Abstract
The nano-Sulphonated Poly (glycidyl methacrylate)-Hexamethyl Pararosaniline Chloride (Crystal Violet; CV) composite (CV-SPGMA) has been developed as a novel adsorbent for treatment of Dichromate and Permanganate Contaminated Waste Water for the first time. The innovative adsorbent has been developed by adsorption of CV dye from wastewater using nano-Sulphonated Poly (glycidyl methacrylate) (SPGMA) particles. The study investigated the impact of various adsorption parameters. The CV content was observed to be linearly increased by variations in the concentration of CV up to 200 mg/L where maximum content obtained; 174.6 mg/g. The equilibrium almost reached after 90 min. An endothermic nature of the CV adsorption process has been noticed where 178 mg/g CV content obtained at 80 °C. The CV content decreased from 240 mg/g to 46 mg/g with the SPGMA adsorbent dose increment from 5 to 40 mg. The pH of adsorption exhibited the most pronounced impact, with the highest CV content achieved at a pH of 10.0 corresponding to 190.4 mg/g. The reusability of the produced CV-SPGMA adsorbent was examined for consecutive adsorption–desorption cycles, revealing a loss of just 13% in its initial adsorption efficiency after 10 cycles. In addition, the alterations in the chemical structure and morphology caused by the development of CV-SPGMA composite were observed through the utilization of characterization techniques including Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDAX). Finally, the developed CV-SPGMA composite adsorbent, for the first time, tested for the removal of Cr (VI) and Mn (VII) metal ions from dichromate and permanganate contaminated waters under mild adsorption conditions where shows seven folds affinity towards removal of the Cr (VI), 84.6 mg/g, than Mn (VII), 11.66 mg/g.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The treatment of wastewater is a significant challenge for the textile dyeing business. The industrial sector, particularly industries involved in plastics, paper, dyestuffs, and textiles, utilize dyes for the purpose of coloring their goods. Additionally, these industries have a significant water consumption requirement. Numerous dyes and their resultant degradation products has the potential to exhibit toxicity towards living organisms. Hence, the process of decolorization or separation of dyes holds considerable importance in the context of wastewater treatment prior to its discharge into natural water sources [1]. Approximately 10–15% of dyes in the form of wastewater are released into the environment during the dyeing process. The public's opinion of water quality is significantly influenced by the aesthetic aspect of water color. Synthetic dyes pose a significant challenge in terms of removal due to their intricate aromatic molecular structures, which contribute to their enhanced stability and hinder their biodegradation [2, 3]. Due to their inherent chemical composition, dyes exhibit a notable resistance to fading when subjected to various external factors such as light, water, and a wide range of chemicals [4,5,6]. Dye wastewater is a particularly complex category of contaminants due to its visible nature and limited biodegradability [7,8,9]. Dyes are chemicals with intricate molecular structures that possess the ability to attenuate light, modulate temperature, and inhibit oxidation. The aforementioned characteristics render the dyes resistant to degradation within the environment, resulting in their accumulation within the food chain. Certain color compounds possess hazardous and carcinogenic properties, leading to the manifestation of allergies and genetic alterations. Cationic dyes, exemplified by crystal violet, have been found to be associated with several health conditions including dizziness, allergic reactions, cyanosis, as well as respiratory and digestive impairments. Cationic dyes pose a greater degree of risk as compared to anionic dyes. Crystal violet (CV), also known as methyl violet, is a widely utilized cationic dye in several fields such as textiles, scientific research, and pharmaceuticals. According to previous studies [10, 11], concentrations of the CV exceeding 1 mg/L have been found to exhibit toxicity towards both people and animals. The presence of several hazardous substances in dyes poses a significant threat to the overall health and welfare of living organisms. The textile business employs a range of dye structures, including reactive dyes, acid dyes, basic dyes, diazo dyes, azo dyes, and anthraquinone dyes. The application of dyes in the textile industry can be categorized into three groups: a) anionic dyes, which include direct, acidic, and reactive dyes, b) non-ionic dyes, specifically disperse dyes, and c) cationic dyes, encompassing all base colors. These dyes find their application in the coloring of silk, leather, and wool [12,13,14]. The utilization of reactive dyes in the dye production process and subsequent application in textile dying might present challenges for both plant personnel engaged in dye production and textile workers involved in the dying process. There is evidence suggesting that certain reactive dyes have the potential to induce contact dermatitis, rhinitis, allergic conjunctivitis, occupational asthma, or other allergic reactions among individuals employed in relevant occupations [15]. Hence, it is imperative to eliminate this color from the wastewater prior to its discharge into the environment.Various techniques are at one's disposal for the attainment of efficient color elimination. A range of physical approaches, including membrane filtration processes such as reverse osmosis, nano-filtration, and electro dialysis, as well as various adsorbents in adsorption techniques, have been employed [16]. Several chemical procedures, including oxidative processes, the use of Fenton reagents, and ozonation, are commonly employed. The biological process removes dissolved materials in a manner similar to self-depuration, but with additional and enhanced effectiveness compared to clariflocculation. The efficacy of dye removal is contingent upon several elements, including the ratio of organic load to biomass in the oxidation tank, the temperature of the system, and the concentration of oxygen [17]. The effectiveness of color eradication by the integration of biological therapy with existing conventional biological treatment procedures has been found to be unsatisfactory. The adsorption procedure is widely recognized as a significant treatment technology due to its cost-effectiveness, potential as an alternative method, and effectiveness in removing dyes [18,19,20,21]. When the objective is only focused on treatment, the utilization of activated carbon as an adsorbent has been identified as an appropriate method for the removal of organic dyes from wastewater. In contrast, when the focus is primarily on the recycling of valuable chemicals or organic compounds, other adsorbents such as polymeric adsorbents that provide a more convenient desorption process (e.g., by the use of solvents) may be employed [22]. Various materials, including nanoparticles, super absorbents, charcoal, and activated carbon, can be employed for the purpose of eliminating organic substances, such as colors, from wastewater. Several adsorbents have been previously documented for the purpose of eliminating harmful dyes, including activated carbon [23], peach gum [24], ginger waste [25], rice husks [26], almond shell [27], biocomposites [1], as well as synthetic polymer-based composites and copolymers [28,29,30,31,32]. For instance, various adsorbents based on polymers, including grafted cotton textiles [33], carboxylated alginate beads [34], and Phosphoric Acid Doped Pyrazole-g-Polyglycidyl Methacrylate [35], have been examined for their efficacy in eliminating Methylene Blue dye from wastewater contaminated with this substance.
