halsey and Freundlich isotherm models. The kinetic study had shown the pseudo-second order as the best fitted kinetic model. Anions are commonly taken up during the process of adsorption of AY11 dye by PPAC because a rise in positively charged ions at acidic pH levels hence producing the attractive electrostatic forces hence making them more attractive. Purbasari et al. [108] conducted the study using geopolymer that are frequently used as adsorbent for heavy metal and dye. The ability of geopolymers to exchange ions can be increased by surface modification with cationic surfactants. In this study, an adsorbent for the removal of anionic methyl orange (MO) dye was produced by combining a geopolymer made from fly ash and modified using cetyltrimethylammonium bromide (CTAB) a cationic surfactant. The equilibrium was reached within 90 min at pH 2, Pseudo-second-order kinetics, and Langmuir isotherm studies showed that the modified geopolymer had a highest adsorption capacity of 19.231 mg/g for MO dye. Recently, Huynh et al. [109] studied the adsorption of methyl orange (MO) from wastewater onto Pine leaves-based biochar (Pinus kesiya). The maximum adsorption capacity was reported as 136.99 mg/g. The kinetic studies showed that the Elovich, pseudo-first-order, pseudo-second-order, and intraparticle diffusion models had played an important role in biosorption of MO onto the biomass of pine leaves. Thermodynamic experiments proved that MO dye adsorption was spontaneous, endothermic and physisorption in nature. At pH lower than 7.5, the adsorbent had a positive surface hence potential electrostatic attraction increased anion MO adsorption. In another study, Kim et al. [110] evaluated the removal of Methyl Orange (MO) and Sunset Yellow FCF (SYF) using pristine biochar prepared from pine sawdust (PSB) and PSB that had been modified with Mg/Al layered double hydroxides (PSB-LDHMgAl). The adsorption of MO and SYF by PSB and PSB-LDHMgAl followed pseudo-second-order kinetics. In comparison to PSB (MO = 2.2 mg/g and SYF = 1.6 mg/g) the adsorption capacity of MO and SYF adsorbed onto PSB-LDHMgAl (MO = 21.8 mg/g and SYF = 23.6 mg/g) was much higher. In terms of isotherm study, the MO and SYF adsorption via PSB-LDHMgAl followed the Freundlich isotherm better than the Langmuir isotherm. Optimal condition for adsorption was reported at the initial dye concentration of 5 mg/L, temperature of 298 K, adsorbent dosage of 0.04–0.4 g/L and contact time of 24 h. Ahmadian et al. [111] had utilised the Lemon peel biochar as an adsorbent to extract chromium (VI) and Acid Orange 7 from acidic and basic solutions. Maximum adsorption occurred under ideal circumstances, with an initial Acid Orange 7 concentration of 100 mg/L, a pH of 2, biochar dose of 0.2 g/L and contact period of 90-min. The maximum adsorption capacity of Acid Orange 7 was observed as 225 mg/g. The Langmuir isotherm and pseudo-second-order kinetics model were observed as the best fit and revealed multilayer adsorption with chemical interactions between pollutants and the surface of biochar. The capability of biochar produced using green pea peel (GPBC) and zinc oxide green pea peel nanocomposite (ZnO/GPBC) for elimination of reactive Congo Red (CR) from water solution was examined. The most excellent monolayer adsorption capacity of 62.11 mg/g using GPBC and 114.94 mg/g using ZnO/GPBC were reported at the optimum temperature of 25 °C. For both the biochar adsorbents, the removal processes have followed pseudo-second-order kinetic model. Freundlich model was the best fit for the ZnO/GPBC, whereas GPBC based adsorption showed Langmuir model as the best fit. At pH 6 and 100 mg of adsorbent dose, the maximum adsorption was achieved [112].
6 The mechanism involves the adsorption of dyes using biochar adsorbents
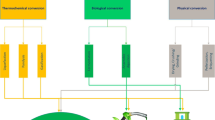
Important characters of biochar are responsible for the effective adsorption of the pollutants from water such as cation exchange capacity, presence of reactive groups on the surface, size of pores as well as high surface area of the biochar adsorbents. However, the production circumstances like temperature, residence time, heating rate, raw materials, etc. may influence the characters of the biochar adsorbents. Further, at higher temperatures, the quantity of biochar will get reduced, but the surface area will increase along with greater carbon fractions [113]. The removal of dyes from wastewater by biochar occurs via both chemisorption and physisorption. The different mechanisms like complexation, electrostatic interaction, pore-filling, cation exchange, and van der Waals interaction are involved in the adsorption of dye on the biochar [114]. Recently, Dwivedi and Dey [115] observed that biochar adsorbents could remove 12,501.98 mg/g which is very high. For dye removal from water the major mechanisms could be electrostatic interaction, hydrogen bonding, π–π interaction, etc. either in combination or alone. It was observed that the adsorption of malachite green dye (MGD) on the activated biochar derived from Opuntia ficus-indica primarily follows pore-filling mechanism [116]. While the functional groups on the MGD and the biochar formed hydrogen bonds that enhance the adsorption process. According to XRD and FTIR spectrum, the activated biochar contains electron donor group like amine and hydroxyl groups, but the MGD contained functional groups as a component of the aromatic ring that were electron-deficient. It is also possible that a large number of functional groups (–OH, –CO, and –CH) could be present on the surface of the biochar, which may have contributed to the adsorption of dyes via-stacking, hydrogen bonds, and van der Waals forces, among other mechanisms [117]. According to Nartey and Zhao [118], presence of porous and vertical channels as observed through SEM images of biochar showed that pore filling might be another factor in dye adsorption. For the adsorption of anionic dyes, specifically for mineral-rich biochar, the displacement of mineral ions of biochar with dyes in the aqueous solution has also been documented [119]. The adsorption efficiency of the anionic dyes may increase in presence of cations like Ca2+, and Mg2+ in the matrix of biochar indicating the presence of ion exchange mechanisms [120]. Figure 3 shows the commonly reported mechanisms for dye removal from water using biochar adsorbents.