Furthermore, the global industry has led to the widespread pervasion of environmental degradation caused by heavy metals. Nickel, copper, cadmium, manganese, and chromium are commonly recognized as dangerous heavy metals. These heavy metals are often employed and are prevalent as environmental contaminants [36, 37]. Low amounts of these metals are necessary as co-factors for enzymes, but high concentrations can be highly harmful to living cells by blocking metabolism.
Potassium permanganate is frequently employed as a powerful oxidizing agent in several fields to treat organic and inorganic chemicals in soil and water solutions [38, 39]. As far as we know, only a limited number of papers have discussed the elimination of permanganate ions. Adsorption is a cost-effective and effective technique for eliminating Mn (VII) from wastewater. Various adsorbents, including activated orange peel powder [40], activated carbon [41, 42], Prosopis cineraria leaf powder [43], and Millet husk [44], are used for this purpose.
Chromium predominantly occurs as either Cr (VI) or Cr (III) in the natural environment. Chromium (III) species exhibit lower solubility and greater stability in comparison to chromium (IV) species, which display excellent solubility and mobility in aqueous solutions [45]. Chromium (VI) has more mobility compared to chromium (III), therefore increasing its ability to contaminate groundwater. The elevated danger posed by chromium (VI) is linked to its heightened reactivity and ability to cause cancer [46]. Short-term exposure to Cr (VI) results in symptoms such as nausea, diarrhea, liver and renal impairment, dermatitis, internal bleeding, and respiratory issues [5]. Inhaling this substance can lead to immediate and severe harm, including irritation, ulceration of the nasal septum, and respiratory sensitization, which can result in asthma [47]. Consumption can impact the functioning of the kidneys and liver. Direct contact with the skin can lead to systemic toxicity, extensive burns, and hinder the healing process of wounds or abrasions. Failure to receive prompt treatment may result in the development of ulceration and severe chronic allergic contact dermatitis. Exposure of the eye might result in irreversible harm. Adsorption is a cost-effective and effective technique for eliminating Cr (VI) from wastewater. Various adsorbents, including charcoal [48], activated carbon from diverse sources [49,50,51], polyaniline and its composites [52], and Chitosan [53], are used for this purpose.
Recently natural and acrylic polymers using diazo acid Blue 11 (AB 113) as a chelating agent were designed to selectively eliminate metal ions, specifically Zn2+, Mn2+, and Cr3+, from acid-contaminated wastewater [54].
The current investigation is a continuation of the prior research focused on the utilization of methylene blue-Sulphonated poly(glycidyl methacrylate) composite adsorbent (MB-SPGMA) for the purpose of removal of Cr (VI) and Mn (VII) metal anions from dichromate and permanganate contaminated waters [55, 56]. The present study examines the various factors affecting the CV content in the developed CV-SPGMA composite adsorbent, including CV concentration, adsorption period, temperature, pH, adsorbent dose, and agitation speed. Furthermore, the chemical and morphological alterations that occurred following the development of CV-SPGMA composite adsorbent were confirmed through FTIR, SEM, and EDAX investigations. The investigation of the potential reusability of the SPGMA adsorbent for the purpose of removing the CV dye was conducted through multiple adsorption–desorption cycles. Finally, the developed CV-SPGMA composite adsorbent, for the first time, tested for the removal of Cr (VI) and Mn (VII) metal ions from dichromate and permanganate contaminated waters under mild adsorption conditions. The adsorption process was verified by using EDAX analysis of the CV-SPGMA, Cr- CV-SPGMA, and Mn-CV-SPGMA samples.
2 Materials and methods
2.1 Materials
Glycidyl methacrylate (GMA) were purchased from ACROS (USA), and Potassium persulfate and sodium bisulfite were obtained from Sigma Chem. Co. (St. Louis, USA), Ethanol absolute perused from Adwic, Egypt, and finally, CV from Aldrich, Germany was perused.
2.2 Preparation of sulphonated Poly (glycidyl methacrylate) adsorbent
The Poly(glycidyl methacrylate) was first polymerized following by the sulphonation process as discussed elsewhere [57, 58] with slight modification where the sulphonation process used DMF:Water (1:1) as a solvent. The resultant SPGMA adsorbent has 12.2 meq.g−1 ions exchange capacity and 43.0 nm an average particle size.
2.3 Fourier transform infrared spectroscopic analysis
The Fourier transform infrared spectroscopic (FTIR) spectra of the SPGMA and CV-SPGMA adsorbents polymers particles were recorded with an FTIR spectrometer in the spectral range 4000–500 cm−1.
2.4 Scanning electron microscopic and EDAX analysis
The scanning electron microscope (SEM) image of the polymer particles was obtained by placing the particles onto carbon tape-attached aluminum SEM stubs after coating them with gold to a few nanometer thicknesses under vacuum. In addition to morphological inspection, the composition (mass ratios) of the SPGMA, CV-SPGMA, Cr- CV-SPGMA, and Mn-CV-SPGMA adsorbents were evaluated by EDAX analysis in conjunction with scanning electron microscopy to assess the adsorption of CV dye, Cr(VI), and Mn(VII) ions.
2.5 Crystal violet batch adsorption process
The CV adsorption was performed by mixing 10 mg of SPGMA polymer with 10 mL CV solution of 10–200 mg/L. The mixture was agitated at RT using a magnetic stirrer for definite time, then centrifuged at 12000 rpm to separate the matrix of the liquid phase. The CV concentration at mg/L, before and after the adsorption, for each solution was determined by measuring the absorbance at the maximum wavelength (ʎmax = 590 nm) [28] using a UV–VIS spectrophotometer and multiplying by constant extracted from the slope of the standard curve. The CV-polymer composites composition as (mg/g) was calculated according to the following formula:
C0 and Ct are the CV initial and final concentrations at definite immobilization time (mg/L), Vis the volume of the CV solution (L), and M is the mass of the SPGMA polymer (g).
2.5.1 Adsorption reusability
To test the reusability of the SPGMA adsorbent, the CV-SPGMA adsorbent was regenerated by mixing with 100 ml of 1 M NaCl solution at room temperature using a mechanical stirrer at 400 rpm for 180 min to desorbs CV molecules, then separated as mentioned previously and washed successive times with distilled water before separating and used in further adsorption experiments, 10 successive cycles, under the same CV adsorption conditions.
2.5.2 Chromium (VI) and Manganese (VII) ions removal [59, 60]
Synthetic dichromate (Cr VI) and permanganate (Mn VII) solutions, 10 mL, with 100 mg/L and 20 mg/L concentrations were mixed with 10 mg of CV-SPGMA composite at room temperature for 90 min, then separated by centrifugation under 12,000 rpm for 30 min, used in batch adsorption experiments. The Cr (VI) and Mn (VII) concentration at mg/L, before and after the adsorption, for each solution was determined by measuring the absorbance at the maximum wavelength (ʎmax = 357 nm and 525 nm) using UV–VIS spectrophotometer and the adsorption capacity was calculated according to Eq. 1.
3 Results and discussion
3.1 Effect of dye concentraion
Figure 1 shows the effect of variation of the CV concentration between 10 and 200 mg/L on the formed CV-SPGMA composites composition. The effect of the initial dye concentration factor depends on the immediate relation between the dye concentration and the available binding sites on a matrix surface. Usually, the percentage of dye immobilization decreases with an increase in initial dye concentration, which may be due to the saturation of immobilization sites on the matrice surface [61]. As a result, there will be unoccupied active sites at low concentrations on the matrix surface. When the initial dye concentration increases, the active sites required immobilizing the dye molecules disappears [62]. In this work, the increase in the initial CV concentration will cause an increase in the CV content of the CV-SPGMA composites, and this may be due to the high driving force for mass at a high initial dye concentration [63]. That indicates a high number of active sites relative to the number of CV molecules in the liquid phase of all the CV concentrations used where free active sites are still available. This postulation has been confirmed by the linear increase of the CV content of the CV-SPGMA composites to reach the highest value; 174.6 mg/g.
3.2 Effect of adsorption time
A progressive increase of the CV content in the CV-SPGMA composite adsorbent has been recognized in Fig. 2. Two steps of adsorption rate were observed. The first step was a linear increment with increase of adsorption time up to 45 min where a fast CV dye removal percentage reached 81%. Further increase of the adsorption time, the rate of adsorption capacity starts to decrease and the equilibrium almost reached after 90 min with CV content of 174 mg/g corresponding to 87.11% CV dye removal percentage. The high available active adsorption sites in the first adsorption step, up to 45 min, explain the fast adsorption rate. Further progress of the adsorption time carried out with much less available active sites and less remaining CV due molecules in the liquid phase which consequently reduced the dye concentration gradient, the main driving force, between the dye liquid phase and the adsorbent solid phase [31]. The obtained results are in accordance with previousely published results [28, 30]. Synthetic copolymer adsorbent of AA and AMP was used in the removal of CV dye [28]. Also, xanthan gum /poly (N-vinyl imidazole) (XG/PVI) hydrogel [30] was used in the adsorption removal of CV where that the adsorption efficiency of CV dye increased with increasing the contact time and reached a maximum (91.0%) within 6 h, after that it leveled off. This suggests that a monolayer of dye molecules on the gel surface is formed. The formations of monolayer of CV dye at external interface on hydrogel resulting in increasing the adsorption driving force. However, the removal of dye remained constant with increasing the adsorption time from 6 to 8 h, because the free adsorption active sites may become saturated. So, increase the adsorption time (above 6 h) did not affect the adsorption efficiency. It is worthy to mention here that all the adsorbents mentioned above have anionic characters which act to attract the cationic CV dye molecules through opposite charge interaction.
3.3 Effect of adsorption temperature
An endothermic nature of the CV adsorption process has been noticed from the adsorption behavior under different temperatures; 30 °C-80°C; Fig. 3. The CV removal percentage (%) enhanced from 78% at 30 °C to 89% at 80 °C. It is worthy to mention here that the adsorption time was only 30 min. This behavior may be referred to facilitate both of the dye molecules adsorption processes on the surface and the diffusion to interior pores of the adsorbent. That keeps the concentration gradient between the dye aqueous phase and the adsorbent solid phase enough as the driveling force of the adsorption process.
3.4 Effect of SPGMA adsorbent dose
The SPGMA adsorbent dose increment from 5 to 40 mg has negatively affected the amount of immobilized CV as seen in Fig. 4. The increment of the available active sites for the immobilization process explains the noticed behavior where the immobilization percentage increased from 60 to 93%. As a result, the immobilization capacity decreased from 240 mg/g to 46 mg/g. This behavior agrees with previous results obtained by Mohy-Eldin and his co-workers in the immobilization of MB dye onto SPGMA [55].
3.5 Effect of agitation speed
The variation of the agitation speed shows the same behavior, while no noticeable effect on the CV content of CV-SPGMA composites was observed with variation from 100 to 500 rpm. That is an indication of the external mass diffusion absence due to the surface immobilization process nature, which is mainly controlled by the ratio between the numbers of active sites available on the polymer particles' surface for the immobilization process of CV molecules from the solution (Table 1). This finding is in agreement with previous results obtained by Mohy-Eldin and his co-workers in the immobilization of MB dye onto SPGMA [55].
3.6 Effect of adsorption pH
The pH of solution is considered one of the most important parameters in adsorption studies of cationic dyes onto adsorbents, because of its effects on both adsorption on active sites and dye molecules ionization [29,30,31,32]. The effect of the solution pH on CV dye adsorption using SPGMA adsorbent was studied using pH range (3–10) and the results are illustrated in Fig. 5. Two phases with different rates of adsorption were observed in the pH ranges 3–7 and 7–10. The turning point was located at pH 7.0; the pka of the functional sulfonated groups of the SPGMA adsorbent. The results showed that the less adsorption linear rate of CV was observed in the CV solution from pH 3.0 up to 7.0 because the concentration of H+ ions is very high which is a competitor for the adsorption of cationic CV dye onto SPGMA adsorbent as a result of the protonation process of the sulfonate groups. However, CV adsorption % increased with increasing the solution pH above 7.0, pka of the functional sulfonated groups, in a higher rate to reach the maximum value (95.2%) at pH 10.0 indicating the de-protonation of the sulfonated groups and having a negative charges sites attract the CV cationic ions. This behavior was similar to the adsorption of CV on Semi-IPN poly(acrylic acid-acrylamide-methacrylate) and amylose hydrogels [29], xanthan gum and poly (N-vinyl imidazole) hydrogel [30], and crosslinked poly (N-vinyl imidazole) grafted xanthan gum [32] as an adsorbents.
3.7 Reusability
An estimate of the extent of recovery of CV absorbed from aqueous solution can be made employing the sorption desorption cycle, as illustrated in Fig. 6. In this respect, the sorption–desorption cycles were analyzed to investigate the SPGMA adsorbent reusability for removing CV from aqueous solutions. The cycle was performed ten times by using a 1 M sodium chloride solution. The figure clearly shows that a continuous, almost linear reduction has been observed after the fourth cycle. However, the reduction in the removal percentage (%) of CV was not drastic; the CV removal percentage reached 76% in the tenth cycle compared with 87.2% in the first one. Only 12.85% of the CV removal efficiency was lost after ten cycles of sorption–desorption processes. The SPGMA adsorbent has a good sorption–desorption performance and can be used confidently without a noticeable reduction in its sorption capacity to remove CV dye.