A variety of adsorbent materials were previously used for Remazol Brilliant Blue R dye (RBBR) adsorption such as chitosan-tripolyphosphate/kaolin clay, macroporous polystyrene resin (Amberlyst A21), leaf powder, lime peel powder, coffee husk-based activated carbon, and bone. However, the biochar adsorbents employed in this study had better adsorption capacities than the other adsorbents (9.6–208 mg/g) except chitosantriphosphate/kaolin clay (687 mg/g) used to remove RBBR [121]. Since these biochar adsorbents were produced from the discarded garbage and observed as effective and economical adsorbent in comparison of chitosan-tripolyphosphate/kaolin clay [122]. Therefore, the biochar adsorbents may also be able to remove multiple types of dyes, but more research is required to determine their precise concentration [123].
7 Anticipated challenges in the applications of the biochar adsorbents
Biochar's potential as an adsorbent for the elimination of dyes has been discussed in detail in the sections described above, but application of biochar had several disadvantages. In some studies, it has been reported that the yield of biochar is low at higher temperature range so it can be taken as challenge so that engineering devices can be developed to produce more quantities of the biochar. Few research had shown that the efficiency of biochar declines with the time on application of dye removal from water [124, 125] it might be due to the saturation of active sites present on the surface of the biochar. Further, desorption of biochar adsorbents seems difficult because of organic nature of the biochar adsorbents. Further, in the mixed composites of biochar adsorbents (especially with inorganic chemicals), leaching metals can be expected in the treated water which needs extra treatment [126,127,128]. Additionally, biochar generated from the sludge, can have some heavy metals (e. g. sludge of swine wastewater), and they can affect the environment and other living creatures as well [126]. According to Zhang et al. [127, 128], some heavy metals may be more volatile at temperatures about > 400 °C. If the biochar adsorbents is developed more than this temperature, it may contaminate the surrounding environment from such volatile heavy metals. Extensive research for the standardization of the biochar adsorbents can be conducted in future to overcome these shortcomings so that the utilization of biochar adsorbents can be promoted greatly.
8 Conclusions and future perspectives
On the basis of above discussions, it can be inferred that the potentials of different biochar adsorbents for the removal of dyes are considerably high. Further, less efficient biochar adsorbents can also be modified through adding some hydroxides/metals which can enhance their efficiency. However, it may increase the cost of water treatment little bit. Regeneration of biochar adsorbents is also possible but it is insignificant as most of the biochar adsorbents can be developed by using waste biomass like crop residues, leaves, sludge etc. Application of biochar has been extensively reported in soil health maintenance, whereas water treatment using biochar adsorbents is relatively less explored especially for dyes. Biochar adsorbents are also having some shortcomings of precision in standardization of practices to produce them. Cost is also among the important factors, however; it may also vary as per the methods used for their production and other parameters. Nevertheless, adsorption of dyes using biochar adsorbents has been a preferred method of water purification. The major feedstocks used for biochar production are agricultural waste, algae biomass, sludge, plant residue, leaves etc. Pyrolysis and hydrothermal carbonization are most common and easy methods to prepare them. Literatures showed that pseudo-second order kinetic model and Langmuir isotherm model were dominated in most of the studies of anionic dye removal from water. Langmuir model fitness with adsorption experiment data is the representation of homogeneous nature of adsorption process. In few studies the adsorption capacities of biochar adsorbents were quite well even > 1000 mg/g for dye adsorption from water. However, extensive research works are still required to achieve the dye removal using biochar in column mode as most of the studies have been conducted in batch experiment mode. Effect pH on the adsorption process and other parameters of biochar production should be validated very carefully so that the drawbacks can be reduced as much as possible.
Data availability
No datasets were generated or analysed during the current study.