3.8 Comparative study
The adsorption capacity of the SPGMA adsorbent is compared with the other adsorbents for crystal violet dye removal (Table 2). The SPGMA adsorbent shows the higher adsorption capacity from soil-silver nanocomposite [64], leaf biomass [65], TLAC/chitosan composite [66], AC-Fe2O3-NPLs [67], starch-g-poly(acrylic acid)/ZnSe [68], and AA-AMP copolymer adsorbent [28]. However, the SPGMA adsorbent shows the lower adsorption capacity than Modified cellulose with glycidyl methacrylate [69], and COOH-functionalized cellulose nanocrystals adsorbents [70].
3.9 Characterization of the adsorbent
The adsorption process of the CV by SPGMA adsorbent was followed by performing FTIR, SEM, and EDAX characterization of the SPGMA adsorbent before and after the adsorption step.
3.9.1 FTIR analysis
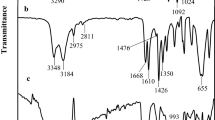
Figure 7 demonstrates the characteristics peaks of the SPGMA adsorbent before CV adsorption and CV- SPGMA adsorbent after CV adsorption step. The FT-IR spectra of SPGMA show the peaks at 1725, 1300–1100 cm−1, caused by the stretching vibration of the ester carbonyl groups, C–O–C stretching in addition to the characteristic peaks of the residual epoxy ring at 1260 and 950–815 cm−1, while the peak at 760 cm−1 of weak intensity still noticed with shift to 780 cm−1. This may be referred to a minor fraction of epoxy rings that may have taken part in the formation of cross-linking structure during polymerization. The characteristic absorption peak of the sulphonate group at 1050–1060 cm−1 was recognized for SPGMA. Since the epoxy groups in poly (GMA) reacted with Na2SO3 to form SPGMA, the epoxy groups would produce -OH groups. The figure shows that the intensity of -OH peak (3500 cm−1) [58]. The adsorption of CV dye exhibits distinctive peaks corresponding to the properties of CV, which are observed in conjunction with those of SPGMA. The chart for CV-SPGMA exhibited distinct absorption peaks that are indicative of disubstituted benzene rings. The absorption peak seen at 2921 cm-1 corresponds to the stretching vibration of hydrocarbon (-C-H) in methyl groups. The spectrum displayed an absorption peak at 1553 cm−1, which can be attributed to the stretching vibration of (C = C) groups present in the benzene ring. Additionally, another absorption peak at 1479 cm−1 was observed, corresponding to the methyl group of the quaternized amine. Furthermore, the absorption peak at 1364 cm−1 indicated the C-H deformation in the methyl groups of the aromatic tertiary amine. Furthermore, it is noteworthy that two distinct absorption peaks were seen at wave numbers 1173 cm−1 and 1117 cm−1, which can be attributed to the C-H bending in benzene rings and the C-N stretching vibration, respectively [71,72,73,74].
3.9.2 SEM and EDAX analyses
The surface morphology of SPGMA was investigated by SEM and presented in Fig. 8. The SEM images showed an irregular particles structure of SPGMA with aggregation. Such aggregation of the SPGMA particles is controlled by its functional groups (sulphonic groups) which are hydrophilic ones absorbed moisture from surrounding environment. The adsorptions of CV dye showing a kind of SPGMA particles surface coating with an increase of the surface smoothing as a result and developing of the aggregates size. That observation is confirming the role of adsorbed CV dye molecules in filling the spaces between the aggregated SPGMA particles and its role as a kind of binder. The sulphonation of PGMA has been verified through EDAX analysis, Fig. 9 and Table 3, where the C, N, and O content of the CV-SPGMA adsorbent has been increased in benefits of Na and S content of the SPGMA adsorbent which proves the adsorption process of the CV. The appearance of the N weight % in the SPGMA adsorbent (9.178%) may be referred to the residues of the DMF used as solvent mixture in the sulphonation step of the PGMA nanoparticles. After CV adsorption, the N weight % in the CV-SPGMA increased to 13.620% due to the N content of the CV adsorbed dye. On the other hand, the both Na and S weight % reduced from 8.793% and 6.438% of the SPGMA adsorbent before adsorption to 1.237% and 2.257% after CV adsorption of the CV-SPGMA adsorbent. The C weight % increased from 31.428% of the SPGMA adsorbent before adsorption to 36.832% of the CV-SPGMA adsorbent.