References
Sharma, A., Siddiqui, Z.M., Dhar, S., Mehta, P., Pathania, D.: Adsorptive removal of Congo red dye (CR) from aqueous solution by Cornulaca monacantha stem and biomass-based activated carbon: isotherm, kinetics and thermodynamics. Sep. Sci. Technol. 54(6), 916–929 (2019)
Ranjbar, D., Raeiszadeh, M., Lewis, L., MacLachlan, M.J., Hatzikiriakos, S.G.: Adsorptive removal of Congo red by surfactant modified cellulose nanocrystals: a kinetic, equilibrium, and mechanistic investigation. Cellulose 27, 3211–3232 (2020)
Reck, I.M., Paixao, R.M., Bergamasco, R., Vieira, M.F., Vieira, A.M.S.: Removal of tartrazine from aqueous solutions using adsorbents based on activated carbon and Moringa oleifera seeds. J. Clean. Prod. 171, 85–97 (2018)
Elgarahy, A.M., Elwakeel, K.Z., Elshoubaky, G.A., Mohammad, S.H.: Untapped sepia shell–based composite for the sorption of cationic and anionic dyes. Water Air Soil Pollut. 230, 1–23 (2019)
Hussain, I., Li, Y., Qi, J., Li, J., Wang, L.: Nitrogen-enriched carbon sheet for methyl blue dye adsorption. J. Environ. Manage. 215, 123–131 (2018)
Javaid, R., Qazi, U.Y.: Catalytic oxidation process for the degradation of synthetic dyes: an overview. Int. J. Environ. Res. Public Health 16(11), 2066 (2019)
Ayed, L., Mahdhi, A., Cheref, A., Bakhrouf, A.: Decolorization and degradation of azo dye methyl red by an isolated Sphingomonas paucimobilis: biotoxicity and metabolites characterization. Desalination 274(1–3), 272–277 (2011)
Bhattacharya, S., Gupta, A.B., Gupta, A., Pandey, A.: Introduction to water remediation: importance and methods. Water Remediat. (2018). https://doi.org/10.1007/978-981-10-7551-3_1
Thangaraj, S., Bankole, P.O., Sadasivam, S.K.: Microbial degradation of azo dyes by textile effluent adapted, Enterobacter hormaechei under microaerophilic condition. Microbiol. Res. 250, 126805 (2021)
Chung, K.T.: The significance of azo-reduction in the mutagenesis and carcinogenesis of azo dyes. Mutat. Res./Rev. Genet. Toxicol. 114(3), 269–281 (1983)
Jiku, M.A.S., Singha, A., Faruquee, M., Rahaman, M.A., Alam, M.A., Ehsanullah, M.: Toxic wastewater status for irrigation usage at Gazipur and Savar industrial vicinity of Bangladesh. Acta Ecol. Sin. 41(4), 358–364 (2021)
Kaur, N., Kaushal, J., Mahajan, P., Mantri, A.: Phytoremediation of methylene blue dye (triarylmethane) and Congo red (diazo) by T. ammi L.: kinetic studies. Int. J. Environ. Sci. Technol. 21, 1697–1714 (2023)
Kaushal, J., Mahajan, P.: Kinetic evaluation for removal of an anionic diazo direct red 28 by using phytoremediation potential of Salvinia molesta Mitchell. Bull. Environ. Contam. Toxicol. 108, 437–442 (2022)
Lellis, B., Fávaro-Polonio, C.Z., Pamphile, J.A., Polonio, J.C.: Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 3(2), 275–290 (2019)
Li, F., Zhao, K., Ng, T.S.A., Dai, Y., Wang, C.H.: Sustainable production of bio-oil and carbonaceous materials from biowaste co-pyrolysis. Chem. Eng. J. 427, 131821 (2022)
Periyasamy, A.P.: Recent advances in the remediation of textile-dye-containing wastewater: prioritizing human health and sustainable wastewater treatment. Sustainability 16(2), 495 (2024)
Yazdani, M.R.: Engineered adsorptive materials for water remediation-Development, characterization, and application (2018)
Lu, J., Zhou, Y., Zhou, Y.: Recent advance in enhanced adsorption of ionic dyes from aqueous solution: a review. Crit. Rev. Environ. Sci. Technol. 53(19), 1709–1730 (2023)
Tunç, Ö., Tanacı, H., Aksu, Z.: Potential use of cotton plant wastes for the removal of Remazol Black B reactive dye. J. Hazard. Mater. 163(1), 187–198 (2009)
Parimelazhagan, V., Jeppu, G., Rampal, N.: Continuous fixed-bed column studies on Congo red dye adsorption-desorption using free and immobilized Nelumbo nucifera leaf adsorbent. Polymers 14, 54 (2021)
Ben Arfi, R., Karoui, S., Mougin, K., Ghorbal, A.: Adsorptive removal of cationic and anionic dyes from aqueous solution by utilizing almond shell as bioadsorbent. Euro-mediterr. J. Environ. Integr. 2, 1–13 (2017). https://doi.org/10.1007/s41207-017-0032-y
Tahir, M.A., Bhatti, H.N., Iqbal, M.: Solar Red and Brittle Blue direct dyes adsorption onto Eucalyptus angophoroides bark: equilibrium, kinetics and thermodynamic studies. J. Environ. Chem. Eng. 4(2), 2431–2439 (2016)
Farah, J.Y., EL-Gendy, N.S.: Performance, kinetics and equilibrium in biosorption of anionic dye acid Red 14 by the waste biomass of Saccharomyces cerevisiae as a low-cost biosorbent. Turk. J. Eng. Environ. Sci. 37(2), 146–161 (2013)