3.10 Dichromate and permanganate metal ions removal
Selected CV-SPGMA composite adsorbent with a CV composition 175 mg/g has been used for the first time in treating synthetic contaminated water with 100 mg/L dichromate solution Cr (VI) and 20 mg/L permanganate solution Mn (VII) ions under batch conditions; Fig. 10. The Figure shows a similar adsorption behavior for both metal ions which a linear increment of the removal percentage was recognized. The CV-SPGMA composite adsorbent shows higher affinity towards the Cr (VI) ions compared with Mn (VII) ions where removed 45% of the first only after 5 min comparing to 45 min of the later one. In spite of the Cr (VI) ions concentration is five folds of the Mn (VII) ions, the adsorption capacity of the Cr (VI) ions is 84.6 mg/g which is seven folds of the adsorption capacity of the Mn (VII) ions; 11.66 mg/g. These results are compared with our previous study where selected MB-SPGMA composite adsorbent with a composition 27.32 mg/g has been used for the first time in treating synthetic contaminated water with dichromate Cr (VI) and permanganate Mn (VII) ions under batch conditions. Synthetic contaminated water with various metal ions concentrations, 2–8 mg/L, has been used in the study; Mohy Eldin and co-workers [55]. In general, the adsorption capacity of the MB-SPGMA composite adsorbent increased exponentially with an increase in the metal ions concentration. The capacity and rate of removal in the case of permanganate ions is higher than the dichromate counter ions. The adsorption capacity of MB-SPGMA composite adsorbent reached to 0.57 mg/g with 8 mg/L dichromate ions concentration. The curve shows two stages. The first stage is an almost linear increase of the adsorption capacity with an increase of the dichromate ions concentration up to 6 mg/L. The second stage is an exponential increase of the adsorption capacity with an increase of the dichromate ions concentration from 6 mg/L to 8 mg/L. In a previous study [59], it was found that the adsorption capacity of 0.2 g commercial Amberlite IRA 420 anion exchanger treating 50 mL Dichromate solution for 30 min in RT at pH 5.6 with a stirring rate 200 rpm reached 1.73 mg/g. The superiority of the commercial Amberlite IRA 420 anion exchanger may be referred to as the pH of the dichromate solution, which is lower than that used in this study; pH 7.4. On the other hand, the adsorption capacity of MB-SPGMA composite adsorbent reached to 0.88 mg/g with an increase of the permanganate ions concentration from 2 mg/L to 8 mg/L. The curve shows two stages. The first stage is a linear increase of the adsorption capacity with an increase of the permanganate ions concentration up to 4 mg/L. The second stage is an exponential increase of the adsorption capacity with an increase of the permanganate ions concentration from 4 mg/L to 8 mg/L, where the adsorption capacity increased by about ten folds to reach 0.88 mg/g. The same adsorption capacity (0.93 mg/g) obtained using 0.1 g of wet Amberlite IRA 420 with 10 mL of 100 mg/L permanganate [60].The mixture was agitated at R·T using a magnetic stirrer for 30 min. The MB-SPGMA composite adsorbent shows superiority to the commercial Amberlite IRA 420 anion exchanger, which has the same adsorption capacity with treating 8 mg/L permanganate solution compared with 100 mg/L permanganate solution used in the case of using commercial Amberlite IRA 420 anion exchanger. The adsorption rate and capacity of the dichromate metal ions are higher than the permanganate metal ions. This superiority could be referred to as the metal ion radius difference, which directly affects the affinity of CV-SPGMA composite adsorbent towards the metal ions. The pH of the metal ions solution ranged between 7.0 and 8.0, which affects the charge of the CV-SPGMA composite adsorbent mainly by the deprotonation process of the nitrogen atom in the CV immobilized molecules, and finally, the hydrophilicity and porosity of the CV-SPGMA composite adsorbent.
In the other hand, the adsorption process of Cr (VI), and Mn (VII) metals ions has been verified through EDAX analysis, Fig. 11 and Table 4. The Cr, and Mn show up in the EDAX analyses of the Cr-CV-SPGMA, and Mn-CV-SPGMA matrices as 0.252 and 0.192 weight %, while show 0.050 and 0.066 atom% in comparison with the CV-SPGMA composite adsorbent before the adsorption process of both Cr (VI), and Mn (VII) metals ions from Dichromate and permanganate contaminated water synthetic solutions.
4 Conclusion
A novel adsorbent, the CV-SPGMA composite, has been created for the first time to treat Dichromate and Permanganate Contaminated Waste Water. This composite is made of nano-Sulphonated Poly(glycidyl methacrylate) and Hexamethyl Pararosaniline Chloride (Crystal Violet; CV). The nano-Sulphonated Poly (glycidyl methacrylate) (SPGMA) particles have been used to build a novel adsorbent for removing CV dye from wastewater through adsorption. The study examined the influence of different adsorption factors. The concentration of CV was found to have a linearly increasing effect on the CV content, reaching a maximum value of 174.6 mg/g at a concentration of 200 mg/L. The equilibrium was nearly achieved after duration of 90 min. The CV adsorption process has been observed to be endothermic, with a CV content of 178 mg/g achieved at a temperature of 80 oC. The concentration of CV reduced from 240 mg/g to 46 mg/g when the dose of SPGMA adsorbent increased from 5 to 40 mg. The pH of adsorption had the greatest influence, resulting in the maximum CV content at a pH of 10.0, which corresponded to 190.4 mg/g. The reusability of the CV-SPGMA adsorbent was evaluated by conducting sequential adsorption–desorption cycles. The results showed that after 10 cycles, there was only a 13% decrease in the original adsorption effectiveness of the adsorbent. The newly developed CV-SPGMA composite adsorbent was tested for the removal of Cr (VI) and Mn (VII) metal ions from water contaminated with dichromate and permanganate. The adsorbent demonstrated a seven-fold higher affinity for removing Cr (VI) at a rate of 84.6 mg/g compared to Mn (VII) at a rate of 11.66 mg/g. These tests were conducted under mild adsorption conditions. Furthermore, the adsorption process was confirmed by examining alterations in the chemical structure, morphology, and elemental composition using FTIR, SEM, and EDAX analysis, respectively, of the SPGMA adsorbent prior to adsorption and the CV-SPGMA adsorbent subsequent to adsorption. The FTIR spectrum of CV-SPGMA displayed prominent absorption peaks characteristic of disubstituted benzene rings found in the structure of CV. The observed absorption peak at 2921 cm-1 corresponds to the stretching vibration of hydrocarbon (-C-H) in methyl groups. The spectrum exhibited a prominent absorption peak at 1553 cm-1, which can be ascribed to the stretching vibration of (C = C) groups that are present in the benzene ring. Furthermore, an additional peak of absorption was detected at a wavenumber of 1479 cm-1, which corresponds to the methyl group of the quaternized amine. The scanning electron microscope (SEM) images of the CV-SPGMA adsorbent reveal that the adsorption of CV dye caused a formation of a surface coating consisting of SPGMA particles. This led to an increase in surface smoothness and the growth of aggregate size. The EDAX tests of the CV-SPGMA after CV adsorption indicate that the nitrogen weight percentage has increased to 13.620% as a result of the nitrogen content of the dye adsorbed onto the CV. However, the weight percentages of both Na and S decreased from 8.793% and 6.438% respectively in the SPGMA adsorbent before adsorption, to 1.237% and 2.257% respectively following adsorption of CV on the CV-SPGMA adsorbent. The carbon weight percentage increased from 31.428% in the SPGMA adsorbent prior to adsorption to 36.832% in the CV-SPGMA adsorbent. The newly developed CV-SPGMA composite adsorbent was tested for the removal of Cr (VI) and Mn (VII) metal ions from dichromate and permanganate contaminated waters. The adsorption conditions were mild, and the results showed that the adsorbent had a seven-fold higher affinity for removing Cr (VI) (84.6 mg/g) compared to Mn (VII) (11.66 mg/g). The adsorption process of Cr (VI) and Mn (VII) metal ions has been confirmed through EDAX analysis. The EDAX analyses of the Cr-CV-SPGMA and Mn-CV-SPGMA matrices reveal the presence of Cr and Mn at concentrations of 0.252% and 0.192% by weight, respectively. Additionally, the CV-SPGMA composite adsorbent shows concentrations of 0.050% and 0.066% by atom for Cr (VI) and Mn (VII), respectively, before the adsorption process of both metal ions from Dichromate and permanganate contaminated water synthetic solutions.