Liang, J., Ning, X.A., Kong, M., Liu, D., Wang, G., Cai, H., Sun, J., Zhang, Y., Lu, X., Yuan, Y.: Elimination and ecotoxicity evaluation of phthalic acid esters from textile-dyeing wastewater. Environ. Pollut. 231, 115–122 (2017)
Khan, M.D., Abdulateif, H., Ismail, I.M., Sabir, S., Khan, M.Z.: Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. PLoS ONE 10(10), e0138448 (2015)
Sarvajith, M., Reddy, G.K.K., Nancharaiah, Y.V.: Textile dye biodecolourization and ammonium removal over nitrite in aerobic granular sludge sequencing batch reactors. J. Hazard. Mater. 342, 536–543 (2018)
Ghaedi, M., Hajjati, S., Mahmudi, Z., Tyagi, I., Agarwal, S., Maity, A., Gupta, V.K.: Modeling of competitive ultrasonic assisted removal of the dyes–methylene blue and Safranin-O using Fe3O4 nanoparticles. Chem. Eng. J. 268, 28–37 (2015)
Berradi, M., Hsissou, R., Lakdioui, M.K.T., Bekhta, A., Rafik, M.E.G.M., El Bachiri, A., El Harfi, A.: Ultrafiltration of wastewater-models loaded with vat dyes (indigo blue) by membranes composed of organic polymers at different percentages: comparative study. Moroc. J. Chem. (2019). https://doi.org/10.48317/IMIST.PRSM/morjchem-v7i2.13841
Sharma, A., Mittal, R., Sharma, P., Pal, K., Mona, S.: Sustainable approach for adsorptive removal of cationic and anionic dyes by titanium oxide nanoparticles synthesized biogenically using algal extract of Spirulina. Nanotechnology 34(48), 485301 (2023)
Jin, X., Wu, C., Tian, X., Wang, P., Zhou, Y., Zuo, J.: A magnetic-void-porous MnFe2O4/carbon microspheres nano-catalyst for catalytic ozonation: preparation, performance and mechanism. Environ. Sci. Ecotechnol. 7, 100110 (2021)
Tounsadi, H., Metarfi, Y., Taleb, M., El Rhazi, K., Rais, Z.: Impact of chemical substances used in textile industry on the employee’s health: epidemiological study. Ecotoxicol. Environ. Saf. 197, 110594 (2020)
Wu, L., Xu, Y., Lv, X., Chang, X., Ma, X., Tian, X., Shi, X., Li, X., Kong, X.: Impacts of an azo food dye tartrazine uptake on intestinal barrier, oxidative stress, inflammatory response and intestinal microbiome in crucian carp (Carassius auratus). Ecotoxicol. Environ. Saf. 223, 112551 (2021)
Clark, M. (ed.): Handbook of Textile and Industrial Dyeing: Principles, Processes and Types of Dyes. Elsevier, Amsterdam (2011)
Hanger, K.: Industrial dyes: Chemistry, properties and applications, health and safety aspects (2003)
Rawat, D., Sharma, R.S., Karmakar, S., Arora, L.S., Mishra, V.: Ecotoxic potential of a presumably non-toxic azo dye. Ecotoxicol. Environ. Saf. 148, 528–537 (2018)
Piaskowski, K., Świderska-Dąbrowska, R., Zarzycki, P.K.: Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 101(5), 1371–1384 (2018)
Afroze, S., Sen, T.K.: A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut. 229, 1–50 (2018)
Ahmad, A., Mohd-Setapar, S.H., Chuong, C.S., Khatoon, A., Wani, W.A., Kumar, R., Rafatullah, M.: Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 5(39), 30801–30818 (2015)
Vimonses, V., Jin, B., Chow, C.W.: Insight into removal kinetic and mechanisms of anionic dye by calcined clay materials and lime. J. Hazard. Mater. 177(1–3), 420–427 (2010)
Singh, K., Arora, S.: Removal of synthetic textile dyes from wastewaters: a critical review on present treatment technologies. Crit. Rev. Environ. Sci. Technol. 41(9), 807–878 (2011)
Jia, L., Liu, H., Kong, Q., Li, M., Wu, S., Wu, H.: Interactions of high-rate nitrate reduction and heavy metal mitigation in iron-carbon-based constructed wetlands for purifying contaminated groundwater. Water Res. 169, 115285 (2020)
Jia, Y., Ding, L., Ren, P., Zhong, M., Ma, J., Fan, X.: Performances and mechanism of methyl orange and Congo red adsorbed on the magnetic ion-exchange resin. J. Chem. Eng. Data 65(2), 725–736 (2020)
Jebapriya, G.R., Gnanadoss, J.J.: Bioremediation of textile dye using white rot fungi: a review. Int. J. Curr. Res. Rev. 5(3), 1 (2013)
Samsami, S., Mohamadizaniani, M., Sarrafzadeh, M.H., Rene, E.R., Firoozbahr, M.: Recent advances in the treatment of dye-containing wastewater from textile industries: overview and perspectives. Process. Saf. Environ. Prot. 143, 138–163 (2020)
Behera, M., Nayak, J., Banerjee, S., Chakrabortty, S., Tripathy, S.K.: A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: an integrated system design approach. J. Environ. Chem. Eng. 9(4), 105277 (2021)