Data availability
No datasets were generated or analysed during the current study.
References
Noreen, S., Bhatti, H.N., Zuber, M., Zahid, M., Asgher, M.: Removal of actacid orange-RL dye using biocomposites: modeling studies. Pol. J. Environ. Stud. 26(5), 1–10 (2017)
Bhatti, H.N., Safa, Y., Yakout, S.M., Shair, O.H., Iqbal, M., Nazir, A.: Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 150(1), 861–870 (2020)
Noreen, S., Bhatti, H.N., Iqbal, M., Hussain, F., Sarim, F.M.: Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 147(15), 439–452 (2020)
Mahmoodi, N.M., Arami, M.: Numerical finite volume modeling of dye decolorization using immobilized Titania nanophotocatalysis. Chem. Eng. J. 146(2), 189–193 (2009)
Ngo, A.C.R., Tischler, D.: Microbial degradation of azo dyes: approaches and prospects for a hazard-free conversion by microorganisms. Int. J. Environ. Res. Public Health 19, 4740 (2022)
Pirkaramia, A., Olya, M.E., Limaee, N.Y.: Decolorization of azo dyes by photo electro adsorption process using polyaniline coated electrode. Prog. Org. Coat. 76, 682–688 (2013)
Umar, K., Mfarrej, M.F.B., Rahman, Q.I., Zuhaib, M., Khan, A., Zia, Q., Banawas, S., Nadeem, H., Khan, M.F., Ahmad, F.: ZnO Nano-swirlings forAzo Dye AR183 photocatalytic degradation and antimycotic activity. Sci. Rep. 2, 14023 (2022)
Maqbool, M., Bhatti, H.N., Sadaf, S., AL-Anazy, M.M., Iqbal, M.: Biocomposite of polyaniline and sodium alginate with Oscillatoria biomass: a potential adsorbent for the removal of basic blue 41. J. Mater. Res. Technol. 9(6), 14729–14741 (2020)
Maqbool, M., Sadaf, S., Bhatti, H.N., Rehmat, S., Kausar, A., Alissa, S.A., Iqbal, M.: Sodium alginate and polypyrrole composites with algal dead biomass for the adsorption of Congo red dye: kinetics, thermodynamics and desorption studies. Surf. Interfaces 25, 101183 (2021)
Foroutan, R., Mohammadi, R., MousaKhanloo, F., Sahebi, S., Ramavandi, B., Kumar, P.S., Vardhan, K.H.: Performance of montmorillonite/graphene oxide/CoFe2O4 as a magnetic and recyclable nanocomposite for cleaning methyl violet dye-laden wastewater. Adv. Powder Technol. 31, 3993–4004 (2020)
Bonyadi, Z., Kumar, P.S., Foroutan, R., Kafaei, R., Arfaeinia, H., Farjadfard, S., Ramavandi, B.: Ultrasonic-assisted synthesis of Populus alba activated carbon for water defluorination: application for real wastewater. Korean J. Chem. Eng. 36, 1595–1603 (2019)
Peighambardoust, S.J., A-Bavil, O., Foroutan, R., Arsalani, N.: Removal of malachite green using carboxymethyl cellulosegpolyacrylamide/montmorillonite nanocomposite hydroge. Int. J. Bio. Macromo. 159, 1122–1131 (2020)
Esvandi, Z., Foroutan, R., Peighambardoust, S.J., Akbari, A., Ramavandi, B.: Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surf. Interfaces 21, 100754 (2020)
Foroutan, R., Peighambardoust, S.J., Esvandi, Z., Khatooni, H., Ramavandi, B.: Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: a comparative study. J. Environ. Chem. Eng. 9(2), 104752 (2021)
Shah, V., Madamwar, D.: Community genomics: Isolation, characterization and expression of gene coding for azoreductase. Int. Biodeter. Biodegrad. 79(4), 1–8 (2013)
Shertate, R.S., Thorat, P., Department of microbiology & Research center.: Biotransformation of textile dyes, a bioremedial aspect of marine environment. Am. J. Environ. Mental (2014)
Balamurugan, B., Thirumarimurugan, M., Kannadasan, T.: Anaerobic degradation of textile dye bath effluent using Halomonas sp. Bioresour. (2011)
Sharma, G., Kumar, A., Sharma, S., Al-Muhtaseb, A.H., Naushad, Mu., Ghfar, A.A., Ahamad, T., Stadler, F.J.: Fabrication and characterization of novel Fe0@Guar gumcrosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Sep. Purif. Technol. 211, 895–908 (2019)
Sharma, G., Naushad, M., Pathania, D., Mittal, A., El-desoky, G.E.: Modification of Hibiscus cannabinus fiber by graft copolymerization: application for dye removal. Desalin. Water Treat. 54(11), 3114–3121 (2015)
Sharma, G., Dionysiou, D.D., Sharma, S., Kumar, A., Al-Muhtaseb, A.H., Naushad, M., Stadler, F.J.: Highly efficient Sr/Ce/activated carbon bimetallic nanocomposite for photoinduced degradation of rhodamine B. Catal. Today 335, 437–451 (2019)
Sharma, G., Pathania, D., Naushad, M., Kothiyal, N.C.: Fabrication, characterization and antimicrobial activity of polyaniline Th(IV) tungstomolybdophosphate nanocomposite material: efficient removal of toxic metal ions from water. Chem. Eng. J. 251, 413–421 (2014)
Worch, E.: Adsorption technology in water treatment: fundamentals, processes, and modeling. De Gruyter, Berlin, Boston (2012). https://doi.org/10.1515/9783110240238
Foroutan, R., Peighambardoust, S.J., Peighambardoust, S.H., Pateiro, M., Lorenzo, J.M.: Adsorption of crystal violet dye using activated carbon of lemon wood and activated carbon/Fe3O4 magnetic nanocomposite from aqueous solutions: a kinetic equilibrium thermodynamic study. Molecules 26, 2241 (2021)
Song, Y., Tan, J., Wang, G., Zhou, Li.: Superior amine-rich gel adsorbent from peach gum polysaccharide for highly efficient removal of anionic dyes. Carbohyd. Polym. 199, 178–185 (2018)
Pandey, V., Singh, S.B., Gupta, M.K., Afroz, M., Agrahari, S., Singh, H., Tandon, P.K.: Removal of the cationic dyes with activated carbon obtained from low-cost ginger waste. Mater Today: Proc. https://doi.org/10.1016/j.matpr.2023.12.056.