Dasgupta, J., Mondal, D., Chakraborty, S., Sikder, J., Curcio, S., Arafat, H.A.: Nanofiltration based water reclamation from tannery effluent following coagulation pretreatment. Ecotoxicol. Environ. Saf. 121, 22–30 (2015)
Dasgupta, J., Singh, M., Sikder, J., Padarthi, V., Chakraborty, S., Curcio, S.: Response surface-optimized removal of reactive red 120 dye from its aqueous solutions using polyethyleneimine enhanced ultrafiltration. Ecotoxicol. Environ. Saf. 121, 271–278 (2015)
Wang, A.J., Wang, H.C., Cheng, H.Y., Liang, B., Liu, W.Z., Han, J.L., Zhang, B., Wang, S.S.: Electrochemistry-stimulated environmental bioremediation: development of applicable modular electrode and system scale-up. Environ. Sci. Ecotechnol. 3, 100050 (2020)
Wang, X., Xia, J., Ding, S., Zhang, S., Li, M., Shang, Z., Lu, J., Ding, J.: Removing organic matters from reverse osmosis concentrate using advanced oxidation-biological activated carbon process combined with Fe3+/humus-reducing bacteria. Ecotoxicol. Environ. Saf. 203, 110945 (2020)
Al-amshawee, S., Yunus, M.Y.B.M.: Influence of light emitting diode (LED) on microalgae. J. Chem. Eng. Ind. Biotechnol. 5(2), 9–16 (2019)
Lafi, R., Montasser, I., Hafiane, A.: Adsorption of Congo red dye from aqueous solutions by prepared activated carbon with oxygen-containing functional groups and its regeneration. Adsorpt. Sci. Technol. 37(1–2), 160–181 (2019)
Wang, J., Wang, S.: Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. J. Chem. Eng. 401, 126158 (2020)
Duan, X., Yang, S., Wacławek, S., Fang, G., Xiao, R., Dionysiou, D.D.: Limitations and prospects of sulfate-radical based advanced oxidation processes. J. Environ. Chem. Eng. 8(4), 103849 (2020)
Babu, A.N., Reddy, D.S., Sharma, P., Kumar, G.S., Ravindhranath, K., Mohan, G.K.: Removal of hazardous indigo carmine dye from waste water using treated red mud. Mater. Today: Proc. 17, 198–208 (2019)
Mokif, L.A.: Removal methods of synthetic dyes from industrial wastewater: a review. Mesop. Environ. J. 5(1), 23–40 (2019)
Khatri, J., Nidheesh, P.V., Singh, T.A., Kumar, M.S.: Advanced oxidation processes based on zero-valent aluminium for treating textile wastewater. Chem. Eng. J. 348, 67–73 (2018)
Cuerda-Correa, E.M., Alexandre-Franco, M.F., Fernández-González, C.: Advanced oxidation processes for the removal of antibiotics from water. An overview. Water 12(1), 102 (2019)
Moosavi, S., Lai, C.W., Gan, S., Zamiri, G., Akbarzadeh Pivehzhani, O., Johan, M.R.: Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega 5(33), 20684–20697 (2020)
Yagub, M.T., Sen, T.K., Afroze, S., Ang, H.M.: Dye and its removal from aqueous solution by adsorption: a review. Adv. Colloid Interface Sci. 209, 172–184 (2014)
Read, D.J., Leake, J.R., Perez-Moreno, J.: Mycorrhizal fungi as drivers of ecosystem processes in heathland and boreal forest biomes. Can. J. Bot. 82(8), 1243–1263 (2004)
Dai, Y., Zhang, N., Xing, C., Cui, Q., Sun, Q.: The adsorption, regeneration and engineering applications of biochar for removal organic pollutants: a review. Chemosphere 223, 12–27 (2019). https://doi.org/10.1016/j.chemosphere.2019.01.161
Sutar, S., Patil, P., Jadhav, J.: Recent advances in biochar technology for textile dyes wastewater remediation: a review. Environ. Res. 209, 112841 (2022)
Mahdi, Z., El Hanandeh, A., Yu, Q.J.: Preparation, characterization and application of surface modified biochar from date seed for improved lead, copper, and nickel removal from aqueous solutions. J. Environ. Chem. Eng. 7(5), 103379 (2019)
Yaashikaa, P.R., Kumar, P.S., Varjani, S., Saravanan, A.: A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 28, e00570 (2020)
Shivaram, P., Leong, Y.K., Yang, H., Zhang, D.K.: Flow and yield stress behaviour of ultrafine Mallee biochar slurry fuels: the effect of particle size distribution and additives. Fuel 104, 326–332 (2013)
Rashidi, N.A., Yusup, S.: A mini review of biochar synthesis, characterization, and related standardization and legislation. Appl. Biochar Environ. Saf. (2020). https://doi.org/10.5772/intechopen.92621
Filiberto, D.M., Gaunt, J.L.: Practicality of biochar additions to enhance soil and crop productivity. Agriculture 3(4), 715–725 (2013)
Senthil Kumar, P., Ramalingam, S., Kirupha, S.D., Murugesan, A., Vidhyadevi, T., Sivanesan, S.: Adsorption behavior of nickel (II) onto cashew nut shell: Equilibrium, thermodynamics, kinetics, mechanism and process design. Chem. Eng. J. 167(1), 122–131 (2011)
Senthil Kumar, P., Senthamarai, C., Sai Deepthi, A.S.L., Bharani, R.: Adsorption isotherms, kinetics and mechanism of Pb (II) ions removal from aqueous solution using chemically modified agricultural waste. Can. J. Chem. Eng. 91(12), 1950–1956 (2013)
Varjani, S., Kumar, G., Rene, E.R.: Developments in biochar application for pesticide remediation: current knowledge and future research directions. J. Environ. Manage. 232, 505–513 (2019)
Lehmann, J., Joseph, S.: Biochar for environmental management: an introduction. In Biochar for Environmental Management, pp. 1–13. Routledge (2015)
Mohanty, S.K., Valenca, R., Berger, A.W., Iris, K.M., Xiong, X., Saunders, T.M., Tsang, D.C.: Plenty of room for carbon on the ground: potential applications of biochar for stormwater treatment. Sci. Total. Environ. 625, 1644–1658 (2018). https://doi.org/10.1016/j.scitotenv.2018.01.037
Gayathri, R., Gopinath, K.P., Kumar, P.S.: Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: application in electroplating industrial wastewater. Chemosphere 262, 128031 (2021). https://doi.org/10.1016/j.chemosphere.2020.128031
Hemavathy, R.V., Kumar, P.S., Kanmani, K., Jahnavi, N.: Adsorptive separation of Cu (II) ions from aqueous medium using thermally/chemically treated Cassia fistula based biochar. J. Clean. Prod. 249, 119390 (2020). https://doi.org/10.1016/j.jclepro.2019.119390
Babu, B.V., Chaurasia, A.S.: Modeling, simulation and estimation of optimum parameters in pyrolysis of biomass. Energy Convers. Manage. 44(13), 2135–2158 (2003). https://doi.org/10.1016/S0196-8904(02)00237-6
Sharma, A., Pareek, V., Zhang, D.: Biomass pyrolysis—a review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 50, 10811096 (2015)
Lou, L., Luo, L., Yang, Q., Cheng, G., Xun, B., Xu, X., Chen, Y.: Release of pentachlorophenol from black carbon-inclusive sediments under different environmental conditions. Chemosphere 88(5), 598–604 (2012)
Sohi, S.P., Krull, E., Lopez-Capel, E., Bol, R.: A review of biochar and its use and function in soil. Adv. Agron. 105, 47–82 (2010)
Kumar, P.S., Ramalingam, S., Abhinaya, R.V., Thiruvengadaravi, K.V., Baskaralingam, P., Sivanesan, S.: Lead (II) adsorption onto sulphuric acid treated cashew nut shell. Sep. Sci. Technol. 46(15), 2436–2449 (2011). https://doi.org/10.1080/01496395.2011.590174
Jothirani, R., Kumar, P.S., Saravanan, A., Narayan, A.S., Dutta, A.: Ultrasonic modified corn pith for the sequestration of dye from aqueous solution. J. Ind. Eng. Chem. 39, 162–175 (2016). https://doi.org/10.1016/j.jiec.2016.05.024
Suganya, S., Saravanan, A., Ravikumar, C.: Computation of adsorption parameters for the removal of dye from wastewater by microwave assisted sawdust: theoretical and experimental analysis. Environ. Toxicol. Pharmacol. 50, 45–57 (2017)
Gautam, R.K., Goswami, M., Mishra, R.K., Chaturvedi, P., Awashthi, M.K., Singh, R.S., Giri, B.S., Pandey, A.: Biochar for remediation of agrochemicals and synthetic organic dyes from environmental samples: a review. Chemosphere 272, 129917 (2021)
Sarangi, P.K., Nanda, S.: Biohydrogen production through dark fermentation. Chem. Eng. Technol. 43, 601–612 (2020)
Patra, B.R., Mukherjee, A., Nanda, S., Dalai, A.K.: Biochar production, activation and adsorptive applications: a review. Environ. Chem. Lett. 19, 2237–2259 (2021)
Elkhalifa, S., Al-Ansari, T., Mackey, H.R., McKay, G.: Food waste to biochars through pyrolysis: a review. Resour. Conserv. Recycl. 144, 310–320 (2019)
Wei, J., Tu, C., Yuan, G., Liu, Y., Bi, D., Xiao, L., Lu, J., Theng, B.K., Wang, H., Zhang, L., Zhang, X.: Assessing the effect of pyrolysis temperature on the molecular properties and copper sorption capacity of a halophyte biochar. Environ. Pollut. 251, 56–65 (2019)
Qureshi, K.M., Lup, A.N.K., Khan, S., Abnisa, F., Daud, W.M.A.W.: Optimization of palm shell pyrolysis parameters in helical screw fluidized bed reactor: effect of particle size, pyrolysis time and vapor residence time. Clean. Eng. Technol. 4, 100174 (2021)
Daful, A.G., Chandraratne, M.R.: Biochar production from biomass waste-derived material. Elsevier, Amsterdam (2020)
Tomczyk, A., Sokołowska, Z., Boguta, P.: Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 19, 191–215 (2020)
Ghani, W.A.W.A.K., Mohd, A., da Silva, G., Bachmann, R.T., Taufiq-Yap, Y.H., Rashid, U., Ala’a, H.: Biochar production from waste rubber-wood-sawdust and its potential use in C sequestration: chemical and physical characterization. Ind. Crops Prod. 44, 18–24 (2013)