Mladenovic, N., Makreski, P., Tarbuk, A., Grgic, K., Boev, B., Mirakovski, D., Toshikj, E., Dimova, V., Dimitrovski, D., Jordanov, I.: Improved dye removal ability of modified rice husk with effluent from alkaline scouring based on the circular economy concept. Processes 8, 653 (2020)
El Khomri, M., El Messaoudi, N., Dbik, A., Bentahar, S., Lacherai, A., Chegini, Z.G., Iqbal, M.: Organic Dyes Adsorption on the Almond Shell (Prunus dulcis) as Agricultural Solid Waste from Aqueous Solution in Single and Binary Mixture Systems. Biointerface Res. Appl. Chem. 12, 2022–2040 (2022)
Saini, B., Dey, A.: Synthesis and characterization of copolymer adsorbent for crystal violet dye removal from water. Mater. Today: Proc. 61, 342–350 (2022)
Li, S.: Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylase. Biores. Technol. 101, 2197–2202 (2010)
Mohamed, R.R., Abu Elella, M.H., Sabaa, M.W., Saad, G.R.: Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 25, 6513–6529 (2018)
Jana, S., Ray, J., Mondal, B., Tripathy, T.: Efficient and selective removal of cationic organic dyes from their aqueous solutions by a nanocomposite hydrogel, katira gum-cl-poly(acrylic acid-co-N, N-dimethylacrylamide)@bentonite. Appl. Clay Sci. 173, 46–64 (2019)
Abu Elella, M.H., Sabaa, M.W., AbdElHafeez, E., Mohamed, R.R.: Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 137, 1086–1101 (2019)
Mohy Eldin, M.S., Gouda, M.H., Abu-Saied, M.A., El-Shazly, Y.M.S., Farag, H.A.: Development of grafted cotton fabrics ions exchanger for dye removal applications: methylene blue model. Desalin. Water Treat. 57, 22049–22060 (2016)
MohyEldin, M.S., Elkady, M.F., Abdel Rahman, A.M., Soliman, E.A., Elzatahry, A.A., Youssef, M.E., Eweida, B.Y.: Preparation and characterization of imino diacetic acid functionalized alginate beads for removal of contaminates from waste water: I. methylene blue cationic dye model. Desalin. Water Treat. 40, 15–23 (2012)
MohyEldin, M.S., Aly, K., Khan, Z.A., Meky, A.E., Saleh, T.S., Elbogamy, A.S.: Development of novel acid-base ions exchanger for basic dye removal: phosphoric acid doped pyrazole-g-polyglycidyl methacrylate. Desalin. Water Treat. 57, 24047–24055 (2016)
Aksu, Z., In, Y.-S., Wong, N.F.Y., Tam: Algae for wastewater treatment. Springer-Verlag and Landes Bioscience, Germany, Biosorption of heavy metals by microalgae in batch and continuous systems pp. 37–53. (1998)
Van Nguyen, P., Thi Hong Truong, H., Pham, T.A., Le Cong, T., Le, T., Thi Nguyen, K.C.: Removal of manganese and copper from aqueous solution by yeast Papiliotrema huenov. Mycobiology 49, 507–520 (2021)
Ahmaruzzaman, Md.: Adsorption of phenolic compounds on low-cost adsorbents: a review. Adv. Coll. Interf. Sci. 143, 48–67 (2008)
Qian, Y., Gao, P., Xue, G., Liu, Z., Chen, J.: Oxidation of cefalexin by permanganate: reaction kinetics. Mech. Residual Antibacterial Act. Mol. 23, 2015 (2018)
Gupta, V., Kumari, S., Virvadiya, C.: Adsorption analysis of Mn(VII) from aqueous medium by activated orange peels powder. Int. Res. J. Pure Appl. Chem. 9, 1–8 (2015)
Ezeugo, J., Anadebe, C.V.: Removal of potassium permanganate from aqueous solution by adsorption onto activated carbon prepared from animal bone and corn cob. Equatorial J. Eng. 14-21 (2018)
Mahmoud, M.E., Yakout, A.A., Saad, S.R., Osman, M.M.: Removal of potassium permanganate from water by modified carbonaceous materials. Desalin. Water Treat. 57, 15559–15569 (2016)
Virvadiya, C., Kumari, S., Choudhary, V., Gupta, V.: Combined bio- and chemosorption of Mn(VII) from aqueous solution by PROSOPIS CINERARIA leaf powder. Eur. Chem. Bull. 3, 315–318 (2014)
Chaudhary, M.: Use of millet husk as a biosorbent for the removal of chromium and manganese ions from the aqueous solutions. Int. J. Chem. Environ. Pharm. Res. 2, 30–33 (2011)
Wan Ngah, W.S., Hanafiah, M.A.K.M.: Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour. Technol. 99, 3935–3948 (2007)
Waranusantigula, P., Pokethitiyook, P., Kruatrachue, M., Upatham, E.S.: Kinetics of cbasic dye (methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ. Pollut. 125, 385–392 (2003)
Vo, A.T., Nguyen, V.P., Ouakouak, A., Nieva, A., Doma, B.T., Jr., Tran, H.N., Chao, H.-P.: Efficient removal of Cr(VI) from water by biochar and activated carbon prepared through hydrothermal carbonization and pyrolysis: adsorption-coupled reduction mechanism. Water 11, 1164 (2019)
Varga, M., Takács, M., Záray, G., Varga, I.: Comparative study of sorption kinetics and equilibrium of chromium (VI) on charcoals prepared from different low-cost materials. Microchem. J. 107, 25–30 (2013)
Srinivasan, K.: Evaluation of rice husk carbon for the removal of trace inorganic form water. Thesis Submitted to I.I.T Madras (1986)
Fawzy, M.A., Darwish, H., Alharthi, S., Zaban, M.I., Noureldeen, A., Hassan, S.H.A.: Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box-Behnken experimental design. Sci. Rep. 12, 3256 (2022)
Malathi, S., Srinivasan, K., Gomathi, M.: Studies on the removal of Cr (VI) from aqueous solution by activated carbon developed from cottonseed activated withsulphuric acid. Int. J. Chem. Tech. Res. 8, 795–802 (2015)
Samani, M.R., Toghraie, D.: Removal of hexavalent chromium from water using polyaniline/ wood sawdust/ poly ethylene glycol composite: an experimental study. J. Environ. Health Sci. Eng. 17, 53–62 (2019)
Jassal, P.S., Raut, V.P., Anand, N.: Removal of chromium (VI) ions from Aqueous solution onto Chitosan and cross-linked chitosan beads. Proc. Indian Natl. Sci. Acad. 76, 1-6 (2010)
Marin, N.M.: Natural and synthetic polymers modified with acid blue 113 for removal of Cr3+, Zn2+ and Mn2+. Polymers 14, 2139 (2022)