Jeyasubramanian, K., Thangagiri, B., Sakthivel, A., Raja, J.D., Seenivasan, S., Vallinayagam, P., Madhavan, D., Devi, S.M., Rathika, B.: A complete review on biochar: production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 292, 120243 (2021)
Godwin, P.M., Pan, Y., Xiao, H., Afzal, M.T.: Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 4(1), 31–42 (2019)
Huang, Y.F., Chiueh, P.T., Lo, S.L.: A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 26(3), 103–109 (2016)
Ingole, P.M., Ranveer, A.C., Deshmukh, S.M., Deshmukh, S.K.: Microwave assisted pyrolysis of biomass: a review. Int. J. Adv. Technol. Eng. Sci 4(6), 78–84 (2016)
Wang, Y., Zeng, Z., Tian, X., Dai, L., Jiang, L., Zhang, S., Wu, Q., Wen, P., Fu, G., Liu, Y., Ruan, R.: Production of bio-oil from agricultural waste by using a continuous fast microwave pyrolysis system. Bioresour. Technol. 269, 162–168 (2018)
Libra, J.A., Ro, K.S., Kammann, C., Funke, A., Berge, N.D., Neubauer, Y., Titirici, M.M., Fühner, C., Bens, O., Kern, J., Emmerich, K.H.: Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2(1), 71–106 (2011)
Cheng, N., Wang, B., Wu, P., Lee, X., Xing, Y., Chen, M., Gao, B.: Adsorption of emerging contaminants from water and wastewater by modified biochar: a review. Environ. Pollut. 273, 116448 (2021)
Hossain, N., Nizamuddin, S., Griffin, G., Selvakannan, P., Mubarak, N.M., Mahlia, T.M.I.: Synthesis and characterization of rice husk biochar via hydrothermal carbonization for wastewater treatment and biofuel production. Sci. Rep. 10(1), 1–15 (2020)
Wilk, M., Magdziarz, A.: Hydrothermal carbonization, torrefaction and slow pyrolysis of Miscanthus giganteus. Energy 140, 1292–1304 (2017)
Ambaye, T.G., Vaccari, M., van Hullebusch, E.D., Amrane, A., Rtimi, S.: Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 18, 3273–3294 (2021)
Gabhane, J.W., Bhange, V.P., Patil, P.D., Bankar, S.T., Kumar, S.: Recent trends in biochar production methods and its application as a soil health conditioner: a review. SN Appl. Sci. 2, 1–21 (2020)
Roy, K., Verma, K.M., Vikrant, K., Goswami, M., Sonwani, R.K., Rai, B.N., Vellingiri, K., Kim, K.H., Giri, B.S., Singh, R.S.: Removal of patent blue (V) dye using Indian bael shell biochar: characterization, application and kinetic studies. Sustainability 10(8), 2669 (2018)
Hanoon, M.A., Ahmed, M.J.: Adsorption of methyl orange from wastewater by using biochar. Iraqi J. Chem. Pet. Eng. 20(3), 23–29 (2019)
Aichour, A., Zaghouane-Boudiaf, H., Khodja, H.D.: Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: characterization, adsorption properties and reuse studies. Arab. J. Chem. 15(1), 103542 (2022)
Kapoor, R.T., Rafatullah, M., Aljuwayid, A.M., Habila, M.A., Wabaidur, S.M., Alam, M.: Removal of patent blue dye using Ananas comosus-derived biochar: equilibrium, kinetics, and phytotoxicity studies. Separations 9(12), 426 (2022)
Eleryan, A., Hassaan, M.A., Aigbe, U.O., Ukhurebor, K.E., Onyancha, R.B., El-Nemr, M.A., Ragab, S., Hossain, I., El Nemr, A.: Kinetic and isotherm studies of acid orange 7 dye absorption using sulphonated mandarin biochar treated with TETA. Biomass Convers. Biorefin. 14, 10599–10610 (2023)
El-Nemr, M.A., Yılmaz, M., Ragab, S., Hassaan, M.A., El Nemr, A.: Isotherm and kinetic studies of acid yellow 11 dye adsorption from wastewater using Pisum sativum peels microporous activated carbon. Sci. Rep. 13(1), 4268 (2023)
Purbasari, A., Ariyanti, D., Fitriani, E.: Adsorption of methyl orange dye by modified fly ash-based geopolymer-characterization, performance, kinetics and isotherm studies. J. Ecol. Eng. 24(3), 90–98 (2023)
Huynh, P.T., Nguyen, D.K., Duong, B.N., Nguyen, P.H., Dinh, V.P.: Methylene orange and methyl blue adsorption behavior on pine leaves biomass (Pinus kesiya). Res. Sq. (2023). https://doi.org/10.21203/rs.3.rs-2642269/v1
Kim, J., Bak, G.H., Yoo, D.Y., Lee, Y.I., Lee, Y.G., Chon, K.: Functionalization of pine sawdust biochars with Mg/Al layered double hydroxides to enhance adsorption capacity of synthetic azo dyes: adsorption mechanisms and reusability. Heliyon (2023). https://doi.org/10.1016/j.heliyon.2023.e14142
Ahmadian, A., Goharrizi, B.A., Shahriari, T., Ahmadi, S.: Adsorption of chromium (VI) and acid orange 7 on lemon peel biochar: a response surface methodology approach. Int. J. Environ. Sci. Technol. 20(3), 2939–2958 (2023)
Rubangakene, N.O., Elwardany, A., Fujii, M., Sekiguchi, H., Elkady, M., Shokry, H.: Biosorption of Congo red dye from aqueous solutions using pristine biochar and ZnO biochar from green pea peels. Chem. Eng. Res. Des. 189, 636–651 (2023)
Sivaranjanee, R., Kumar, P.S., Chitra, B., Rangasamy, G.: A critical review on biochar for the removal of toxic pollutants from water environment. Chemosphere 360, 142382 (2024)
Goswami, L., Kushwaha, A., Kafle, S.R., Kim, B.S.: Surface modification of biochar for dye removal from wastewater. Catalysts 12(8), 817 (2021). https://doi.org/10.3390/catal12080817
Dwivedi, S., Dey, S.: Review on biochar as an adsorbent material for removal of dyes from waterbodies. Int. J. Environ. Sci. Technol. 20(8), 9335–9350 (2023)
Choudhary, M., Kumar, R., Neogi, S.: Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 392, 122441 (2020). https://doi.org/10.1016/j.jhazmat.2020.122441
Sathishkumar, P., Li, Z., Govindan, R., Jayakumar, R., Wang, C., Gu, F.L.: Zinc oxide-quercetin nanocomposite as a smart nano-drug delivery system: molecular-level interaction studies. Appl. Surf. Sci. 536, 147741 (2021)
Nartey, O.D., Zhao, B.: Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: an overview. Adv. Mater. Sci. Eng. (2014). https://doi.org/10.1155/2014/715398
Ravenni, G., Cafaggi, G., Sárossy, Z., Nielsen, K.R., Ahrenfeldt, J., Henriksen, U.B.: Waste chars from wood gasification and wastewater sludge pyrolysis compared to commercial activated carbon for the removal of cationic and anionic dyes from aqueous solution. Bioresour. Technol. Rep. 10, 100421 (2020)
Pormazar, S.M., Dalvand, A.: Adsorption of direct red 23 dye from aqueous solution by means of modified montmorillonite nanoclay as a superadsorbent: mechanism, kinetic and isotherm studies. Korean J. Chem. Eng. 37, 2192–2201 (2020)
Jawad, A.H., Abdulhameed, A.S.: Mesoporous Iraqi red kaolin clay as an efficient adsorbent for methylene blue dye: adsorption kinetic, isotherm and mechanism study. Surf. Interfaces 18, 100422 (2020)
Gokulan, R., Prabhu, G.G., Avinash, A., Jegan, J.: Experimental and chemometric analysis of bioremediation of remazol dyes using biochar derived from green seaweeds. Desalin. Water Treat. 184, 340–353 (2020)
Singh, A.L., Chaudhary, S., Kumar, S., Kumar, A., Singh, A., Yadav, A.: Biodegradation of reactive yellow-145 azo dye using bacterial consortium: a deterministic analysis based on degradable metabolite, phytotoxicity and genotoxicity study. Chemosphere 300, 134504 (2022)
Oladipo, A.A., Ifebajo, A.O.: Highly efficient magnetic chicken bone biochar for removal of tetracycline and fluorescent dye from wastewater: two-stage adsorber analysis. J. Environ. Manage. 209, 9–16 (2018)
Tran, H.N.: Comments on “high-efficiency removal of dyes from wastewater by fully recycling litchi peel biochar.” Chemosphere 257, 126444 (2020)
Odinga, E.S., Waigi, M.G., Gudda, F.O., Wang, J., Yang, B., Hu, X., Li, S., Gao, Y.: Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ. Int. 134, 105172 (2020)
Zhang, Q., Ye, X., Li, H., Chen, D., Xiao, W., Zhao, S., Xiong, R., Li, J.: Cumulative effects of pyrolysis temperature and process on properties, chemical speciation, and environmental risks of heavy metals in magnetic biochar derived from coagulation-flocculation sludge of swine wastewater. J. Environ. Chem. Eng. 8(6), 104472 (2020)
Zhang, Y., Chen, Z., Xu, W., Liao, Q., Zhang, H., Hao, S., Chen, S.: Pyrolysis of various phytoremediation residues for biochars: chemical forms and environmental risk of Cd in biochar. Bioresour. Technol. 299, 122581 (2020)
Funding
No funding was received to carry out present work.
Author information
Authors and Affiliations
Contributions
ALS: writing and conceptualization, LR: editing and diagram preparations, PS: literature review and data collection, AP: editing and value additions, NP: editing and value additions, VKC: mentoring and supervision.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any personal and financial interest in publishing this paper.
Consent to participate
Not applicable.
Consent to publish
All authors agree with publishing.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srivastav, A.L., Rani, L., Sharda, P. et al. Sustainable biochar adsorbents for dye removal from water: present state of art and future directions. Adsorption (2024). https://doi.org/10.1007/s10450-024-00522-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00522-2