El-Aassar, M.R., Tamer, T.M., El-Sayed, M.Y., Omer, A.M., Althobaiti, I.O., Youssef, M.E., Alolaimi, R.F., El Agammy, E.F., Alruwaili, M.S., Mohy-Eldin, M.S.: Development of azo dye immobilized poly (Glycidyl methacrylate-co-Methyl Methacrylate) polymers composites as novel adsorbents for water treatment applications: methylene blue-polymers composites. Polymers 14(21), 4672 (2022)
El-Aassar, M.R., Tamer, T.M., El-Sayed, M.Y., Omer, A.M., Althobaiti, I.O., Youssef, M.E., Alolaimi, R.F., El Agammy, E.F., Alruwaili, M.S., Mohy-Eldin, M.S.: Development of azo dye immobilized sulfonated poly (Glycidyl methacrylate) polymers composites as novel adsorbents for water treatment applications: methylene blue adsorption isotherm, kinetic, thermodynamic, and simulations studies. Molecules 27(23), 8418 (2022)
Mohy Eldin, M.S., Elkady, M.F., Abu-Saied, M.A., Abdel Rahman, A.M., Soliman, E.A., Elzatahry, A.A., Youssef, M.E.: Removal of cadmium ions from synthetic aqueous solutions using a novel nano-sulphonated poly glycidylmethacrylate cation exchanger: kinetic and equilibrium studies. J. App. Poly. Sci. 118, 3111–3122 (2010)
Elkady, M.F., Abu-Saied, M.A., Abdel Rahman, A.M., Soliman, E.A., Elzatahry, A.A., Youssef, M.E., Mohy Eldin, M.S.: Novel nano-sulphonated polyglycidyl methacrylate cation exchanger for removal of heavy metals: optimization of the operational conditions. Desalination 279, 152–162 (2011)
MohyEldin, M.S., Al-Bogami, A.S., Aly, K.M., Khan, Z.A., Mekky, A.E.M., Saleh, T.S., HakamyA, A.-W.: Removal of chromium (VI) metal ions using amberlite IRA-420 anions exchanger. Desalin. Water Treat. 60, 335–342 (2017)
Mohy Eldin, M.S., Alamry, K.A., Al-Malki, M.A.: Kinetic and isothermal studies of manganese (VII) ions removal using amberlite IRA-420 anions exchanger. Desalin. Water Treat. 72, 30–40 (2017)
Eren, Z., Acar, F.N.: Adsorption of reactive black 5 from an aqueous solution: equilibrium and kinetic studies. Desalination 194, 1–10 (2006)
Kannan, N., Sundaram, M.M.: Kinetics and mechanism of removal of methylene blue by adsorption on various carbons: a comparative study. Dyes Pigm. 51, 25–40 (2001)
Bulut, Y., Aydin, A.: A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 194, 259–267 (2006)
Satapathy, M.K., Das, P.: Optimization of crystal violet dye removal using novel soil-silver nanocomposite as nanoadsorbent using response surface methodology. J. Environ. Chem. Eng. 2, 708–714 (2014)
Ali, H., Muhammad, S.K.: Biosorption of crystal violet from water on leaf biomass of Calotropis procera. J. Environ. Sci. Technol. 1, 143–150 (2008)
Kumari, J., Krishnamoorthy, H., Arumugam, P., Radhakrishnan, T.K., Vasudevan, S.D.: An efficient removal of crystal violet dye from waste water by adsorption onto TLAC/Chitosan composite: a novel low cost adsorbent. Int. J. Biol. Macromol. 96, 324–333 (2017)
Hamidzadeh, S., Torabbeigi, M., Shahtaheri, S.J.: Removal of crystal violet from water by magnetically modified activated carbon and nanomagnetic iron oxide. J. Environ. Heal. Sci. Eng. 13, 8 (2015)
Abdolahi, G., Dargahi, M., Ghasemzadeh, H.: Synthesis of starch-g-poly (acrylic acid)/ZnSe quantum dot nanocomposite hydrogel, for effective dye adsorption and photocatalytic degradation: thermodynamic and kinetic studies. Cellulose 27, 6467–6483 (2020)
Zhou, Y., Zhang, M., Wang, X., Huang, Q., Min, Y., Ma, T., Niu, J.: Removal of crystal violet by a novel cellulose-based adsorbent: comparison with native cellulose. Ind. Eng. Chem. Res. 53, 5498–5506 (2014)
Qiao, H., Zhou, Y., Yu, F., Wang, E., Min, Y., Huang, Q., Pang, L., Ma, T.: Effective removal of cationic dyes using carboxylate-functionalized cellulose nanocrystals. Chemosphere 141, 297–303 (2015)
Abu Elella, M.H., Sabaa, M.W., AbdElHafeez, E., Mohamed, R.R.: Crystal violet dye removal using crosslinked grafted xanthan gum. Int. J. Biol. Macromol. 137, 1086–1101 (2019)
Mohamed, R.R., Abu Elella, M.H., Sabaa, M.W., Saad, G.R.: Synthesis of an efficient adsorbent hydrogel based on biodegradable polymers for removing crystal violet dye from aqueous solution. Cellulose 25, 6513–6529 (2018)
Bajpai, S., Jain, A.: Equilibrium and thermodynamic studies for adsorption of crystal violet onto spent tea leaves (STL). Water 4, 52–71 (2012)
Cheriaa, J., Khaireddine, M., Rouabhia, M., Bakhrouf, A.: Removal of triphenylmethane dyes by bacterial consortium. Sci. World J. 2012, 1–9 (2012)
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU), (grant number IMSIU-RP23098).
Author information
Authors and Affiliations
Contributions
T.T. performed the investigation and wrote the first draft, M. A. requested the fund and project management, A. O. performed the investigation and wrote the first draft, A. A. helped in project management, M.Y. applied the models for results and wrote the first draft, T.Y. applied the models for results and wrote the first draft, R.K. applied the models for results and wrote the first draft, M.S. tabulated the results and produced the figures, M. M. design and supervise the work, reviewed & edited the final version and submitted the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable. The research did not involve human participants or animals.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamer, T., Abou-Krisha, M., Omer, A. et al. Nano-Sulphonated Poly (glycidyl methacrylate)-Hexamethyl Pararosaniline chloride novel composite adsorbent development for treatment of dichromate and permanganate contaminated waste water. Adsorption 30, 877–890 (2024). https://doi.org/10.1007/s10450-024-00460-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-024-00460-z